Abstract
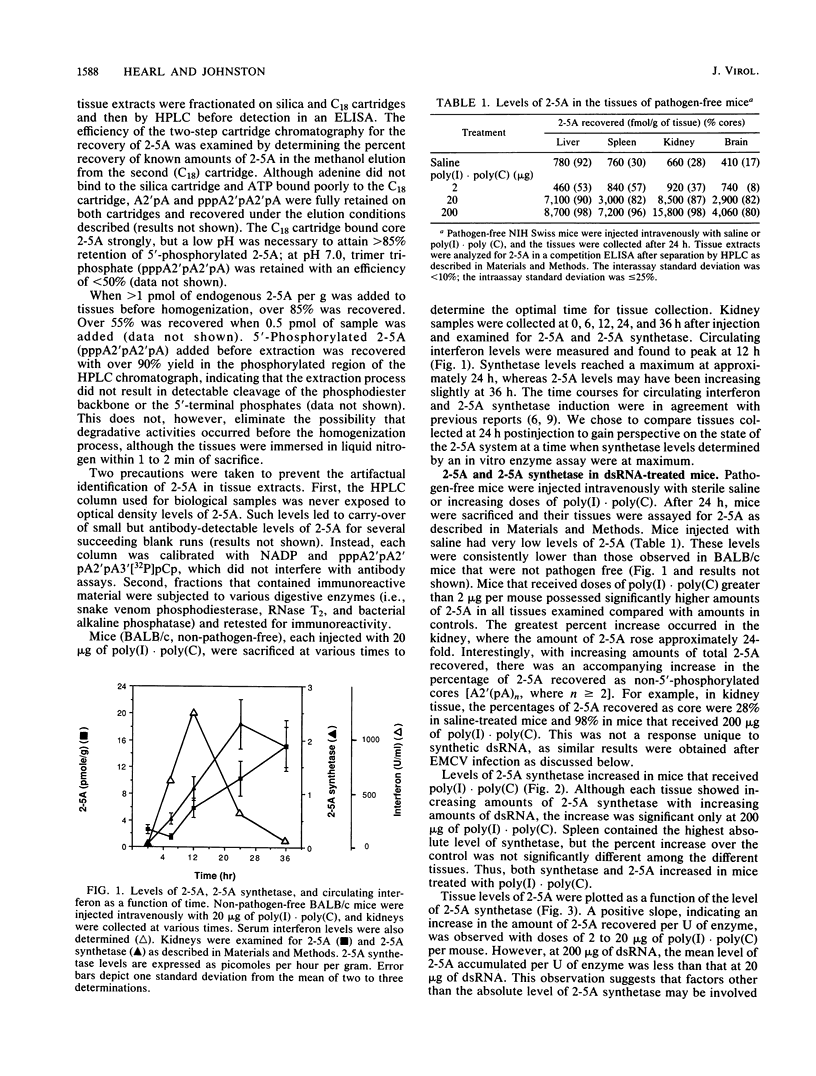
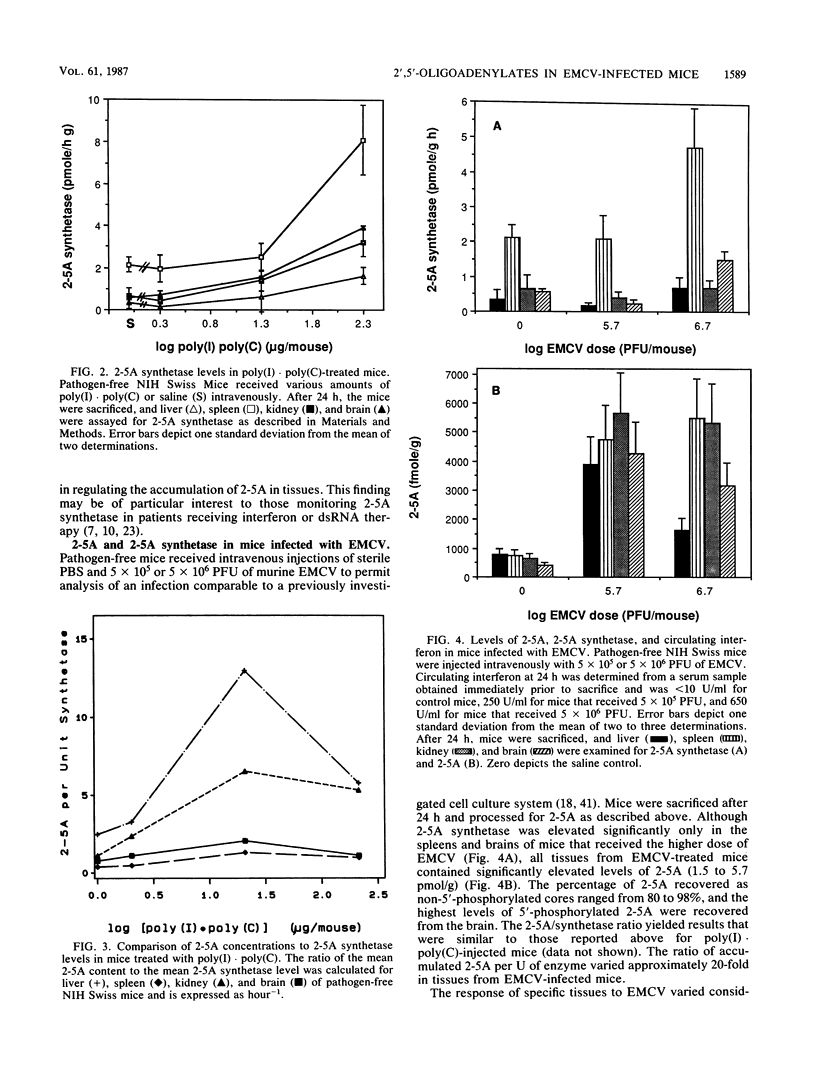
Levels of 2',5'-oligoadenylates (2-5A) in various tissues of murine encephalomyocarditis virus (EMCV)-infected mice were determined and compared with those found in pathogen-free mice and in mice treated with the interferon inducer poly(I).poly(C). In control, pathogen-free mice, liver, spleen, brain, and kidney tissues possessed levels of 2-5A below 1 pmol/g of tissue, demonstrating that 2-5A was not a major component of uninfected mouse tissue. All control tissues had low basal levels (0.3 to 2.0 pmol/h per g) of 2-5A synthetase, the enzyme responsible for 2-5A production. After mice were injected intravenously with the interferon inducer poly(I).poly(C), circulating interferon, 2-5A synthetase, and 2-5A were elevated with increasing doses of double-stranded RNA. The greatest response to poly(I).poly(C) occurred in the kidney, in which enzyme levels increased 5-fold and 2-5A levels increased 24-fold to 15 pmol/g. Mice that were infected with EMCV also possessed elevated levels of 2-5A and 2-5A synthetase in the four tissues examined, although the relative distribution differed from that observed with poly(I).poly(C), indicating that the interferon inducer affects the concentration and location of intracellular 2-5A. Brain, spleen, and kidney tissues from EMCV-infected mice contained seven- to eightfold more 2-5A than control tissues did. The nanomolar levels of 2-5A in the tissues of EMCV-infected mice provide evidence that 2-5A may play a role in the antiviral response in an intact animal. In both poly(I).poly(C)- and EMCV-treated mice, the levels of 2-5A recovered from the tissues were not directly proportional to the amount of 2-5A synthetase present. These results indicate that factors other than the level of 2-5A synthetase controlled the accumulation of 2-5A in tissues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A. Induction, purification, and properties of 2'5' oligoadenylate synthetase. Ann N Y Acad Sci. 1980;350:486–496. doi: 10.1111/j.1749-6632.1980.tb20651.x. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. E., Kerr I. M. (p)pp(A2'p)nA is rare in normal mouse tissues while (A2'p)nA but not (p)pp(A2'p)nA appears to be present in E. coli. Prog Clin Biol Res. 1985;202:3–10. [PubMed] [Google Scholar]
- Dougherty J. P., Samanta H., Farrell P. J., Lengyel P. Interferon, double-stranded RNA, and RNA degradation. Isolation of homogeneous pppA(2'p5'A)n-1 synthetase from Ehrlich ascites tumor cells. J Biol Chem. 1980 May 10;255(9):3813–3816. [PubMed] [Google Scholar]
- Dougherty J. P., Samanta H., Farrell P. J., Lengyel P. Interferon, double-stranded RNA, and RNA degradation. Isolation of homogeneous pppA(2'p5'A)n-1 synthetase from Ehrlich ascites tumor cells. J Biol Chem. 1980 May 10;255(9):3813–3816. [PubMed] [Google Scholar]
- Floyd-Smith G., Slattery E., Lengyel P. Interferon action: RNA cleavage pattern of a (2'-5')oligoadenylate--dependent endonuclease. Science. 1981 May 29;212(4498):1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- Galaktidou G., Kortsaris A., Koliais S. I. Induction of 2',5' oligo(A) synthetase in tumor-bearing mice with encephalomyocarditis (EMC) virus or poly(I)poly(C). FEBS Lett. 1983 Nov 28;164(1):161–165. doi: 10.1016/0014-5793(83)80041-6. [DOI] [PubMed] [Google Scholar]
- Hersh C. L., Brown R. E., Roberts W. K., Swyryd E. A., Kerr I. M., Stark G. R. Simian virus 40-infected, interferon-treated cells contain 2',5'-oligoadenylates which do not activate cleavage of RNA. J Biol Chem. 1984 Feb 10;259(3):1731–1737. [PubMed] [Google Scholar]
- Hovanessian A. G., Youn J. K., Buffet-Janvresse C., Riviere Y., Michelson M., Lacour J., Lacour F. Enhancement of natural killer cell activity and 2-5A synthetase in operable breast cancer patients treated with polyadenylic; polyuridylic acid. Cancer. 1985 Jan 15;55(2):357–362. doi: 10.1002/1097-0142(19850115)55:2<357::aid-cncr2820550210>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Imai J., Lesiak K., Torrence P. F. Respective role of each of the purine N-6 amino groups of 5'-O-triphosphoryladenylyl(2'----5')adenylyl(2----5')adenosine in binding to and activation of RNase L. J Biol Chem. 1985 Feb 10;260(3):1390–1393. [PubMed] [Google Scholar]
- Johnston M. I., Imai J., Lesiak K., Jacobsen H., Sawai H., Torrence P. F. Antibody-nucleic acid interactions. Monoclonal antibodies define different antigenic domains in 2',5'-oligoadenylates. Biochemistry. 1985 Aug 13;24(17):4710–4718. doi: 10.1021/bi00338a033. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., Cayley P. J., Silverman R. H., Wreschner D. H., Gilbert C. S., Brown R. E., Kerr I. M. Radioimmune, radiobinding and HPLC analysis of 2-5A and related oligonucleotides from intact cells. Nature. 1980 Nov 13;288(5787):189–192. doi: 10.1038/288189a0. [DOI] [PubMed] [Google Scholar]
- Krause D., Silverman R. H., Jacobsen H., Leisy S. A., Dieffenbach C. W., Friedman R. M. Regulation of ppp(A2'p)nA-dependent RNase levels during interferon treatment and cell differentiation. Eur J Biochem. 1985 Feb 1;146(3):611–618. doi: 10.1111/j.1432-1033.1985.tb08695.x. [DOI] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. Elevated levels of (2'-5')oligoadenylic acid polymerase activity in growth-arrested human lymphoblastoid Namalva cells. Mol Cell Biol. 1981 Oct;1(10):932–938. doi: 10.1128/mcb.1.10.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan I., Baglioni C. Increased levels of (2'-5')oligo(A) polymerase activity in human lymphoblastoid cells treated with glucocorticoids. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6506–6510. doi: 10.1073/pnas.77.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence L., Marti J., Roux D., Cailla H. Immunological evidence for the in vivo occurrence of (2'-5')adenylyladenosine oligonucleotides in eukaryotes and prokaryotes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2322–2326. doi: 10.1073/pnas.81.8.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodemann E., Kornhuber B., Gerein V., von Il'berg C. (2'-5')oligo(A) synthetase as a monitor of interferon action in juvenile laryngeal papillomatosis. J Interferon Res. 1984 Spring;4(2):283–290. doi: 10.1089/jir.1984.4.283. [DOI] [PubMed] [Google Scholar]
- Reid T. R., Hersh C. L., Kerr I. M., Stark G. R. Analysis of 2',5'-oligoadenylates in cells and tissues. Anal Biochem. 1984 Jan;136(1):136–141. doi: 10.1016/0003-2697(84)90315-4. [DOI] [PubMed] [Google Scholar]
- Samanta H., Dougherty J. P., Lengyel P. Synthesis of (2'-5')(A)n from ATP. Characteristics of the reaction catalyzed by (2'-5')(A)n synthetase purified from mouse Ehrlich ascites tumor cells treated with interferon. J Biol Chem. 1980 Oct 25;255(20):9807–9813. [PubMed] [Google Scholar]
- Sawai H., Ishibashi K., Itoh M., Watanabe S. 2',5'-Oligoadenylate and 2',5'-oligoadenylate phosphodiesterase in human plasma. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1061–1066. doi: 10.1016/0006-291x(84)91391-3. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Chernajovsky Y., Shulman L., Federman P., Berissi H., Revel M. An interferon-induced phosphodiesterase degrading (2'-5') oligoisoadenylate and the C-C-A terminus of tRNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4788–4792. doi: 10.1073/pnas.76.10.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Sokawa Y. 2',5'-Oligoadenylate synthetase activity in lymphocytes from normal mouse. J Biol Chem. 1979 Dec 10;254(23):12034–12037. [PubMed] [Google Scholar]
- Silverman R. H., Cayley P. J., Knight M., Gilbert C. S., Kerr I. M. Control of the ppp(a2'p)nA system in HeLa cells. Effects of interferon and virus infection. Eur J Biochem. 1982 May;124(1):131–138. doi: 10.1111/j.1432-1033.1982.tb05915.x. [DOI] [PubMed] [Google Scholar]
- Smekens-Etienne M., Goldstein J., Ooms H. A., Dumont J. E. Variation of (2'-5')oligo(adenylate) synthetase activity during rat-liver regeneration. Eur J Biochem. 1983 Feb 1;130(2):269–273. doi: 10.1111/j.1432-1033.1983.tb07146.x. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Friedman R. M. Are double-stranded RNA-directed inhibition of protein synthesis in interferon-treated cells and interferon induction related phenomena? J Biol Chem. 1979 Feb 25;254(4):1259–1267. [PubMed] [Google Scholar]
- Torrence P. F., Imai J., Johnston M. I. 5'-O-Monophosphoryladenylyl(2' goes to 5')adenylyl(2' goes to 5')adenosine is an antagonist of the action of 5'-O-triphosphoryladenylyl-(2' goes to 5')adenylyl(2' goes to 5')adenosine and double-stranded RNA. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5993–5997. doi: 10.1073/pnas.78.10.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence P. F., Imai J., Johnston M. I. Assay of 2',5'-oligoadenylate phosphodiesterase activity in mouse L-cell extracts. Anal Biochem. 1983 Feb 15;129(1):103–110. doi: 10.1016/0003-2697(83)90058-1. [DOI] [PubMed] [Google Scholar]
- Verhaegen-Lewalle M., Content J. 2'-Phosphodiesterase activity in human cell lines treated or untreated with human interferon. Eur J Biochem. 1982 Sep 1;126(3):639–643. doi: 10.1111/j.1432-1033.1982.tb06828.x. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., English K. E., O'Brien A. D. Silica enhancement of murine endotoxin sensitivity. Infect Immun. 1982 Nov;38(2):681–685. doi: 10.1128/iai.38.2.681-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J. A., Swyryd E. A., Stark G. R. An improved method for purifying 2',5'-oligoadenylate synthetases. J Biol Chem. 1984 Jan 25;259(2):1363–1370. [PubMed] [Google Scholar]
- Williams B. R., Golgher R. R., Brown R. E., Gilbert C. S., Kerr I. M. Natural occurrence of 2-5A in interferon-treated EMC virus-infected L cells. Nature. 1979 Dec 6;282(5739):582–586. doi: 10.1038/282582a0. [DOI] [PubMed] [Google Scholar]
- Williams G. J., De Benedetti A., Baglioni C. Inhibition of 2',5'-oligo(A)-dependent endoribonuclease by 2',5'-oligo(A) degradation products. Virology. 1986 Jun;151(2):233–242. doi: 10.1016/0042-6822(86)90045-0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., McCauley J. W., Skehel J. J., Kerr I. M. Interferon action--sequence specificity of the ppp(A2'p)nA-dependent ribonuclease. Nature. 1981 Jan 29;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]


