Abstract
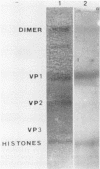
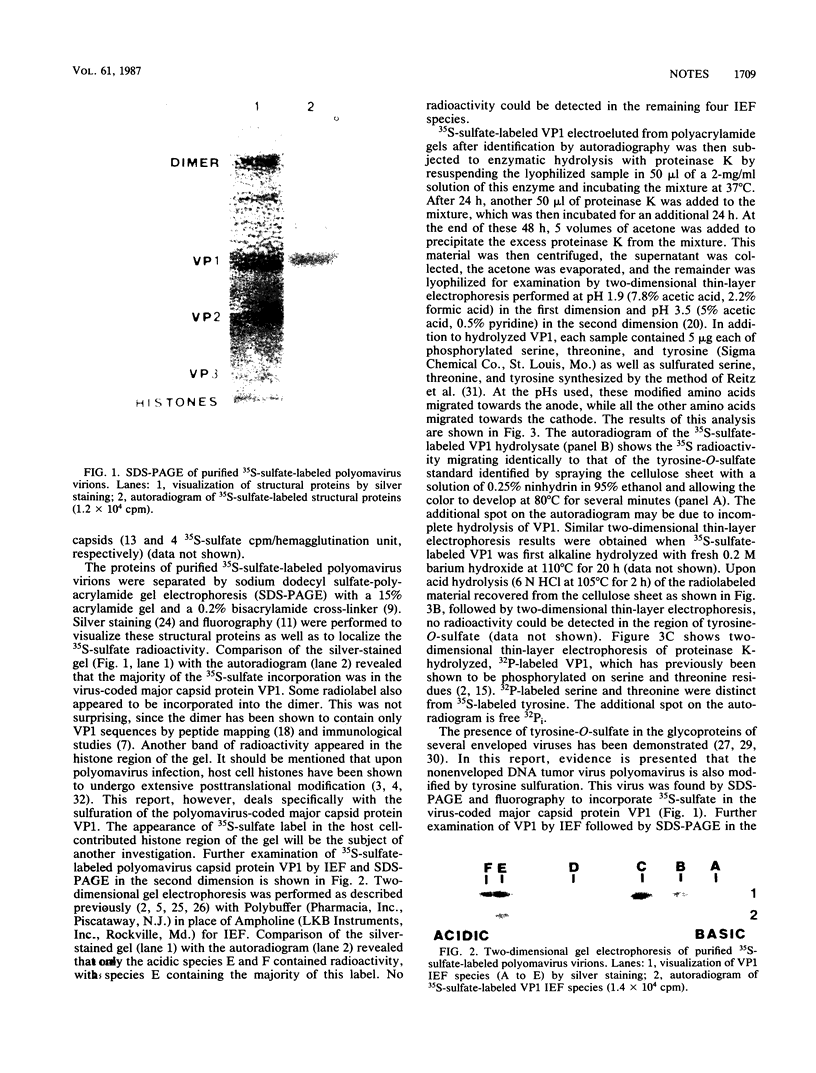
Polyomavirus was propagated in primary mouse kidney cell monolayers and 35S-sulfate labeled by maintaining the infected cells in serum-free Eagle medium supplemented with 35S-labeled sodium sulfate. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of CsCI gradient-purified 35S-sulfate-labeled virions followed by fluorography indicated that the polyomavirus-coded major capsid protein VP1 incorporated this radiolabel. Two-dimensional gel electrophoresis followed by fluorography revealed 35S-sulfate incorporation into only two of the six VP1 isoelectric species (E and F). Amino acid analysis of 35S-sulfate labeled VP1 by enzymatic hydrolysis followed by two-dimensional thin-layer electrophoresis revealed the presence of 35S-sulfate-labeled tyrosine-O-sulfate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Consigli R. A. Chemical cleavage of polyomavirus major structural protein VP1: identification of cleavage products and evidence that the receptor moiety resides in the carboxy-terminal region. J Virol. 1983 Oct;48(1):197–205. doi: 10.1128/jvi.48.1.197-205.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Consigli R. A. Comparison of nonphosphorylated and phosphorylated species of polyomavirus major capsid protein VP1 and identification of the major phosphorylation region. J Virol. 1983 Oct;48(1):206–217. doi: 10.1128/jvi.48.1.206-217.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhorn R., Jackson V., Granner D., Chalkey R. Phosphorylation of the lysine-rich histones throughout the cell cycle. Biochemistry. 1975 Jun 3;14(11):2504–2511. doi: 10.1021/bi00682a033. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Rieke W. O., Chalkley R. Rapid electrophoretic analysis for histone phosphorylation. A reinvestigation of phosphorylation of lysine-rich histone during rat liver regeneration. Biochemistry. 1971 Oct 12;10(21):3952–3959. doi: 10.1021/bi00797a024. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Anders D. G., Trempy J., Consigli R. A. Differences in the subpopulations of the structural proteins of polyoma virions and capsids: biological functions of the multiple VP1 species. J Virol. 1981 Jan;37(1):80–91. doi: 10.1128/jvi.37.1.80-91.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Consigli R. A. Separation of neutralizing and hemagglutination-inhibiting antibody activities and specificity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1980 Apr;34(1):119–129. doi: 10.1128/jvi.34.1.119-129.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N'-tetraacetic acid and dithiothreitol. J Virol. 1978 Jul;27(1):193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J. N., Winston V. D., Consigli R. A. Dissociation of polyoma virus by the chelation of calcium ions found associated with purified virions. J Virol. 1977 Sep;23(3):717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Frost E., Bourgaux P. Electrophoretic analysis of the structural polypeptides of polyoma virus mutants. Virology. 1975 May;65(1):286–288. doi: 10.1016/0042-6822(75)90032-x. [DOI] [PubMed] [Google Scholar]
- Fukui S., Numata Y., Yamashina I. Comparison of protein sulfation in control and virus-transformed baby hamster kidney cells. J Biochem. 1984 Dec;96(6):1783–1788. doi: 10.1093/oxfordjournals.jbchem.a135011. [DOI] [PubMed] [Google Scholar]
- Garcea R. L., Ballmer-Hofer K., Benjamin T. L. Virion assembly defect of polyomavirus hr-t mutants: underphosphorylation of major capsid protein VP1 before viral DNA encapsidation. J Virol. 1985 May;54(2):311–316. doi: 10.1128/jvi.54.2.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Prager M. D. The effect of butyrate on sulfated glycoprotein biosynthesis by human kidney tumor cells. J Biol Chem. 1981 Jul 10;256(13):6529–6532. [PubMed] [Google Scholar]
- Hewick R. M., Fried M., Waterfield M. D. Nonhistone virion proteins of polyoma: characterisation of the particle proteins by tryptic peptide analysis by use of ion-exchange columns. Virology. 1975 Aug;66(2):408–419. doi: 10.1016/0042-6822(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Determination and occurrence of tyrosine O-sulfate in proteins. Methods Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Center M. S., Consigli R. A. Origin of the polyoma virus-associated endonuclease. J Virol. 1975 Jan;17(1):127–131. doi: 10.1128/jvi.17.1.127-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. R., Free S. J. Protein modification and its biological role. Int J Biochem. 1985;17(3):283–289. doi: 10.1016/0020-711x(85)90202-2. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- Pinter A., Compans R. W. Sulfated components of enveloped viruses. J Virol. 1975 Oct;16(4):859–866. doi: 10.1128/jvi.16.4.859-866.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Robbins A. K., Crawford L. V. Phophorylation of polyoma and SV40 virus proteins. J Gen Virol. 1977 Oct;37(1):75–83. doi: 10.1099/0022-1317-37-1-75. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Raghow R., Portner A., Hsu C. H., Clark S. B., Kingsbury D. W. Charge heterogeneity in polypeptides of negative strand RNA viruses. Virology. 1978 Oct 15;90(2):214–225. doi: 10.1016/0042-6822(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Consigli R. A. Transient inhibition of polyoma virus synthesis by Sendai virus (parainfluenza I). I. Demonstration and nature of the inhibition by inactivated virus. J Virol. 1972 Dec;10(6):1091–1097. doi: 10.1128/jvi.10.6.1091-1097.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Generation of capsids from unstable polyoma virions. J Virol. 1983 Sep;47(3):620–625. doi: 10.1128/jvi.47.3.620-625.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L. K., Consigli R. A. Identification and protein analysis of polyomavirus assembly intermediates from infected primary mouse embryo cells. Virology. 1985 Jul 15;144(1):127–138. doi: 10.1016/0042-6822(85)90311-3. [DOI] [PubMed] [Google Scholar]