Abstract
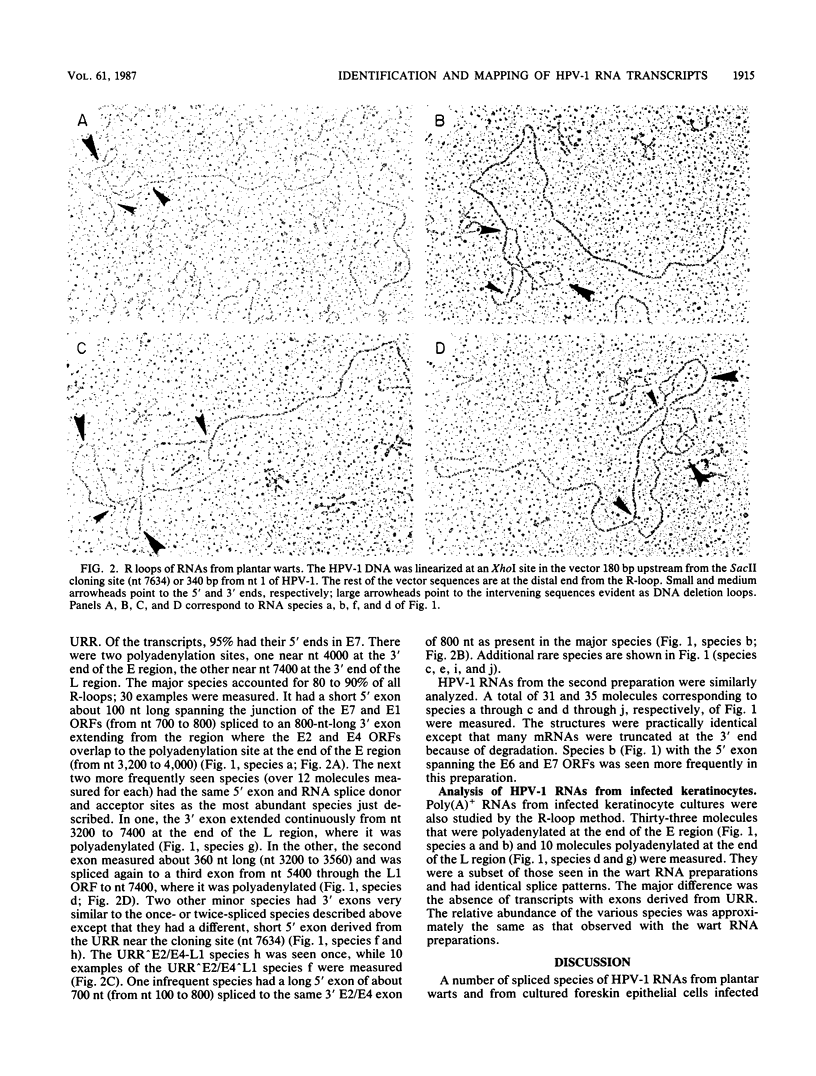
Multiple spliced transcripts of human papillomavirus type 1 were detected by electron microscopic analysis of R-loops formed with total RNA extracted from plantar warts and with poly(A)+ RNA isolated from cultured keratinocytes infected with human papillomavirus type 1. The 5' ends of the RNAs were mapped to sites in the E7 open reading frame (ORF), just upstream of the E6 ORF and in the upstream regulatory region. Species with 5' ends in E7 accounted for over 95% of all transcripts seen. Two polyadenylation sites were used, one at the end of the early (E) region of the viral DNA, the other at the end of the late (L) region. The most abundant species had a short 5' exon of approximately 100 nucleotides spanning the junction of the E7 and E1 ORFs spliced to a 3' exon of 800 nucleotides in the region with overlapping E2 and E4 ORFs; it was polyadenylated at the end of the E region. This species probably encodes the abundant E4 protein found in plantar warts (F. Breitburd, O. Croissant, and G. Orth, Cancer Cells, vol. 5, in press; J. Doorbar, D. Campbell, R. J. A. Grand, and P. H. Gallimore, EMBO J. 5:355-362, 1986). Other transcripts had exons spanning the E6-E7 ORFs, the E4-E5-L2-L1 ORFs, or the L1 ORF. The infrequent L1 transcript, probably the mRNA coding for the major capsid protein, had the same 5' exon in E7 as the abundant mRNA spliced from E1 and E4 ORFs, suggesting genetic regulation via the choice of the alternative polyadenylation sites or mRNA processing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg L. J., Singh K., Botchan M. Complementation of a bovine papilloma virus low-copy-number mutant: evidence for a temporal requirement of the complementing gene. Mol Cell Biol. 1986 Mar;6(3):859–869. doi: 10.1128/mcb.6.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett T. S., Gallimore P. H. Introduction of cloned human papillomavirus 1a DNA into rat fibroblasts: integration, de novo methylation and absence of cellular morphological transformation. J Gen Virol. 1985 May;66(Pt 5):1063–1072. doi: 10.1099/0022-1317-66-5-1063. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R. The spliced structures of adenovirus 2 fiber message and the other late mRNAs. Cell. 1978 Oct;15(2):497–510. doi: 10.1016/0092-8674(78)90019-3. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Gelinas R. E., Broker T. R., Roberts R. J. An amazing sequence arrangement at the 5' ends of adenovirus 2 messenger RNA. Cell. 1977 Sep;12(1):1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Streeck R. E. Genome organization and nucleotide sequence of human papillomavirus type 33, which is associated with cervical cancer. J Virol. 1986 Jun;58(3):991–995. doi: 10.1128/jvi.58.3.991-995.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant O., Breitburd F., Orth G. Specificity of cytopathic effect of cutaneous human papillomaviruses. Clin Dermatol. 1985 Oct-Dec;3(4):43–55. doi: 10.1016/0738-081x(85)90048-3. [DOI] [PubMed] [Google Scholar]
- Danos O., Georges E., Orth G., Yaniv M. Fine structure of the cottontail rabbit papillomavirus mRNAs expressed in the transplantable VX2 carcinoma. J Virol. 1985 Mar;53(3):735–741. doi: 10.1128/jvi.53.3.735-741.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C., Yaniv M. Production of spliced DNA copies of the cottontail rabbit papillomavirus genome in a retroviral vector. Ciba Found Symp. 1986;120:68–82. doi: 10.1002/9780470513309.ch6. [DOI] [PubMed] [Google Scholar]
- Dartmann K., Schwarz E., Gissmann L., zur Hausen H. The nucleotide sequence and genome organization of human papilloma virus type 11. Virology. 1986 May;151(1):124–130. doi: 10.1016/0042-6822(86)90110-8. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Campbell D., Grand R. J., Gallimore P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 1986 Feb;5(2):355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E., Croissant O., Bonneaud N., Orth G. Physical state and transcription of the cottontail rabbit papillomavirus genome in warts and transplantable VX2 and VX7 carcinomas of domestic rabbits. J Virol. 1984 Aug;51(2):530–538. doi: 10.1128/jvi.51.2.530-538.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I., Danos O., Yaniv M. Genomic structure of the cottontail rabbit (Shope) papillomavirus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1580–1584. doi: 10.1073/pnas.82.6.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Molecular cloning and nucleotide sequence of deer papillomavirus. J Virol. 1985 Oct;56(1):85–91. doi: 10.1128/jvi.56.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska S., Orth G., Obalek S., Croissant O. Cutaneous warts. Clinical, histologic, and virologic correlations. Clin Dermatol. 1985 Oct-Dec;3(4):71–82. doi: 10.1016/0738-081x(85)90051-3. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorta R. F., Taichman L. B. Human papilloma viral DNA replicates as a stable episome in cultured epidermal keratinocytes. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3393–3397. doi: 10.1073/pnas.79.11.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. Cottontail rabbit papillomavirus-specific transcripts in transplantable tumors with integrated DNA. Virology. 1984 Oct 30;138(2):362–367. doi: 10.1016/0042-6822(84)90362-3. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. Differences exist between viral transcripts in cottontail rabbit papillomavirus-induced benign and malignant tumors as well as non-virus-producing and virus-producing tumors. J Virol. 1984 Sep;51(3):706–712. doi: 10.1128/jvi.51.3.706-712.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Leary S. L., Faras A. J. Shope papillomavirus transcription in benign and malignant rabbit tumors. Virology. 1985 Oct 15;146(1):120–129. doi: 10.1016/0042-6822(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Zabielski J., Ahola H., Moreno-Lopez J., Pettersson U. Messenger RNAs from the transforming region of bovine papilloma virus type I. J Mol Biol. 1985 Apr 20;182(4):541–554. doi: 10.1016/0022-2836(85)90240-2. [DOI] [PubMed] [Google Scholar]
- Syrjänen K. J. Human papillomavirus (HPV) infections of the female genital tract and their associations with intraepithelial neoplasia and squamous cell carcinoma. Pathol Annu. 1986;21(Pt 1):53–89. [PubMed] [Google Scholar]
- Taichman L., Reilly S., Garant P. R. In-vitro cultivation of human oral keratinocytes. Arch Oral Biol. 1979;24(5):335–341. doi: 10.1016/0003-9969(79)90099-2. [DOI] [PubMed] [Google Scholar]
- Tsunokawa Y., Takebe N., Kasamatsu T., Terada M., Sugimura T. Transforming activity of human papillomavirus type 16 DNA sequence in a cervical cancer. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2200–2203. doi: 10.1073/pnas.83.7.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts S. L., Phelps W. C., Ostrow R. S., Zachow K. R., Faras A. J. Cellular transformation by human papillomavirus DNA in vitro. Science. 1984 Aug 10;225(4662):634–636. doi: 10.1126/science.6330900. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Burkhardt A. L., Doniger J., DiPaolo J. A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986 Feb;57(2):572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L., Schlehofer J. R. Viruses in the etiology of human genital cancer. Prog Med Virol. 1984;30:170–186. [PubMed] [Google Scholar]




