Abstract
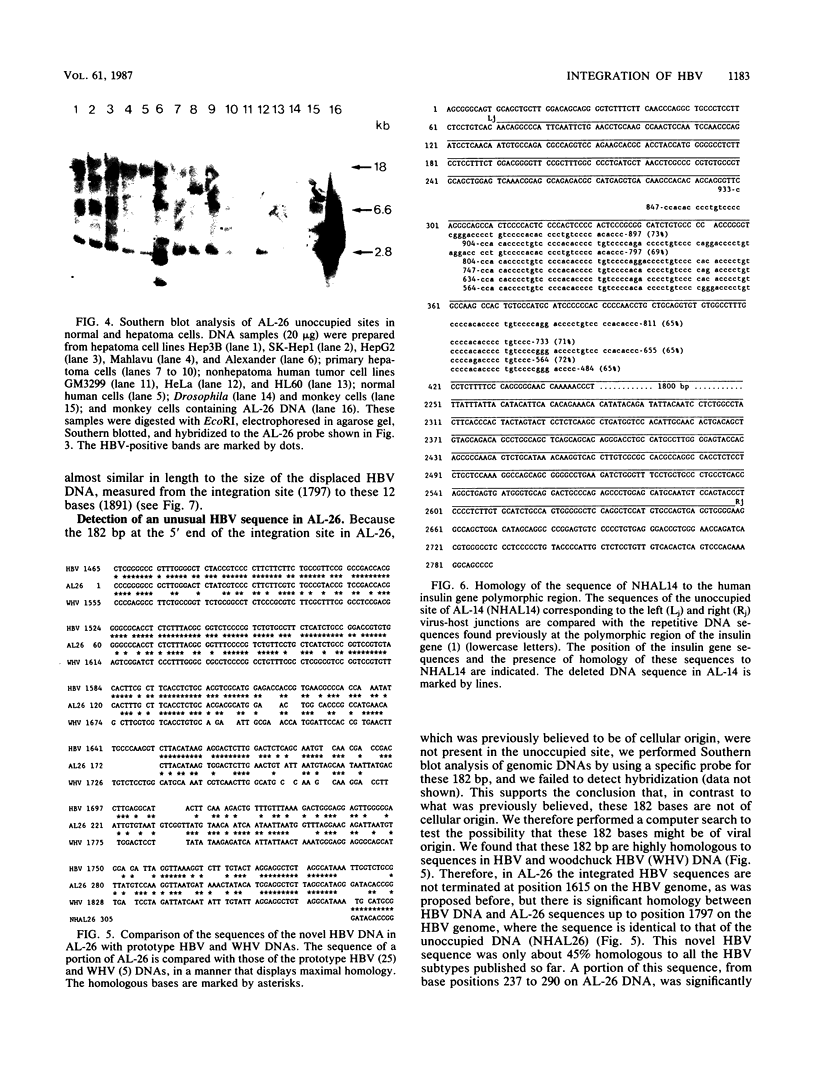
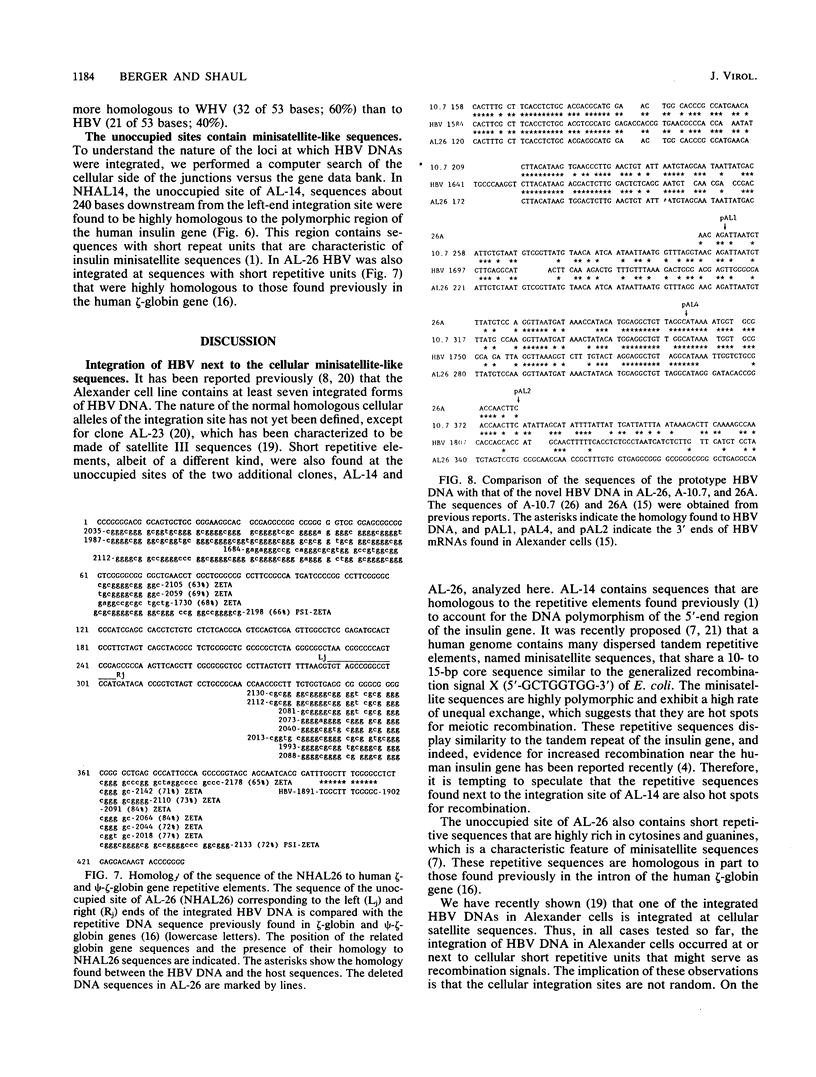
Hepatitis B virus (HBV) sequences integrated in the PLC/PRF/5 cell line (Alexander cells), which was derived from a human primary liver carcinoma, were previously extensively studied. Here we describe the analysis of the unoccupied sites of two linearly integrated forms of HBV DNA, AL-14 and AL-26, that were characterized previously. No major cellular DNA rearrangements were seen at the integration sites except for small deletions of host sequences: 2 kilobases of DNA in AL-14 and 17 base pairs (bp) in AL-26. The unoccupied site of AL-26 was found to be missing 182 bp, which previously mapped next to the right end of the integration sites of several independent clones. These were believed to be of cellular origin, but we show here that these 182 bp are in fact from unusual HBV sequences. Surprisingly, a region of this newly detected HBV DNA sequence is more homologous to that of woodchuck HBV DNA. Our analysis shows that the normal counterparts of both AL-14 and AL-26 contain minisatellite-like repetitive sequences. Based on the data presented here and our previous finding of HBV DNA integration at satellite sequences, we propose that genomic simple repetitive sequences are hot spots for HBV DNA integration.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bullock P., Forrester W., Botchan M. DNA sequence studies of simian virus 40 chromosomal excision and integration in rat cells. J Mol Biol. 1984 Mar 25;174(1):55–84. doi: 10.1016/0022-2836(84)90365-6. [DOI] [PubMed] [Google Scholar]
- Chakravarti A., Elbein S. C., Permutt M. A. Evidence for increased recombination near the human insulin gene: implication for disease association studies. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1045–1049. doi: 10.1073/pnas.83.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S. E., Higgs D. R., Clegg J. B., Weatherall D. J. Molecular basis of length polymorphism in the human zeta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5022–5026. doi: 10.1073/pnas.80.16.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Koch S., Freytag von Loringhoven A., Kahmann R., Hofschneider P. H., Koshy R. The genetic organization of integrated hepatitis B virus DNA in the human hepatoma cell line PLC/PRF/5. Nucleic Acids Res. 1984 Sep 11;12(17):6871–6886. doi: 10.1093/nar/12.17.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S., von Loringhoven A. F., Hofschneider P. H., Koshy R. Amplification and rearrangement in hepatoma cell DNA associated with integrated hepatitis B virus DNA. EMBO J. 1984 Sep;3(9):2185–2189. doi: 10.1002/j.1460-2075.1984.tb02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy R., Maupas P., Müller R., Hofschneider P. H. Detection of hepatitis B virus-specific DNA in the genomes of human hepatocellular carcinoma and liver cirrhosis tissues. J Gen Virol. 1981 Nov;57(Pt 1):95–102. doi: 10.1099/0022-1317-57-1-95. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Marvo S. L., King S. R., Jaskunas S. R. Role of short regions of homology in intermolecular illegitimate recombination events. Proc Natl Acad Sci U S A. 1983 May;80(9):2452–2456. doi: 10.1073/pnas.80.9.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Rutter W. J. Hybrid hepatitis B virus-host transcripts in a human hepatoma cell. Proc Natl Acad Sci U S A. 1985 Jan;82(1):83–87. doi: 10.1073/pnas.82.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Garcia P. D., Schonberg S., Rutter W. J. Integration of hepatitis B virus DNA in chromosome-specific satellite sequences. J Virol. 1986 Sep;59(3):731–734. doi: 10.1128/jvi.59.3.731-734.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ziemer M., Garcia P. D., Crawford R., Hsu H., Valenzuela P., Rutter W. J. Cloning and analysis of integrated hepatitis virus sequences from a human hepatoma cell line. J Virol. 1984 Sep;51(3):776–787. doi: 10.1128/jvi.51.3.776-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Loringhoven A. F., Koch S., Hofschneider P. H., Koshy R. Co-transcribed 3' host sequences augment expression of integrated hepatitis B virus DNA. EMBO J. 1985 Jan;4(1):249–255. doi: 10.1002/j.1460-2075.1985.tb02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]