Abstract
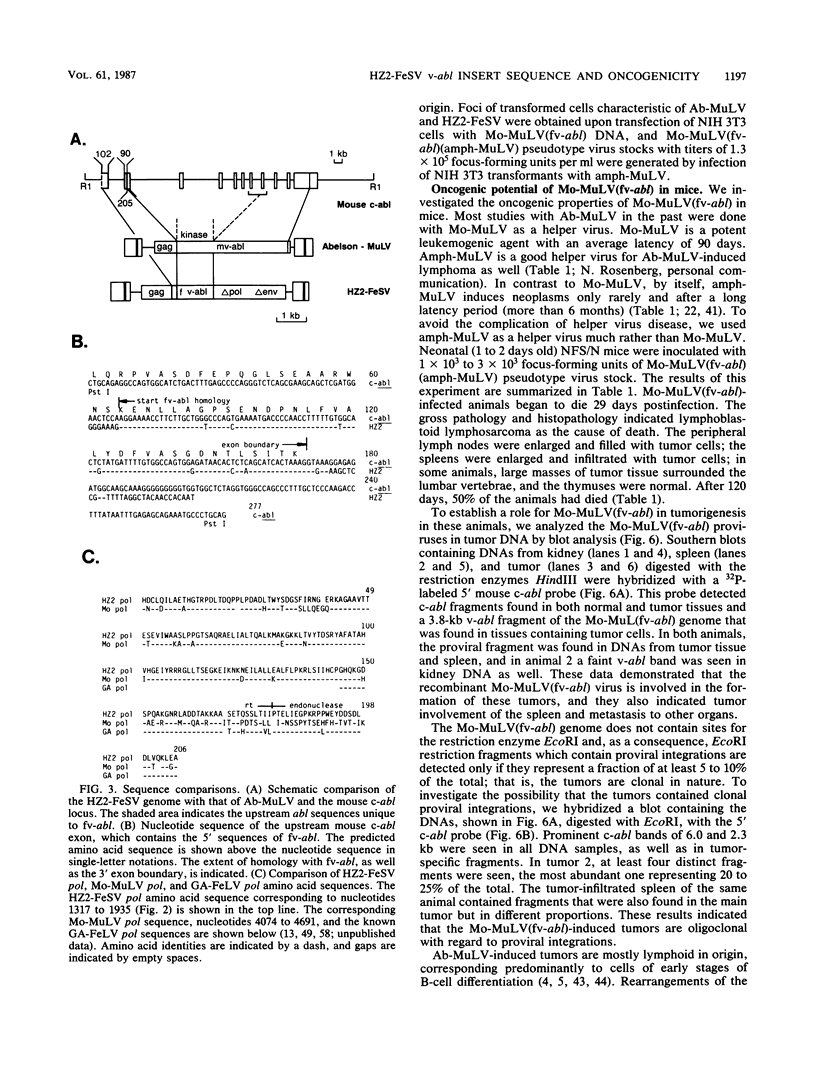
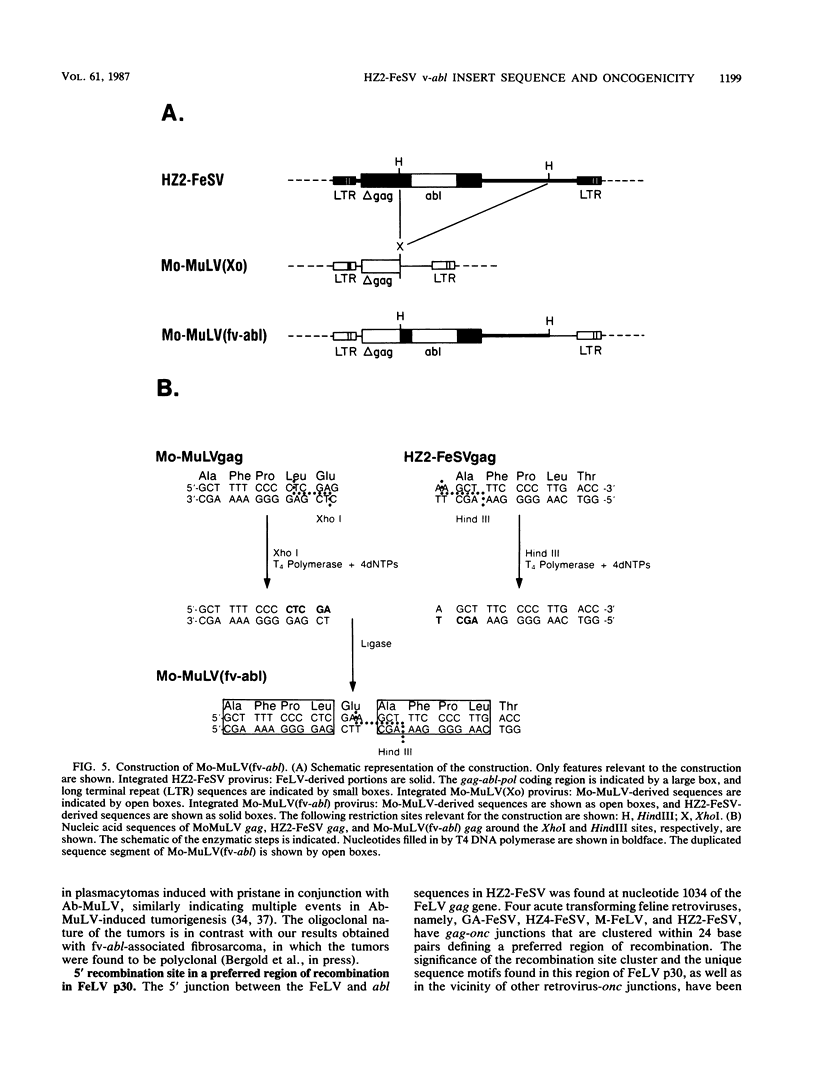
Hardy-Zuckerman 2 feline sarcoma virus (HZ2-FeSV), isolated from a multicentric feline fibrosarcoma is a replication-defective acute transforming feline retrovirus which originated by transduction of feline c-abl sequences with feline leukemia virus (FeLV) and is known to encode a 110-kilodalton gag-abl fusion protein with tyrosine-specific protein kinase activity (P. Besmer, W. D. Hardy, E. E. Zuckerman, P. J. Bergold, L. Lederman, and H. W. Snyder, Nature (London) 303:825-828, 1983). The nucleotide sequence of the abl segment in the HZ2-FeSV genome was determined and compared with the murine and human v-abl and c-abl sequences. The predicted transforming protein consists of 344 amino acids (aa) of FeLV gag origin, 439 aa of abl origin, and at least 200 aa of FeLV pol origin (p110gag-abl-pol). The 1,317-base-pair HZ2-FeSV v-abl segment (fv-abl) corresponds to 5' abl sequences which include the region known to specify the protein kinase domain. The 5' 189 base pairs of fv-abl correspond to 5' c-abl sequences not contained in Abelson murine leukemia virus (MuLV) v-abl. The mouse c-abl exon which contains these segments was identified, and its nucleotide sequence was determined. Comparison of the predicted amino acid sequence of fv-abl with those of Abelson MuLV v-abl and c-abl revealed five aa differences. The 5' junction between FeLV and abl was found to involve a preferred region in FeLV gag p30 (P. Besmer, J. E. Murphy, P. C. George, F. H. Qiu, P. J. Bergold, L. Lederman, H. W. Snyder, D. Brodeur, E. E. Zuckerman, and W. D. Hardy, Nature (London) 320:415-421, 1986). A six-base homology exists at the recombination site between the parental FeLV and the c-abl sequences. The 3' junction between fv-abl and FeLV pol predicts an in-frame fusion of fv-abl and FeLV pol. A transformed cell line containing a truncated gag-abl-pol protein, p85, that lacks most of the FeLV pol sequences was obtained by transfection of NIH 3T3 mouse cells. This result implies that the pol sequences of the p110gag-abl-pol protein are dispensable for fibroblast transformation. To assess whether the fv-abl segment specifies the unique biological properties of HZ2-FeSV, we constructed a Moloney MuLV-based version of HZ2-FeSV, Mo-MuLV(fv-abl), in which the fv-abl sequences were contained in a genetic context similar to that in HZ2-FeSV.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Influence of prednisolone on Moloney leukemogenic virus in BALB-c mice. Cancer Res. 1970 Aug;30(8):2208–2212. [PubMed] [Google Scholar]
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Ben-Neriah Y., Daley G. Q., Mes-Masson A. M., Witte O. N., Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986 Jul 11;233(4760):212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- Besmer P. Acute transforming feline retroviruses. Curr Top Microbiol Immunol. 1983;107:1–27. doi: 10.1007/978-3-642-69075-4_1. [DOI] [PubMed] [Google Scholar]
- Besmer P., Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977 Mar;21(3):965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Hardy W. D., Jr, Zuckerman E. E., Bergold P., Lederman L., Snyder H. W., Jr The Hardy-Zuckerman 2-FeSV, a new feline retrovirus with oncogene homology to Abelson-MuLV. Nature. 1983 Jun 30;303(5920):825–828. doi: 10.1038/303825a0. [DOI] [PubMed] [Google Scholar]
- Besmer P., Lader E., George P. C., Bergold P. J., Qiu F. H., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus with fms homology specifies a C-terminally truncated version of the c-fms protein that is different from SM-feline sarcoma virus v-fms protein. J Virol. 1986 Oct;60(1):194–203. doi: 10.1128/jvi.60.1.194-203.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Besmer P., Snyder H. W., Jr, Murphy J. E., Hardy W. D., Jr, Parodi A. The Parodi-Irgens feline sarcoma virus and simian sarcoma virus have homologous oncogenes, but in different contexts of the viral genomes. J Virol. 1983 May;46(2):606–613. doi: 10.1128/jvi.46.2.606-613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Kerby S. B., Sutrave P., Gunnell M. A., Mark G., Rapp U. R. Structure and biological activity of human homologs of the raf/mil oncogene. Mol Cell Biol. 1985 Jun;5(6):1400–1407. doi: 10.1128/mcb.5.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Gerard G. F., Hixson C. W., Oroszlan S. Amino- and carboxyl-terminal sequence of Moloney murine leukemia virus reverse transcriptase. Virology. 1985 Jun;143(2):676–679. doi: 10.1016/0042-6822(85)90411-8. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Johnson M. S., Doolittle R. F. Aligning amino acid sequences: comparison of commonly used methods. J Mol Evol. 1984;21(2):112–125. doi: 10.1007/BF02100085. [DOI] [PubMed] [Google Scholar]
- Feuerman M. H., Davis B. R., Pattengale P. K., Fan H. Generation of a recombinant Moloney murine leukemia virus carrying the v-src gene of avian sarcoma virus: transformation in vitro and pathogenesis in vivo. J Virol. 1985 Jun;54(3):804–816. doi: 10.1128/jvi.54.3.804-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Generation of novel, biologically active Harvey sarcoma viruses via apparent illegitimate recombination. J Virol. 1981 Apr;38(1):136–150. doi: 10.1128/jvi.38.1.136-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Stephenson J. R., Groffen J., Hansen P. F., de Klein A., Bartram C. R., Grosveld G. Localization of the c-ab1 oncogene adjacent to a translocation break point in chronic myelocytic leukaemia. Nature. 1983 Nov 17;306(5940):239–242. doi: 10.1038/306239a0. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. 5' viral and human cellular sequences corresponding to the transforming gene of simian sarcoma virus. Science. 1983 Feb 4;219(4584):503–505. doi: 10.1126/science.6297002. [DOI] [PubMed] [Google Scholar]
- Laprevotte I., Hampe A., Sherr C. J., Galibert F. Nucleotide sequence of the gag gene and gag-pol junction of feline leukemia virus. J Virol. 1984 Jun;50(3):884–894. doi: 10.1128/jvi.50.3.884-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman L., Singhal M. C., Besmer P., Zuckerman E. E., Hardy W. D., Jr, Snyder H. W., Jr Immunological and biochemical characterization of HZ2 feline sarcoma virus and Abelson murine leukaemia virus translation products. J Gen Virol. 1985 Sep;66(Pt 9):2057–2063. doi: 10.1099/0022-1317-66-9-2057. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Rapp U. R. Primary structure of v-raf: relatedness to the src family of oncogenes. Science. 1984 Apr 20;224(4646):285–289. doi: 10.1126/science.6324342. [DOI] [PubMed] [Google Scholar]
- Mushinski J. F., Potter M., Bauer S. R., Reddy E. P. DNA rearrangement and altered RNA expression of the c-myb oncogene in mouse plasmacytoid lymphosarcomas. Science. 1983 May 20;220(4599):795–798. doi: 10.1126/science.6687762. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- Ohno S., Migita S., Wiener F., Babonits M., Klein G., Mushinski J. F., Potter M. Chromosomal translocations activating myc sequences and transduction of v-abl are critical events in the rapid induction of plasmacytomas by pristane and abelson virus. J Exp Med. 1984 Jun 1;159(6):1762–1777. doi: 10.1084/jem.159.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Agranovsky O., McKinney M. D., Murty V. V., Bauchwitz R. Friend murine leukemia virus-immortalized myeloid cells are converted into tumorigenic cell lines by Abelson leukemia virus. Proc Natl Acad Sci U S A. 1985 May;82(10):3306–3310. doi: 10.1073/pnas.82.10.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. The pathogenesis of Abelson virus lymphomas of the mouse. Biochim Biophys Acta. 1982 Jun 28;651(4):213–244. doi: 10.1016/0304-419x(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. Abelson leukemia virus. Curr Top Microbiol Immunol. 1982;101:95–126. doi: 10.1007/978-3-642-68654-2_5. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr Biochemical characterization of protein kinase activities associated with transforming gene products of the Snyder-Theilen and Gardner-Arnstein strains of feline sarcoma virus. Virology. 1982 Feb;117(1):165–172. doi: 10.1016/0042-6822(82)90516-5. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Parker R. C., Varmus H. E., Bishop J. M. Transduction of a cellular oncogene: the genesis of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 May;80(9):2519–2523. doi: 10.1073/pnas.80.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Walther N., Lurz R., Patschinsky T., Jansen H. W., Bister K. Molecular cloning of proviral DNA and structural analysis of the transduced myc oncogene of avian oncovirus CMII. J Virol. 1985 May;54(2):576–585. doi: 10.1128/jvi.54.2.576-585.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Keyes L., Rosenberg N. Abelson virus drives the differentiation of Harvey virus-infected erythroid cells. Cell. 1986 Jan 31;44(2):337–344. doi: 10.1016/0092-8674(86)90768-3. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Ledley F., Goff S., Lee R., Groner Y., Baltimore D. The mouse c-abl locus: molecular cloning and characterization. Cell. 1984 Feb;36(2):349–356. doi: 10.1016/0092-8674(84)90228-9. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. The complexity of virus--cell interactions in Abelson virus infection of lymphoid and other hematopoietic cells. Adv Immunol. 1985;37:73–98. doi: 10.1016/s0065-2776(08)60338-7. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Witte O. N. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol Cell Biol. 1983 Apr;3(4):596–604. doi: 10.1128/mcb.3.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K. C., Eggleton K., Temin H. M. Nucleic acid sequences of the oncogene v-rel in reticuloendotheliosis virus strain T and its cellular homolog, the proto-oncogene c-rel. J Virol. 1984 Oct;52(1):172–182. doi: 10.1128/jvi.52.1.172-182.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Nelson N. C., Taylor S. S. Affinity labeling of cAMP-dependent protein kinase with p-fluorosulfonylbenzoyl adenosine. Covalent modification of lysine 71. J Biol Chem. 1981 Nov 10;256(21):10837–10842. [PubMed] [Google Scholar]