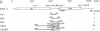Abstract
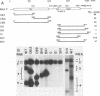
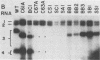
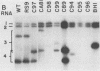
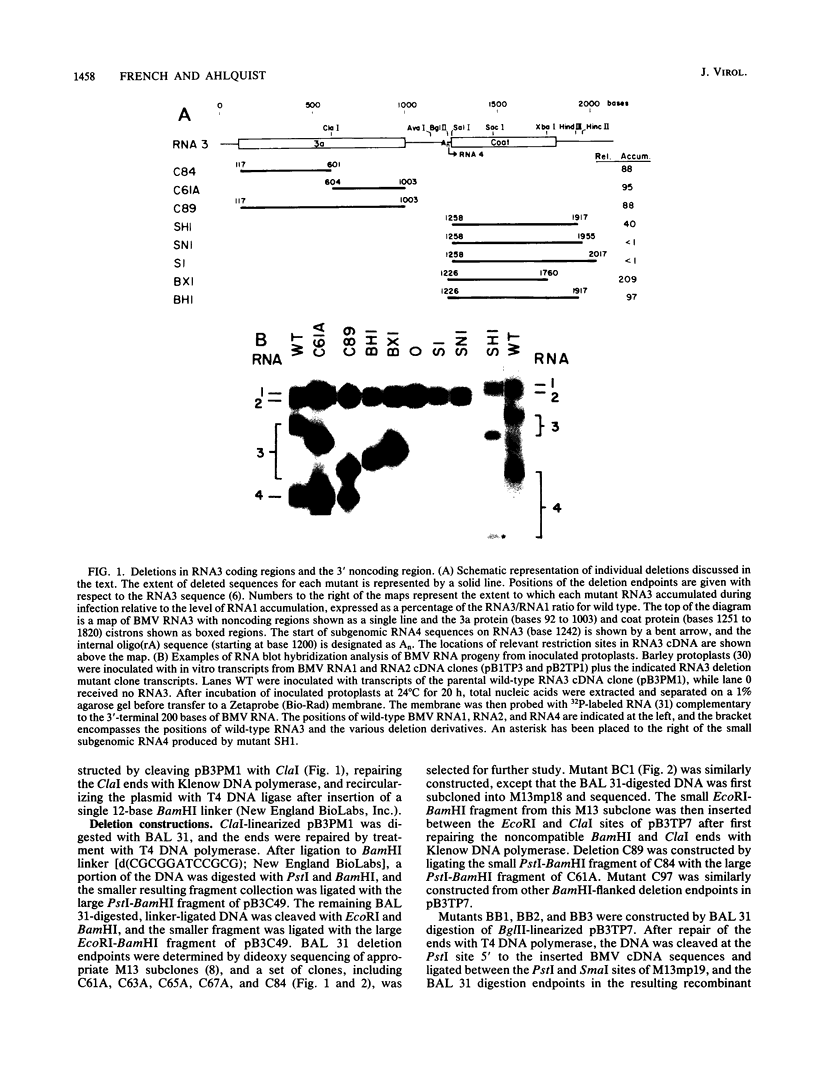
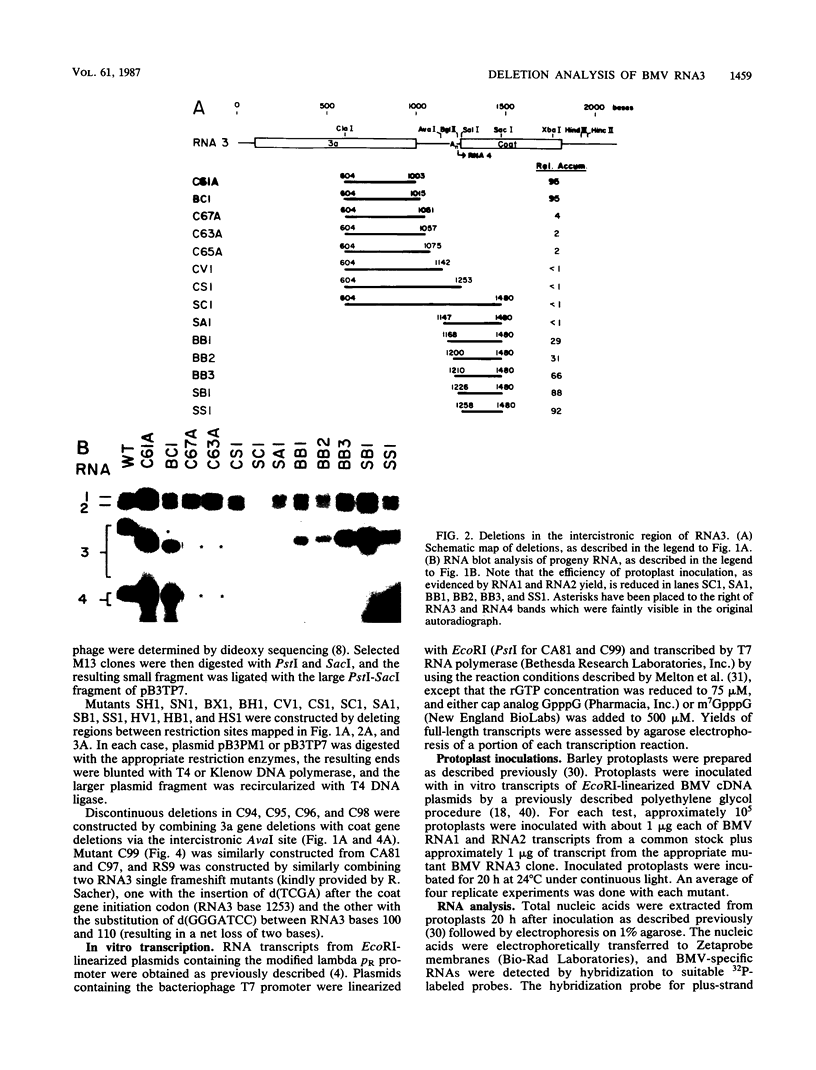
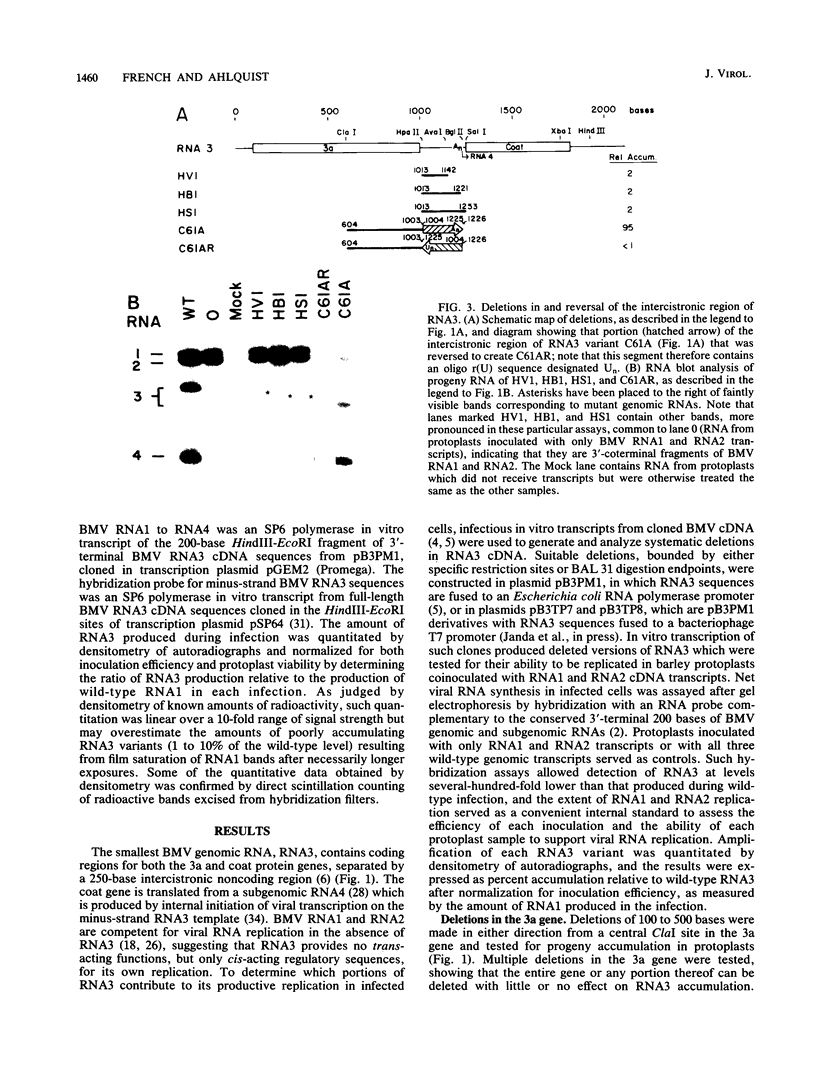
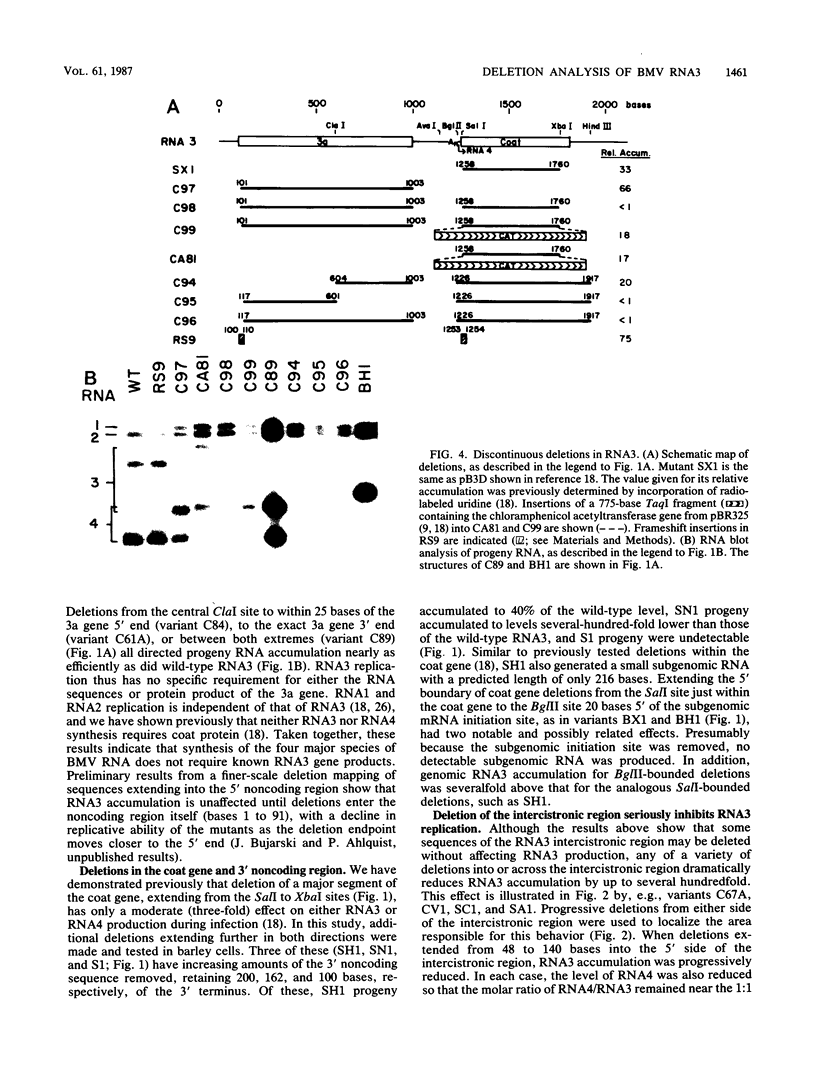
The genome of brome mosaic virus (BMV) is divided among messenger polarity RNA1, RNA2, and RNA3 (3.2, 2.9, and 2.1 kilobases, respectively). cis-Acting sequences required for BMV RNA amplification were investigated with RNA3. By using expressible cDNA clones, deletions were constructed throughout RNA3 and tested in barley protoplasts coinoculated with RNA1 and RNA2. In contrast to requirements for 5'- and 3'-terminal noncoding sequences, either of the two RNA3 coding regions can be deleted individually and both can be simultaneously inactivated by N-terminal frameshift mutations without significantly interfering with amplification of RNA3 or production of its subgenomic mRNA. However, simultaneous major deletions in both coding regions greatly attenuate RNA3 accumulation. RNA3 levels can be largely restored by insertion of a heterologous, nonviral sequence in such mutants, suggesting that RNA3 requires physical separation of its terminal domains or a minimum overall size for normal replication or stability. Unexpectedly, deletions in a 150-base segment of the intercistronic noncoding region drastically reduce RNA3 accumulation. This segment contains a sequence element homologous to sequences found near the 5' ends of BMV RNA1 and RNA2 and in analogous positions in the three genomic RNAs of the related cucumber mosaic virus, suggesting a possible role in plus-strand synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dreher T. W., Hall T. C. Deletions in the 3'-terminal tRNA-like structure of brome mosaic virus RNA differentially affect aminoacylation and replication in vitro. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5636–5640. doi: 10.1073/pnas.82.17.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Hardy S. F., Miller W. A., Hall T. C. Use of dodecyl-beta-D-maltoside in the purification and stabilization of RNA polymerase from brome mosaic virus-infected barley. Virology. 1982 Jun;119(2):465–473. doi: 10.1016/0042-6822(82)90105-2. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P. J. The current picture of the structure and assembly of tobacco mosaic virus. J Gen Virol. 1984 Feb;65(Pt 2):253–279. doi: 10.1099/0022-1317-65-2-253. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Gerlach W. L., Bruening G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8859–8862. doi: 10.1073/pnas.83.23.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Gorski K., Roch J. M., Prentki P., Krisch H. M. The stability of bacteriophage T4 gene 32 mRNA: a 5' leader sequence that can stabilize mRNA transcripts. Cell. 1985 Dec;43(2 Pt 1):461–469. doi: 10.1016/0092-8674(85)90176-x. [DOI] [PubMed] [Google Scholar]
- Gould A. R., Symons R. H. Cucumber mosaic virus RNA 3. Determination of the nucleotide sequence provides the amino acid sequences of protein 3A and viral coat protein. Eur J Biochem. 1982 Aug;126(2):217–226. [PubMed] [Google Scholar]
- Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983 Dec;35(3 Pt 2):849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Joshi S., Chapeville F. tRNA-like structures in the genomes of RNA viruses. Prog Nucleic Acid Res Mol Biol. 1982;27:85–104. doi: 10.1016/s0079-6603(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R. L., Joshi S., Chapeville F., Haenni A. L. tRNA-like structures of plant viral RNAs: conformational requirements for adenylation and aminoacylation. EMBO J. 1983;2(7):1123–1127. doi: 10.1002/j.1460-2075.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiberstis P. A., Loesch-Fries L. S., Hall T. C. Viral protein synthesis in barley protoplasts inoculated with native and fractionated brome mosaic virus RNA. Virology. 1981 Jul 30;112(2):804–808. doi: 10.1016/0042-6822(81)90331-7. [DOI] [PubMed] [Google Scholar]
- Kinniburgh A. J., Maquat L. E., Schedl T., Rachmilewitz E., Ross J. mRNA-deficient beta o-thalassemia results from a single nucleotide deletion. Nucleic Acids Res. 1982 Sep 25;10(18):5421–5427. doi: 10.1093/nar/10.18.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Weiss B. G., Tsiang M., Huang H., Schlesinger S. Deletion mapping of Sindbis virus DI RNAs derived from cDNAs defines the sequences essential for replication and packaging. Cell. 1986 Jan 17;44(1):137–145. doi: 10.1016/0092-8674(86)90492-7. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F., Weber H., Weissmann C. Interactions of Q beta replicase with Q beta RNA. J Mol Biol. 1981 Dec 15;153(3):631–660. doi: 10.1016/0022-2836(81)90411-3. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Bujarski J. J., Dreher T. W., Hall T. C. Minus-strand initiation by brome mosaic virus replicase within the 3' tRNA-like structure of native and modified RNA templates. J Mol Biol. 1986 Feb 20;187(4):537–546. doi: 10.1016/0022-2836(86)90332-3. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Hall T. C. Use of micrococcal nuclease in the purification of highly template dependent RNA-dependent RNA polymerase from brome mosaic virus-infected barley. Virology. 1983 Feb;125(1):236–241. doi: 10.1016/0042-6822(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Truong K. Cleavage within an RNase III site can control mRNA stability and protein synthesis in vivo. Nucleic Acids Res. 1985 Apr 11;13(7):2227–2240. doi: 10.1093/nar/13.7.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science. 1981 Nov 20;214(4523):916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Symons R. H. Nucleotide sequence of cucumber mosaic virus RNA. 1. Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur J Biochem. 1985 Jul 15;150(2):331–339. doi: 10.1111/j.1432-1033.1985.tb09025.x. [DOI] [PubMed] [Google Scholar]
- Samac D. A., Nelson S. E., Sue Loesch-Fries L. Virus protein synthesis in alfalfa mosaic virus infected alfalfa protoplasts. Virology. 1983 Dec;131(2):455–462. doi: 10.1016/0042-6822(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. The intervening sequence RNA of Tetrahymena is an enzyme. Science. 1986 Jan 31;231(4737):470–475. doi: 10.1126/science.3941911. [DOI] [PubMed] [Google Scholar]