Abstract
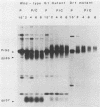
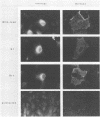
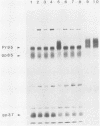
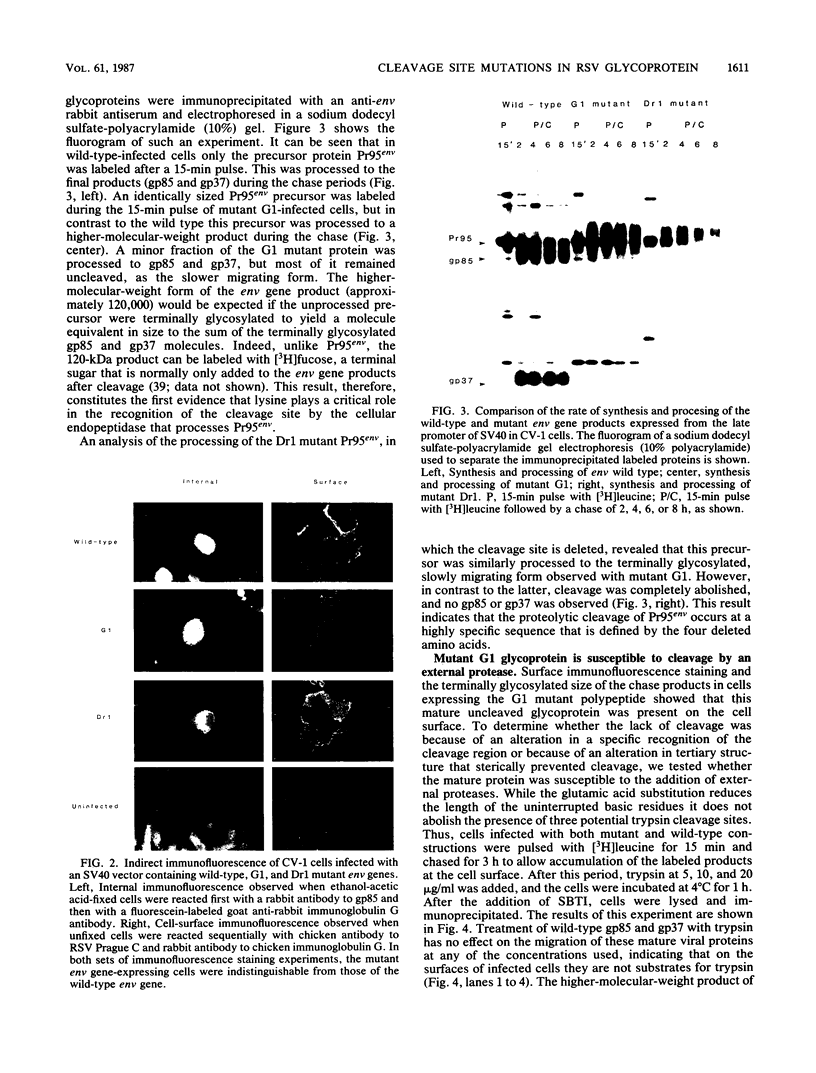
We have investigated the specificity of the proteolytic cleavage of the Rous sarcoma virus glycoprotein precursor by introducing two mutations into the putative cleavage region (Arg-Arg-Lys-Arg). We show that neither a deletion of the cleavage sequence nor a glutamic acid for lysine substitution altered intracellular transport or surface expression of the env gene products. However, both the four-amino-acid deletion and the glutamic acid substitution block processing of the env precursor. Susceptibility of the glutamic acid-substituted env precursor to proteases indicated that tertiary protein structure was unaffected. While inhibitor experiments suggested that more than one endopeptidase might be capable of mediating the proteolytic cleavage, the results presented here point to the presence in the Golgi apparatus of a novel endopeptidase, required for retroviral glycoprotein cleavage, that has a high specificity for lysine-containing peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso-Caplen F. V., Compans R. W. Modulation of glycosylation and transport of viral membrane glycoproteins by a sodium ionophore. J Cell Biol. 1983 Sep;97(3):659–668. doi: 10.1083/jcb.97.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Buchhagen D. L., Hanafusa H. Intracellular precursors to the major glycoprotein of avian oncoviruses in chicken embryo fibroblasts. J Virol. 1978 Mar;25(3):845–851. doi: 10.1128/jvi.25.3.845-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England J. M., Bolognesi D. P., Dietzschold B., Halpern M. S. Evidence that a precursor glycoprotein is cleaved to yield the major glycoprotein of avian tumor virus. J Virol. 1977 Feb;21(2):810–814. doi: 10.1128/jvi.21.2.810-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Gluschankof P., Morel A., Gomez S., Nicolas P., Fahy C., Cohen P. Enzymes processing somatostatin precursors: an Arg-Lys esteropeptidase from the rat brain cortex converting somatostatin-28 into somatostatin-14. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6662–6666. doi: 10.1073/pnas.81.21.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. A., Tracy H. J. Isolation of two "big gastrins" from Zollinger-Ellison tumour tissue. Lancet. 1972 Oct 14;2(7781):797–799. doi: 10.1016/s0140-6736(72)92151-4. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Chang H. T., Potts J. T., Jr Enzymic processing of proparathyroid hormone by cell-free extracts of parathyroid glands. Biochemistry. 1977 Aug 23;16(17):3910–3917. doi: 10.1021/bi00636a029. [DOI] [PubMed] [Google Scholar]
- Hayman M. Synthesis and processing of avian sarcoma virus glycoproteins. Virology. 1978 Apr;85(2):475–486. doi: 10.1016/0042-6822(78)90454-3. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kemmler W., Steiner D. F., Borg J. Studies on the conversion of proinsulin to insulin. 3. Studies in vitro with a crude secretion granule fraction isolated from rat islets of Langerhans. J Biol Chem. 1973 Jul 10;248(13):4544–4551. [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. The generation of the two envelope glycoproteins of Rous sarcoma virus from a common precursor polypeptide. Virology. 1978 Mar;85(1):63–74. doi: 10.1016/0042-6822(78)90411-7. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Wöllert W., Rott R., Scholtissek C. Association of influenza virus proteins with cytoplasmic fractions. Virology. 1974 Jan;57(1):28–41. doi: 10.1016/0042-6822(74)90105-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Bursztajn S., Goldberg A. L. Degradation of the acetylcholine receptor in cultured muscle cells: selective inhibitors and the fate of undegraded receptors. Cell. 1980 Feb;19(2):481–491. doi: 10.1016/0092-8674(80)90523-1. [DOI] [PubMed] [Google Scholar]
- Lindberg I., Yang H. Y., Costa E. An enkephalin-generating enzyme in bovine adrenal medulla. Biochem Biophys Res Commun. 1982 May 14;106(1):186–193. doi: 10.1016/0006-291x(82)92076-9. [DOI] [PubMed] [Google Scholar]
- Lindberg I., Yang H. Y., Costa E. Further characterization of an enkephalin-generating enzyme from adrenal medullary chromaffin granules. J Neurochem. 1984 May;42(5):1411–1419. doi: 10.1111/j.1471-4159.1984.tb02802.x. [DOI] [PubMed] [Google Scholar]
- Linial M., Fenno J., Burnette W. N., Rohrschneider L. Synthesis and processing of viral glycoproteins in two nonconditional mutants of Rous sarcoma virus. J Virol. 1980 Oct;36(1):280–290. doi: 10.1128/jvi.36.1.280-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Hayami M. Analysis of precursors to the envelope glycoproteins of avian RNA tumor viruses in chicken and quail cells. J Virol. 1977 Jun;22(3):598–607. doi: 10.1128/jvi.22.3.598-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Sullivan S. J., Bolognesi D. P. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virology. 1978 Jan;84(1):19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Neff N. T., DeMartino G. N., Goldberg A. L. The effect of protease inhibitors and decreased temperature on the degradation of different classes of proteins in cultured hepatocytes. J Cell Physiol. 1979 Dec;101(3):439–457. doi: 10.1002/jcp.1041010311. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., Geller D. M. The structure of rat proalbumin. J Biol Chem. 1975 May 10;250(9):3409–3413. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]