Abstract
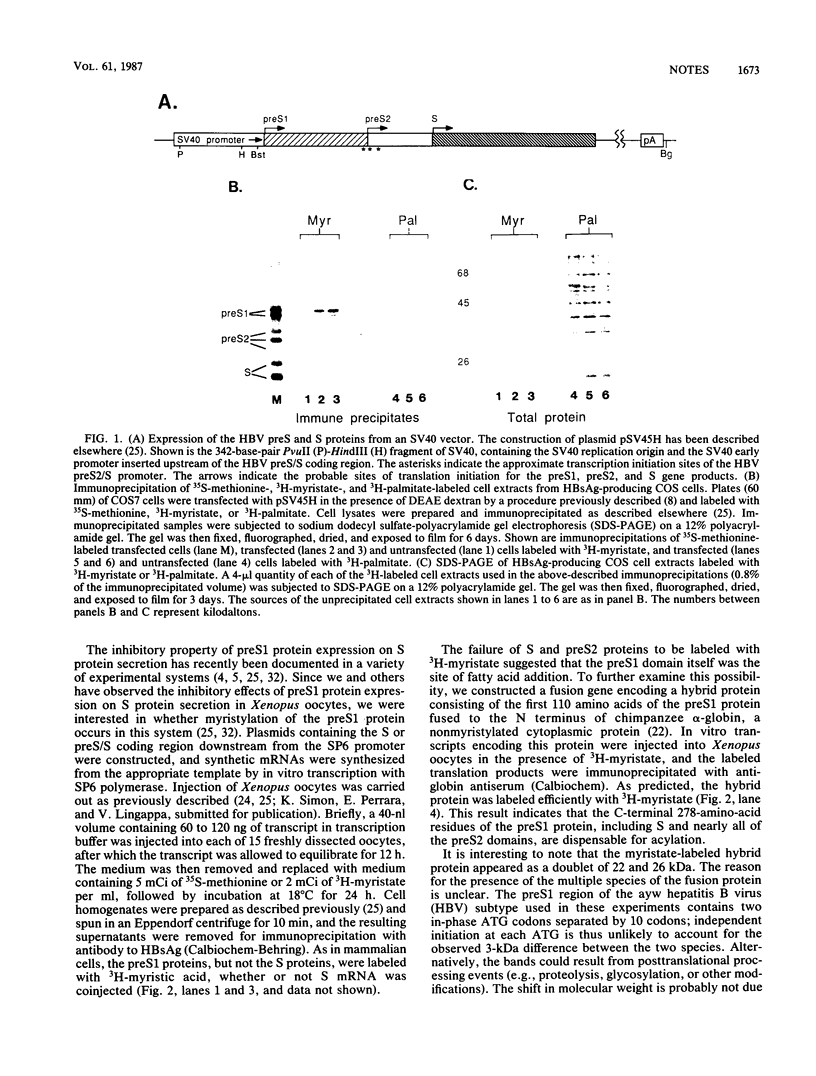
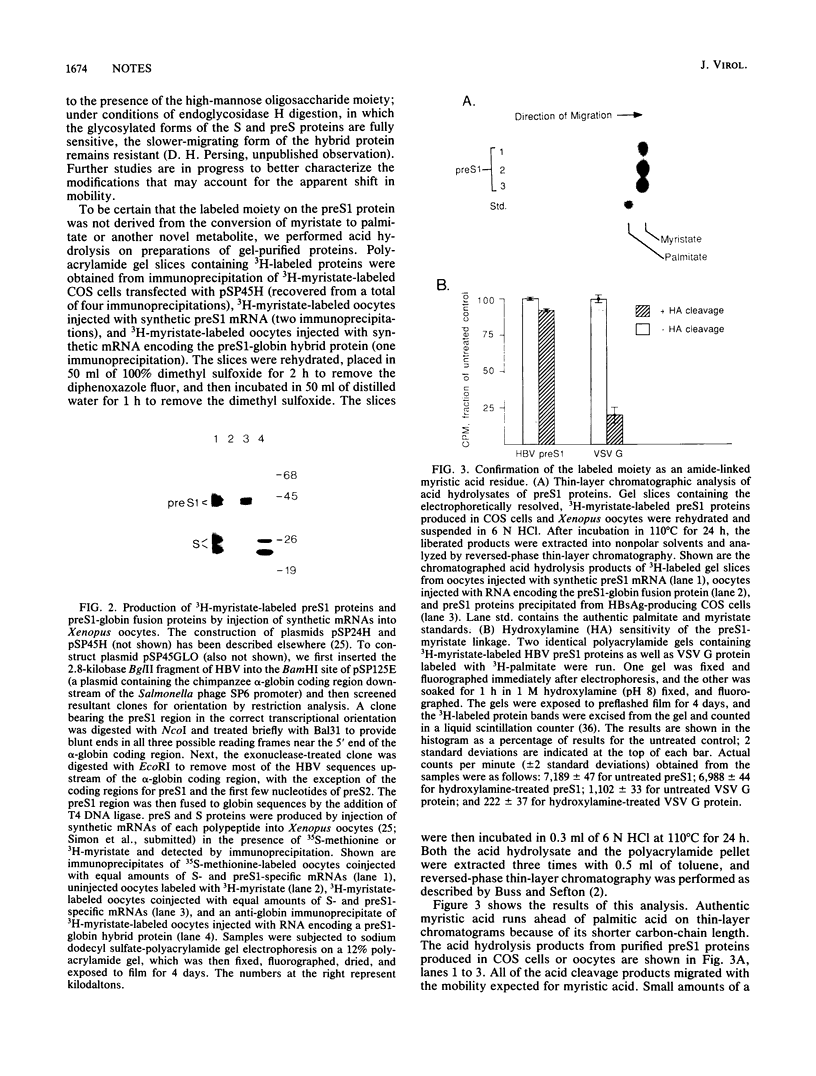
The preS/S coding region of hepatitis B virus encodes two polypeptides (preS1 and preS2) that are larger in size but less abundant than the major viral surface antigen (S) protein. Unlike the preS2 and S proteins, the preS1 protein is preferentially localized on circulating virus particles but is not efficiently secreted from mammalian cells in culture. To search for differences in protein processing that might relate to these properties, we determined whether any of the hepatitis B virus surface proteins are acylated with long-chain fatty acids. Transfected COS cells expressing all three proteins were incubated with 3H-palmitate or 3H-myristate, and the cell extracts were examined by immunoprecipitation. While none of these proteins was labeled with 3H-palmitate, the preS1 protein but not the preS2 or S protein incorporated 3H-myristate via a hydroxylamine-resistant amide linkage. Comparison of the N-terminal amino acid sequences of hepadnaviral preS1 proteins with those of known myristylated proteins suggests that this unusual modification may be a common feature of all hepadnaviral preS1 proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Smith G. L., Moss B. Hepatitis B virus large surface protein is not secreted but is immunogenic when selectively expressed by recombinant vaccinia virus. J Virol. 1986 Nov;60(2):337–344. doi: 10.1128/jvi.60.2.337-344.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Filippi P., McLachlan A., Milich D. R., Riggs M., Lee S., Palmiter R. D., Pinkert C. A., Brinster R. L. Expression of hepatitis B virus large envelope polypeptide inhibits hepatitis B surface antigen secretion in transgenic mice. J Virol. 1986 Dec;60(3):880–887. doi: 10.1128/jvi.60.3.880-887.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C. W., Liu C. C., Levinson A. D. Plasmid-directed synthesis of hepatitis B surface antigen in monkey cells. Mol Cell Biol. 1983 Jan;3(1):44–55. doi: 10.1128/mcb.3.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Miyamoto H., Baba K., Oda K., Nakamura T., Miyakawa Y., Mayumi M. A hepatitis B surface antigen polypeptide (P31) with the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1983 Aug;85(2):268–274. [PubMed] [Google Scholar]
- Magee A. I., Courtneidge S. A. Two classes of fatty acid acylated proteins exist in eukaryotic cells. EMBO J. 1985 May;4(5):1137–1144. doi: 10.1002/j.1460-2075.1985.tb03751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchildon G. A., Casnellie J. E., Walsh K. A., Krebs E. G. Covalently bound myristate in a lymphoma tyrosine protein kinase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7679–7682. doi: 10.1073/pnas.81.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986 Aug 1;46(3):429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Simonsen C. C., Levinson A. D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986 Jun;58(3):884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. An N-terminal peptide from p60src can direct myristylation and plasma membrane localization when fused to heterologous proteins. 1985 Mar 28-Apr 3Nature. 314(6009):374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. A frameshift mutation in the pre-S region of the human hepatitis B virus genome allows production of surface antigen particles but eliminates binding to polymerized albumin. Proc Natl Acad Sci U S A. 1985 May;82(10):3440–3444. doi: 10.1073/pnas.82.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Antibodies to pre-S and X determinants arise during natural infection with ground squirrel hepatitis virus. J Virol. 1986 Oct;60(1):177–184. doi: 10.1128/jvi.60.1.177-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science. 1986 Dec 12;234(4782):1388–1391. doi: 10.1126/science.3787251. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoji S., Parmelee D. C., Wade R. D., Kumar S., Ericsson L. H., Walsh K. A., Neurath H., Long G. L., Demaille J. G., Fischer E. H. Complete amino acid sequence of the catalytic subunit of bovine cardiac muscle cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Feb;78(2):848–851. doi: 10.1073/pnas.78.2.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H., Helenius A. How an animal virus gets into and out of its host cell. Sci Am. 1982 Feb;246(2):58–66. doi: 10.1038/scientificamerican0282-58. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Rutter W. J. Assembly of viral particles in Xenopus oocytes: pre-surface-antigens regulate secretion of the hepatitis B viral surface envelope particle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9338–9342. doi: 10.1073/pnas.83.24.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Structural relationships between minor and major proteins of hepatitis B surface antigen. J Virol. 1983 May;46(2):626–628. doi: 10.1128/jvi.46.2.626-628.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Jackson R. C., Marcus M. M., Lingappa V. R., Blobel G. Tryptic dissection and reconstitution of translocation activity for nascent presecretory proteins across microsomal membranes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1795–1799. doi: 10.1073/pnas.76.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]