Abstract
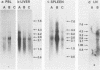
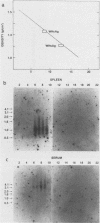
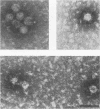
Lymphoid cells were purified from the spleens of 15 woodchucks and examined for the presence of woodchuck hepatitis virus (WHV). Lymphoid cells from the spleens of eight of eight chronically infected animals contained high levels of WHV RNA and DNA. A 100-fold lower level of WHV DNA was found in the spleen from one of five animals that had recovered from acute WHV infections 2 years before this analysis. No WHV nucleic acids were observed in either of two uninfected animals. WHV DNA patterns in the lymphoid cells from the spleens of the chronically infected animals, which included the presence of single-stranded DNA and RNA-DNA hybrid molecules, were identical to those observed in WHV-infected liver. WHV DNA in these cells was present in intact, 27-nm core particles which also contained the endogenous DNA polymerase activity. These results indicate that the spleen is a site of active WHV DNA replication and is most likely a major source of WHV-infected cells in the circulating lymphoid cell population.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cote P. J., Engle R. E., Langer C. A., Ponzetto A., Gerin J. L. Antigenic analysis of woodchuck hepatitis virus surface antigen with site-specific radioimmunoassays. J Virol. 1984 Mar;49(3):701–708. doi: 10.1128/jvi.49.3.701-708.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., England J. M., Deery D. T., Petcu D. J., Mason W. S., Molnar-Kimber K. L. Viral nucleic acid synthesis and antigen accumulation in pancreas and kidney of Pekin ducks infected with duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4865–4869. doi: 10.1073/pnas.80.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. M., Ford E. C., Purcell R. H., Gerin J. L. Demonstration of subpopulations of Dane particles. J Virol. 1976 Mar;17(3):885–893. doi: 10.1128/jvi.17.3.885-893.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K., Ogasawara N., Yoshikawa H., Murakami S. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J Virol. 1985 Dec;56(3):978–986. doi: 10.1128/jvi.56.3.978-986.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Yoakum G. H., Purcell R. H., Gerin J. L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986 Apr;58(1):1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure F., Zagury D., Saimot A. G., Gallo R. C., Hahn B. H., Brechot C. Hepatitis B virus DNA sequences in lymphoid cells from patients with AIDS and AIDS-related complex. Science. 1985 Aug 9;229(4713):561–563. doi: 10.1126/science.2410981. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Marion P. L., Robinson W. S. Hepatitis B viral DNA-RNA hybrid molecules in particles from infected liver are converted to viral DNA molecules during an endogenous DNA polymerase reaction. Virology. 1984 Nov;139(1):64–72. doi: 10.1016/0042-6822(84)90330-1. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Tran C. T., Robinson W. S. Hepatitis B virus particles of plasma and liver contain viral DNA-RNA hybrid molecules. Virology. 1984 Nov;139(1):53–63. doi: 10.1016/0042-6822(84)90329-5. [DOI] [PubMed] [Google Scholar]
- Noonan C. A., Yoffe B., Mansell P. W., Melnick J. L., Hollinger F. B. Extrachromosomal sequences of hepatitis B virus DNA in peripheral blood mononuclear cells of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5698–5702. doi: 10.1073/pnas.83.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Gonzalez-Vitale J. C., Fagan C. J. Polycystic liver disease: a study of cyst fluid constituents. Hepatology. 1982 Jul-Aug;2(4):475–478. doi: 10.1002/hep.1840020414. [DOI] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Ford E. C., Engle R., Cicmanec J., Shapiro M., Purcell R. H., Gerin J. L. Radioimmunoassay and characterization of woodchuck hepatitis virus core antigen and antibody. Virus Res. 1985 Jun;2(4):301–315. doi: 10.1016/0168-1702(85)90027-9. [DOI] [PubMed] [Google Scholar]
- Rizzetto M., Hoyer B., Canese M. G., Shih J. W., Purcell R. H., Gerin J. L. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6124–6128. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romet-Lemonne J. L., McLane M. F., Elfassi E., Haseltine W. A., Azocar J., Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983 Aug 12;221(4611):667–669. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Shen H. D., Choo K. B., Lee S. D., Tsai Y. T., Han S. H. Hepatitis B virus DNA in leukocytes of patients with hepatitis B virus-associated liver diseases. J Med Virol. 1986 Mar;18(3):201–211. doi: 10.1002/jmv.1890180302. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M., Omata M., Yokosuka O., Uchiumi K., Imazeki F., Okuda K. Early events in duck hepatitis B virus infection. Sequential appearance of viral deoxyribonucleic acid in the liver, pancreas, kidney, and spleen. Gastroenterology. 1985 Dec;89(6):1224–1229. [PubMed] [Google Scholar]
- Yoffe B., Noonan C. A., Melnick J. L., Hollinger F. B. Hepatitis B virus DNA in mononuclear cells and analysis of cell subsets for the presence of replicative intermediates of viral DNA. J Infect Dis. 1986 Mar;153(3):471–477. doi: 10.1093/infdis/153.3.471. [DOI] [PubMed] [Google Scholar]