Abstract
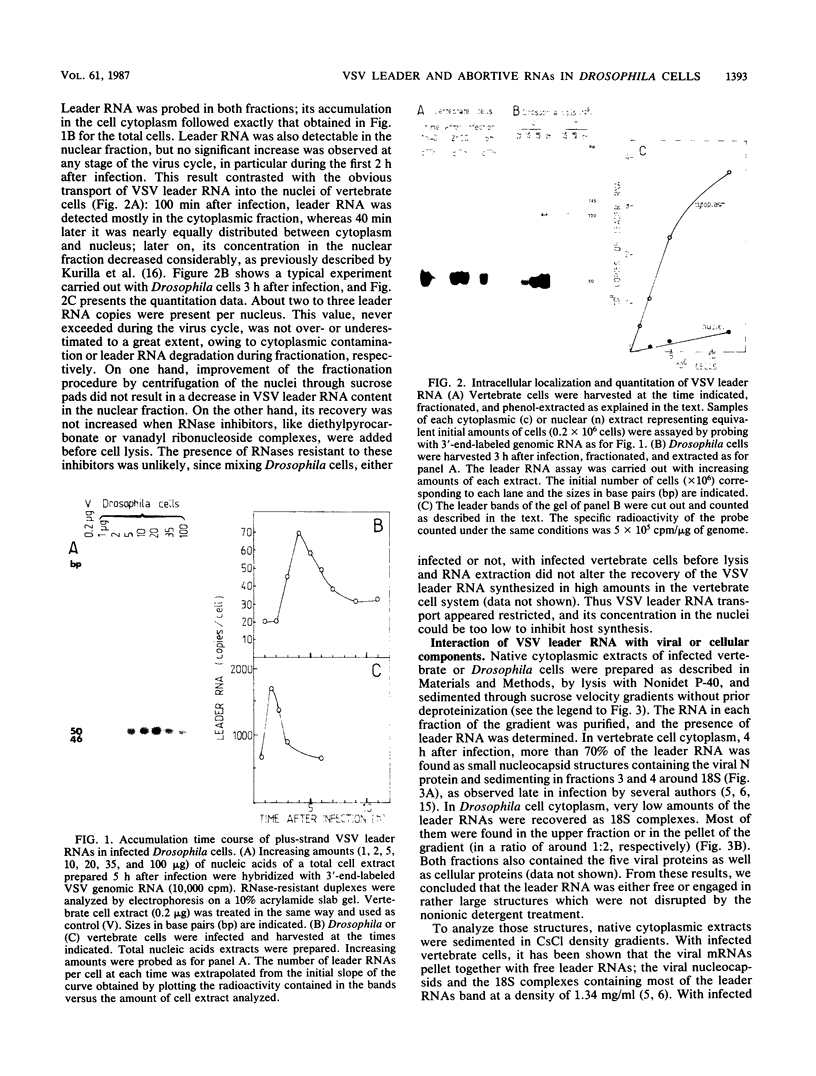
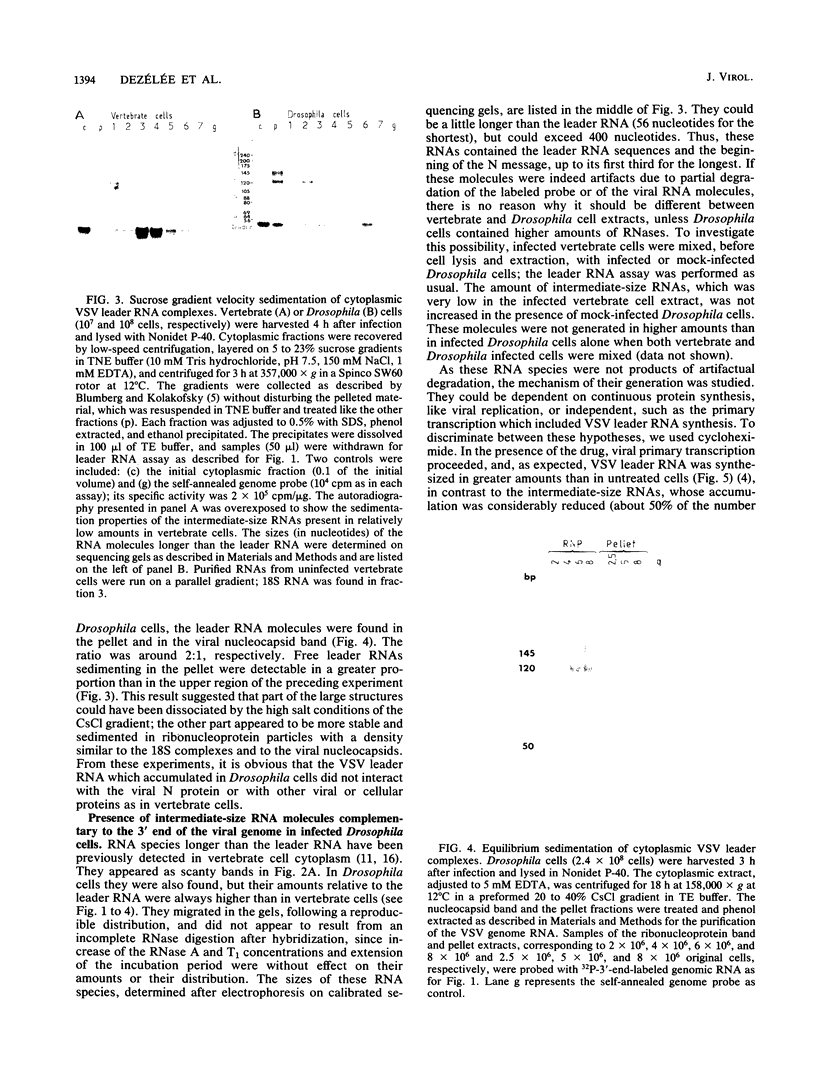
In cultured Drosophila melanogaster cells, vesicular stomatitis virus (VSV) establishes a persistent, noncytopathic infection. No inhibition of host macromolecular synthesis occurs. We studied the synthesis of VSV plus-strand leader RNA, which may be directly involved in vertebrate host synthesis shut-off. Leader RNA accumulated in Drosophila cell cytoplasm, but in low amounts, it was either free or associated to structures larger than the leader RNA-N protein complexes found in vertebrate cells. Only a few leader RNA copies migrated into the cell nucleus; no increase of this transport was observed at any time during the virus cycle. Viral RNAs complementary to the 3' end of the genome and ranging in size from the leader to several hundred nucleotides were found to accumulate in Drosophila cell cytoplasm. Their synthesis was inhibited in the presence of cycloheximide, which blocks all protein synthesis and VSV replication. Correlation between the absence of VSV cytopathogenicity in Drosophila cells and the lack of leader RNA transport into their nuclei is discussed, as well as the possible relationship between the restriction of viral synthesis and the frequent initiation of an abortive replication step.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Blondel D., Dezelee S., Wyers F. Vesicular stomatitis virus growth in Drosophila melanogaster cells. II. Modifications of viral protein phosphorylation. J Gen Virol. 1983 Aug;64(Pt 8):1793–1799. doi: 10.1099/0022-1317-64-8-1793. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Kolakofsky D. Intracellular vesicular stomatitis virus leader RNAs are found in nucleocapsid structures. J Virol. 1981 Nov;40(2):568–576. doi: 10.1128/jvi.40.2.568-576.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Chinchar V. G., Amesse L. S., Portner A. Linked transcripts of the genes for leader and N message are synthesized in vitro by vesicular stomatitis virus. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1296–1302. doi: 10.1016/0006-291x(82)90927-5. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Baird S., Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986 Apr 15;150(1):231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S., Stollar V. The production of high yields of infectious vesicular stomatitis virus in A. albopictus cells and comparisons with replication in BHK-21 cells. Virology. 1980 Dec;107(2):509–513. doi: 10.1016/0042-6822(80)90317-7. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983 Oct;48(1):88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Inhibition of DNA-dependent transcription by the leader RNA of vesicular stomatitis virus: role of specific nucleotide sequences and cell protein binding. Mol Cell Biol. 1985 Oct;5(10):2502–2513. doi: 10.1128/mcb.5.10.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Wagner R. R. Inhibition of cellular DNA synthesis by vesicular stomatitis virus. J Virol. 1981 Apr;38(1):356–367. doi: 10.1128/jvi.38.1.356-367.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Leavitt R. W., Kingsbury D. T., Holland J. J. Natural selection of mutants of vesicular stomatitis virus by cultured cells of Drosophila melanogaster. J Gen Virol. 1973 Sep;20(3):341–351. doi: 10.1099/0022-1317-20-3-341. [DOI] [PubMed] [Google Scholar]
- O'Hara P. J., Horodyski F. M., Nichol S. T., Holland J. J. Vesicular stomatitis virus mutants resistant to defective-interfering particles accumulate stable 5'-terminal and fewer 3'-terminal mutations in a stepwise manner. J Virol. 1984 Mar;49(3):793–798. doi: 10.1128/jvi.49.3.793-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J., Clinton G. M., McClure M. A. RNP template of vesicular stomatitis virus regulates transcription and replication functions. Cell. 1983 Nov;35(1):175–185. doi: 10.1016/0092-8674(83)90220-9. [DOI] [PubMed] [Google Scholar]
- Perrault J., McLear P. W. ATP dependence of vesicular stomatitis virus transcription initiation and modulation by mutation in the nucleocapsid protein. J Virol. 1984 Sep;51(3):635–642. doi: 10.1128/jvi.51.3.635-642.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot M. K., Schnitzlein W. M., Reichmann M. E. The requirement of protein synthesis for VSV inhibition of host cell RNA synthesis. Virology. 1985 Jan 15;140(1):91–101. doi: 10.1016/0042-6822(85)90448-9. [DOI] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Mosquito cells infected with vesicular stomatitis virus yield unsialylated virions of low infectivity. J Virol. 1975 Apr;15(4):1029–1032. doi: 10.1128/jvi.15.4.1029-1032.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H., Mifune K., Motohashi T. Isolation and assay of rabies serogroup viruses in CER cells. Intervirology. 1977;8(2):92–99. doi: 10.1159/000148883. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. In vitro synthesis of the full-length complement of the negative-strand genome RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jan;77(1):294–298. doi: 10.1073/pnas.77.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz J., Keene J. D. Interactions of plus and minus strand leader RNAs of the New Jersey serotype of vesicular stomatitis virus with the cellular La protein. Virology. 1984 May;135(1):65–73. doi: 10.1016/0042-6822(84)90117-x. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]
- Wyers F., Richard-Molard C., Blondel D., Dezelee S. Vesicular stomatitis virus growth in Drosophila melanogaster cells: G protein deficiency. J Virol. 1980 Jan;33(1):411–422. doi: 10.1128/jvi.33.1.411-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]







