Abstract
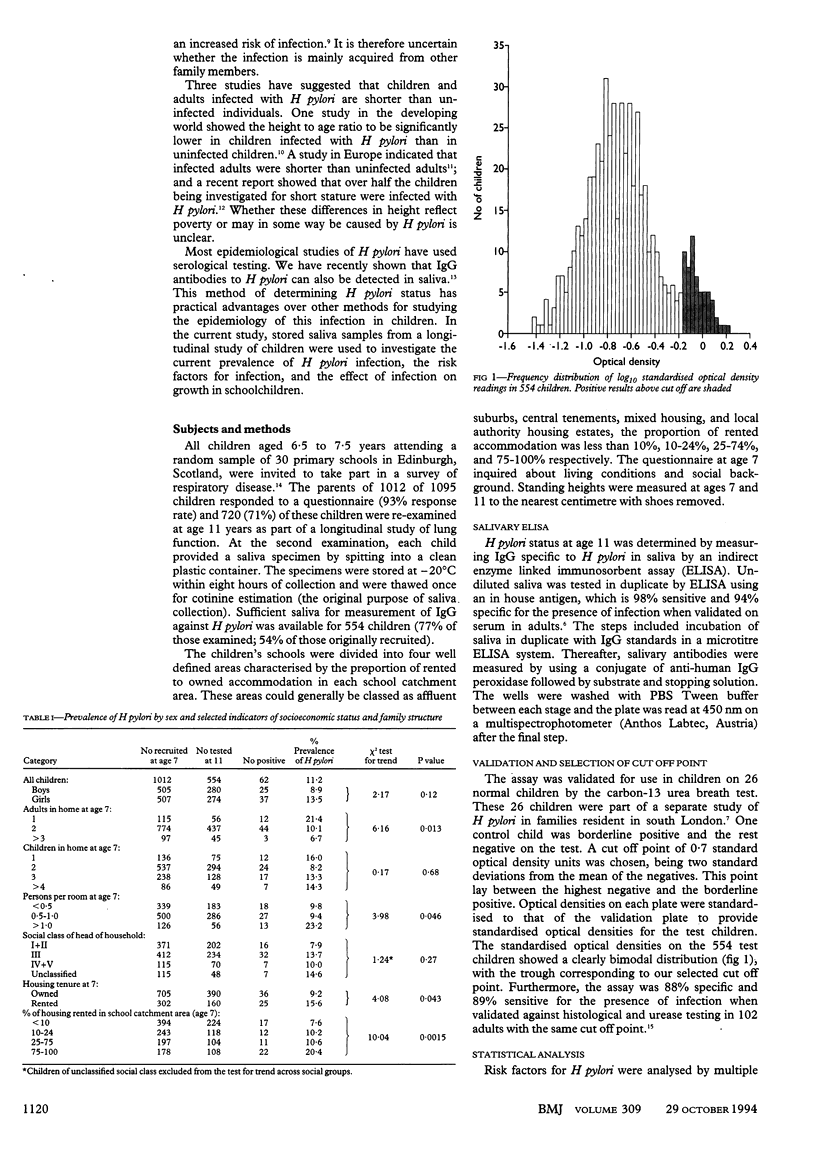
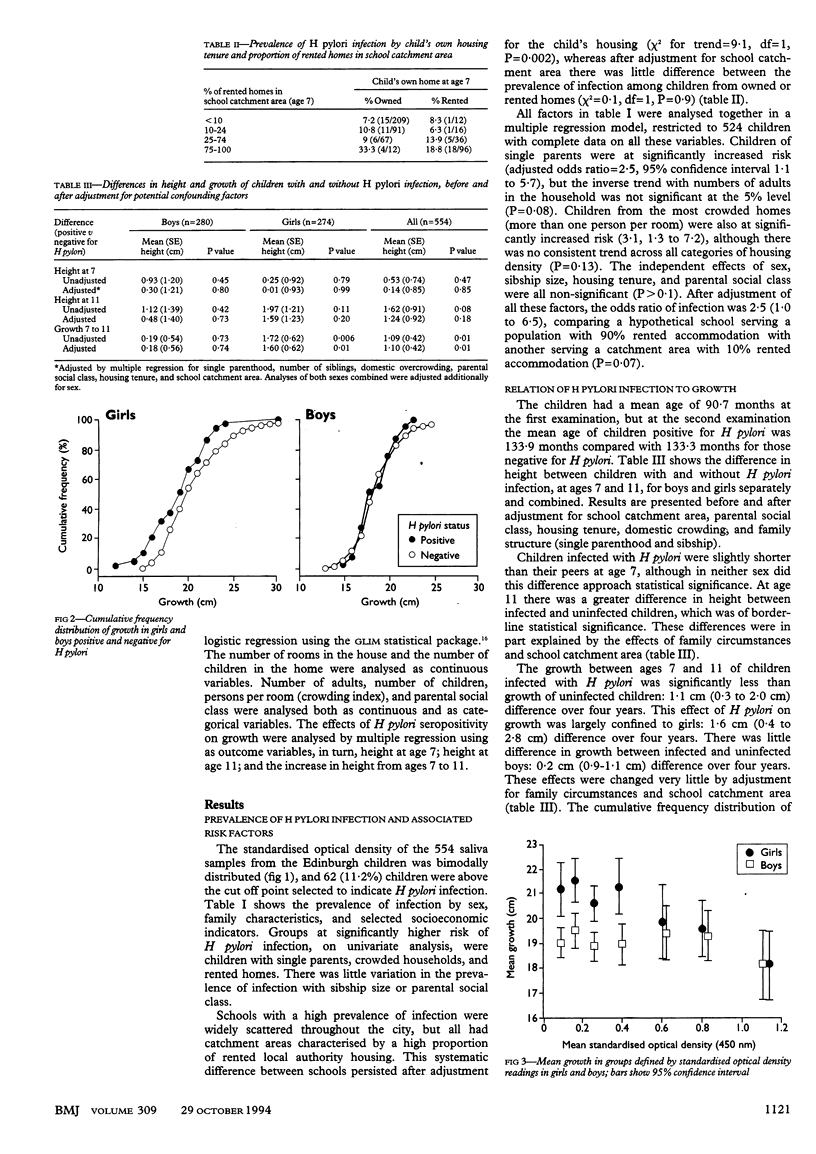
OBJECTIVE--To investigate the current prevalence of Helicobacter pylori infection in childhood, the risk factors for infection, and the effect of infection on growth in preadolescent schoolchildren. DESIGN--Population based sample of 7 year old schoolchildren followed up at age 11; data on risk factors for infection collected at age 7; presence of infection at age 11 determined by measurement of salivary IgG against H pylori by a newly developed enzyme linked immunosorbent assay (ELISA). Height was measured at 7 and 11 years of age. SUBJECTS--554 schoolchildren from Edinburgh. RESULTS--62 (11%) children had H pylori infection. Independent risk factors for infection were single parent families (adjusted odds ratio = 2.5; 95% confidence interval 1.1 to 5.7), the 10% most crowded homes (3.1; 1.3 to 7.2), and schools serving predominantly rented housing estates (2.5; 1.0 to 6.5). School catchment area was more important than parental social class or housing tenure. Growth in height between 7 and 11 was diminished in infected children by a mean of 1.1 cm (0.3 to 2.0 cm) over four years. This growth reduction was largely confined to girls (1.6 cm over four years), among whom it correlated with salivary IgG (P = 0.015). CONCLUSION--Data from salivary assay to investigate the epidemiology of H pylori suggest that factors relating to the type of community in which the child lives may now be as important for acquisition of this infection as features of the family home. The greater reduction of growth among infected girls raises the possibility that H pylori infection may delay or diminish the pubertal growth spurt.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkholz S., Knipp U., Nietzki C., Adamek R. J., Opferkuch W. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol Med Microbiol. 1993 Apr;6(4):317–324. doi: 10.1111/j.1574-695X.1993.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Peichl P., Wyatt J. I., Stachl U., Lindley I. J. Gastric interleukin-8 and IgA IL-8 autoantibodies in Helicobacter pylori infection. Scand J Immunol. 1993 Jan;37(1):65–70. doi: 10.1111/j.1365-3083.1993.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Crowne E. C., Shalet S. M., Wallace W. H., Eminson D. M., Price D. A. Final height in girls with untreated constitutional delay in growth and puberty. Eur J Pediatr. 1991 Aug;150(10):708–712. doi: 10.1007/BF01958760. [DOI] [PubMed] [Google Scholar]
- Drumm B., Perez-Perez G. I., Blaser M. J., Sherman P. M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990 Feb 8;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Adam E., Reddy G. T., Agarwal J. P., Agarwal R., Evans D. J., Jr, Malaty H. M., Evans D. G. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig Dis Sci. 1991 Aug;36(8):1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- Hill I. D., Sinclair-Smith C., Lastovica A. J., Bowie M. D., Emms M. Transient protein losing enteropathy associated with acute gastritis and campylobacter pylori. Arch Dis Child. 1987 Dec;62(12):1215–1219. doi: 10.1136/adc.62.12.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine D. L., Chambers S. T., Hart D. J., Chapman B. A. Primary biliary cirrhosis presenting with granulomatous skin lesions. Gut. 1994 Apr;35(4):564–566. doi: 10.1136/gut.35.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jivani S. K., Rayner P. H. Does control influence the growth of diabetic children? Arch Dis Child. 1973 Feb;48(2):109–115. doi: 10.1136/adc.48.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. D., Graham D. Y., Gaillour A., Opekun A. R., Smith E. O. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet. 1991 Jun 22;337(8756):1503–1506. doi: 10.1016/0140-6736(91)93196-g. [DOI] [PubMed] [Google Scholar]
- Kuh D. L., Power C., Rodgers B. Secular trends in social class and sex differences in adult height. Int J Epidemiol. 1991 Dec;20(4):1001–1009. doi: 10.1093/ije/20.4.1001. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Mendall M. A., Goggin P. M., Molineaux N., Levy J., Toosy T., Strachan D., Northfield T. C. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet. 1992 Apr 11;339(8798):896–897. doi: 10.1016/0140-6736(92)90931-r. [DOI] [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991 Aug;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégraud F., Brassens-Rabbé M. P., Denis F., Belbouri A., Hoa D. Q. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989 Aug;27(8):1870–1873. doi: 10.1128/jcm.27.8.1870-1873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsonnet J., Blaser M. J., Perez-Perez G. I., Hargrett-Bean N., Tauxe R. V. Symptoms and risk factors of Helicobacter pylori infection in a cohort of epidemiologists. Gastroenterology. 1992 Jan;102(1):41–46. doi: 10.1016/0016-5085(92)91782-y. [DOI] [PubMed] [Google Scholar]
- Raymond J., Bergeret M., Benhamou P. H., Mensah K., Dupont C. A 2-year study of Helicobacter pylori in children. J Clin Microbiol. 1994 Feb;32(2):461–463. doi: 10.1128/jcm.32.2.461-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J. The endocrine significance of cytokines. J Endocrinol. 1991 Feb;128(2):171–173. doi: 10.1677/joe.0.1280171. [DOI] [PubMed] [Google Scholar]
- Strachan D. P. Damp housing and childhood asthma: validation of reporting of symptoms. BMJ. 1988 Nov 12;297(6658):1223–1226. doi: 10.1136/bmj.297.6658.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. M. Trend towards earlier menarche in London, Olso, Copenhagen, the Netherlands and Hungary. Nature. 1973 May 11;243(5402):95–96. doi: 10.1038/243095a0. [DOI] [PubMed] [Google Scholar]
- Tanner J. M., Whitehouse R. H., Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child. 1966 Oct;41(219):454–471. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Webb P. M., Knight T., Greaves S., Wilson A., Newell D. G., Elder J., Forman D. Relation between infection with Helicobacter pylori and living conditions in childhood: evidence for person to person transmission in early life. BMJ. 1994 Mar 19;308(6931):750–753. doi: 10.1136/bmj.308.6931.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


