Abstract
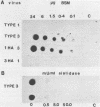
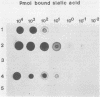
The interaction of mammalian reoviruses with sialylated glycoproteins was studied and found to be highly serotype specific in that attachment of type 3 Dearing reovirus to murine L cell receptors could be strongly inhibited by bovine submaxillary mucin (BSM), fetuin, and alpha 1 acid glycoprotein, albeit at different efficiencies, whereas attachment of type 1 Lang reovirus was inhibited only by fetuin. We subsequently demonstrated, by using reassortants between type 3 and 1 reoviruses, that inhibition of reovirus attachment to cell receptors was specified by the viral attachment protein gene S1. Using a solid-phase binding assay, we further demonstrated that the ability of reovirus type 3 or reassortant 1HA3 and the inability of reovirus type 1 or reassortant 3HA1 to bind avidly to BSM was a property of the viral S1 genome segment and required the presence of sialic acid residues on BSM oligosaccharides. Taken together, these results demonstrated that there is a serotype-specific difference in the ability of the reovirus attachment protein, sigma 1, to interact with sialylated oligosaccharides of glycoproteins. Interaction of reovirus type 3 with sialylated oligosaccharides of BSM is dramatically affected by the degree of O-acetylation of their sialic acid residues, as indicated by the findings that chemical removal of O-acetyl groups stimulated reovirus type 3 attachment to BSM, whereas preferential removal of residues lacking or possessing reduced amounts of O-acetyl groups per sialic acid molecule with Vibrio cholerae sialidase abolished binding. We also demonstrated that BSM was 10 times more potent in inhibiting attachment of infectious reovirus to L cells than was V. cholerae-treated BSM. The results are consistent with the hypothesis that sialylated oligosaccharides on host cells or erythrocytes may act as binding sites or components of binding sites for type 3 reovirus through a specific interaction with the virus attachment protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Graham A. F. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol. 1977 Aug;23(2):250–262. doi: 10.1128/jvi.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. D., Paul R. W., Lee P. W. Studies on reovirus receptors of L cells: virus binding characteristics and comparison with reovirus receptors of erythrocytes. Virology. 1984 Oct 15;138(1):37–48. doi: 10.1016/0042-6822(84)90145-4. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Spriggs D. R., Fields B. N. Evidence for functional domains on the reovirus type 3 hemagglutinin. Virology. 1982 Feb;117(1):146–155. doi: 10.1016/0042-6822(82)90514-1. [DOI] [PubMed] [Google Scholar]
- Carroll S. M., Paulson J. C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985 Sep;3(2):165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Fields B. N., Greene M. I. Isolation and biochemical characterization of the mammalian reovirus type 3 cell-surface receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1494–1498. doi: 10.1073/pnas.82.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Co M. S., Gaulton G. N., Tominaga A., Homcy C. J., Fields B. N., Greene M. I. Structural similarities between the mammalian beta-adrenergic and reovirus type 3 receptors. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5315–5318. doi: 10.1073/pnas.82.16.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N., Joklik W. K. Isolation and preliminary genetic and biochemical characterization of temperature-sensitive mutants of reovirus. Virology. 1969 Mar;37(3):335–342. doi: 10.1016/0042-6822(69)90217-7. [DOI] [PubMed] [Google Scholar]
- Fried H., Cahan L. D., Paulson J. C. Polyoma virus recognizes specific sialyligosaccharide receptors on host cells. Virology. 1981 Feb;109(1):188–192. doi: 10.1016/0042-6822(81)90485-2. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. Reactive sites of reovirus type 3 and their interaction with receptor substances. Virology. 1962 Jul;17:455–461. doi: 10.1016/0042-6822(62)90140-x. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A., GRAHAM E. R. 6-alpha-D-Sialyl-N-acetyl-galactosamine: the neuraminidase-susceptible prosthetic group of bovine salivary mucoprotein. Biochim Biophys Acta. 1959 Aug;34:380–391. doi: 10.1016/0006-3002(59)90290-2. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Hatfield J. W. Saturable attachment sites for type 3 mammalian reovirus on murine L cells and human HeLa cells. Virus Res. 1984;1(5):401–414. doi: 10.1016/0168-1702(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Gentsch J. R., Pacitti A. F. Effect of neuraminidase treatment of cells and effect of soluble glycoproteins on type 3 reovirus attachment to murine L cells. J Virol. 1985 Nov;56(2):356–364. doi: 10.1128/jvi.56.2.356-364.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAFF R. F., STEWART R. C. ROLE OF SIALIC ACID RECEPTORS IN ADSORPTION OF INFLUENZA VIRUS TO CHICK EMBRYO CELLS. J Immunol. 1965 Jun;94:842–851. [PubMed] [Google Scholar]
- Herbrink P., Van Bussel F. J., Warnaar S. O. The antigen spot test (AST): a highly sensitive assay for the detection of antibodies. J Immunol Methods. 1982;48(3):293–298. doi: 10.1016/0022-1759(82)90330-1. [DOI] [PubMed] [Google Scholar]
- Herrler G., Rott R., Klenk H. D., Müller H. P., Shukla A. K., Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985 Jun;4(6):1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa H. H., Rogers G. N., Paulson J. C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology. 1985 Jul 15;144(1):279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kathan R. H., Adamany A. Comparison of human MM, NN, and MN blood group antigens. J Biol Chem. 1967 Apr 25;242(8):1716–1722. [PubMed] [Google Scholar]
- LUDOWIEG J., DORFMAN A. A micromethod for the colorimetric determination of N-acetyl groups in acid mucopolysaccharides. Biochim Biophys Acta. 1960 Feb 26;38:212–218. doi: 10.1016/0006-3002(60)91233-6. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Protein sigma 1 is the reovirus cell attachment protein. Virology. 1981 Jan 15;108(1):156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Paulson J. C. Sendai virus utilizes specific sialyloligosaccharides as host cell receptor determinants. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5693–5697. doi: 10.1073/pnas.77.10.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Svennerholm L., Paulson J. C. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- Noseworthy J. H., Fields B. N., Dichter M. A., Sobotka C., Pizer E., Perry L. L., Nepom J. T., Greene M. I. Cell receptors for the mammalian reovirus. I. Syngeneic monoclonal anti-idiotypic antibody identifies a cell surface receptor for reovirus. J Immunol. 1983 Nov;131(5):2533–2538. [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roboz J., Suttajit M., Bekesi J. G. Elimination of 2-deoxyribose interference in the thiobarbituric acid determination of N-acetylneuraminic acid in tumor cells by pH-dependent extraction with cyclohexanone. Anal Biochem. 1981 Jan 15;110(2):380–388. doi: 10.1016/0003-2697(81)90207-4. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Herrler G., Paulson J. C., Klenk H. D. Influenza C virus uses 9-O-acetyl-N-acetylneuraminic acid as a high affinity receptor determinant for attachment to cells. J Biol Chem. 1986 May 5;261(13):5947–5951. [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Sarris A. H., Palade G. E. The sialoglycoproteins of murine erythrocyte ghosts. A modified periodic acid-Schiff stain procedure staining nonsubstituted and O-acetylated sialyl residues on glycopeptides. J Biol Chem. 1979 Jul 25;254(14):6724–6731. [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Spriggs D. R., Fields B. N. Attenuated reovirus type 3 strains generated by selection of haemagglutinin antigenic variants. Nature. 1982 May 6;297(5861):68–70. doi: 10.1038/297068a0. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Kaye K., Fields B. N. Topological analysis of the reovirus type 3 hemagglutinin. Virology. 1983 May;127(1):220–224. doi: 10.1016/0042-6822(83)90385-9. [DOI] [PubMed] [Google Scholar]
- Superti F., Girmenta C., Seganti L., Orsi N. Role of sialic acid in cell receptors for vesicular stomatitis virus. Acta Virol. 1986 Jan;30(1):10–18. [PubMed] [Google Scholar]
- Superti F., Hauttecoeur B., Morelec M. J., Goldoni P., Bizzini B., Tsiang H. Involvement of gangliosides in rabies virus infection. J Gen Virol. 1986 Jan;67(Pt 1):47–56. doi: 10.1099/0022-1317-67-1-47. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structural studies on human erythrocyte glycoproteins. Alkali-labile oligosaccharides. J Biol Chem. 1969 Nov 10;244(21):5943–5946. [PubMed] [Google Scholar]
- Varki A., Diaz S. A neuraminidase from Streptococcus sanguis that can release O-acetylated sialic acids. J Biol Chem. 1983 Oct 25;258(20):12465–12471. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Powers M. L., Fields B. N. Absolute linkage of virulence and central nervous system cell tropism of reoviruses to viral hemagglutinin. J Infect Dis. 1980 May;141(5):609–616. doi: 10.1093/infdis/141.5.609. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Furthmayr H., Kobata A. Structures of the asparagine-linked sugar chains of glycophorin A. J Biol Chem. 1980 Oct 25;255(20):9713–9718. [PubMed] [Google Scholar]