Abstract
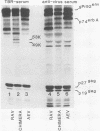
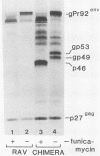
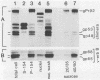
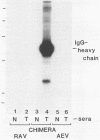
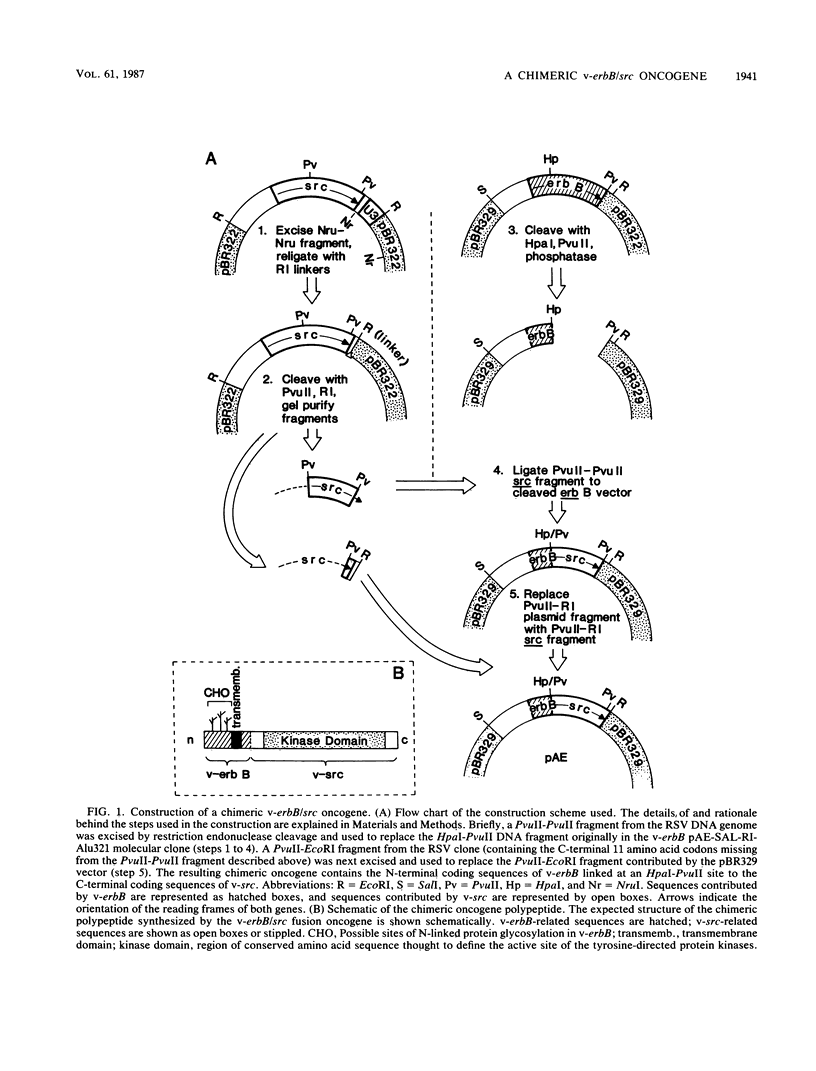
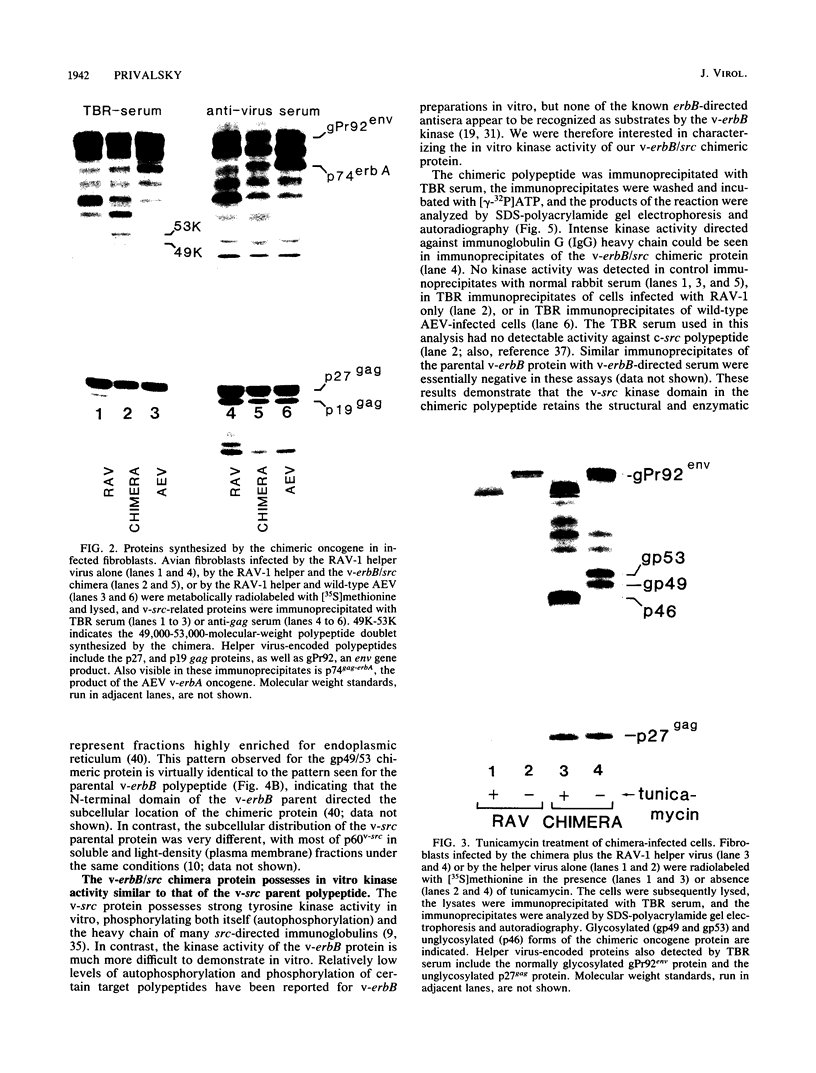
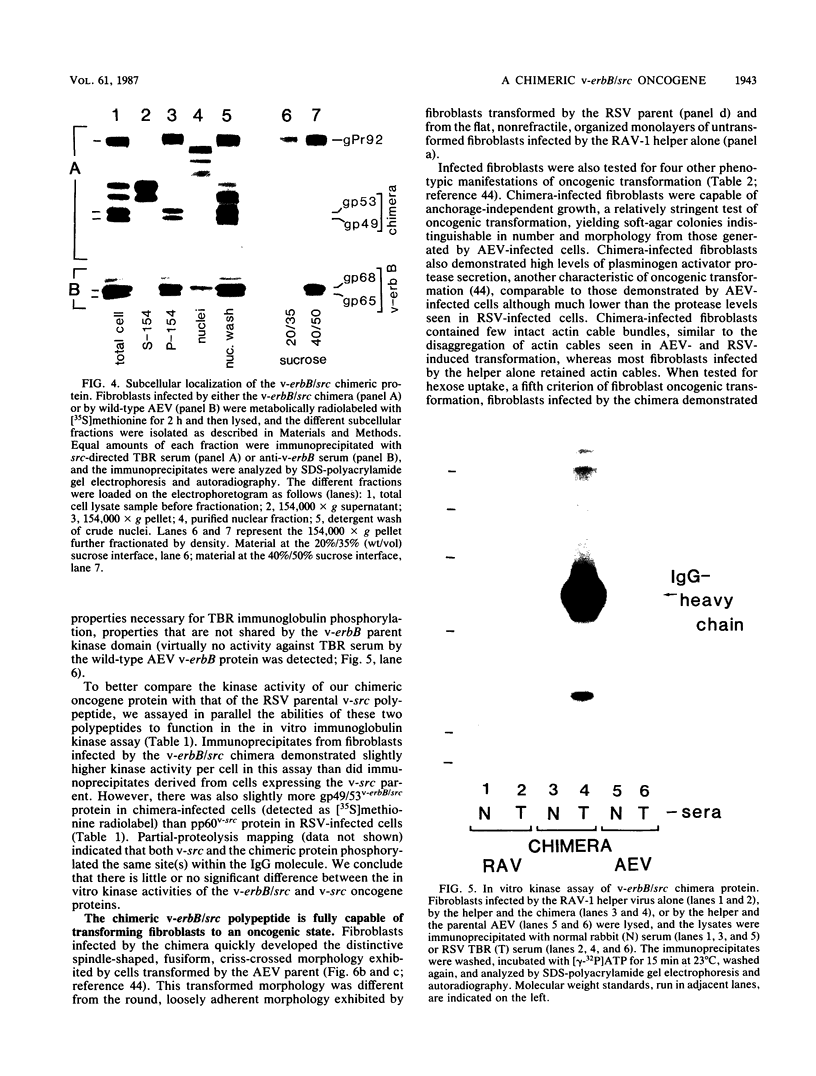
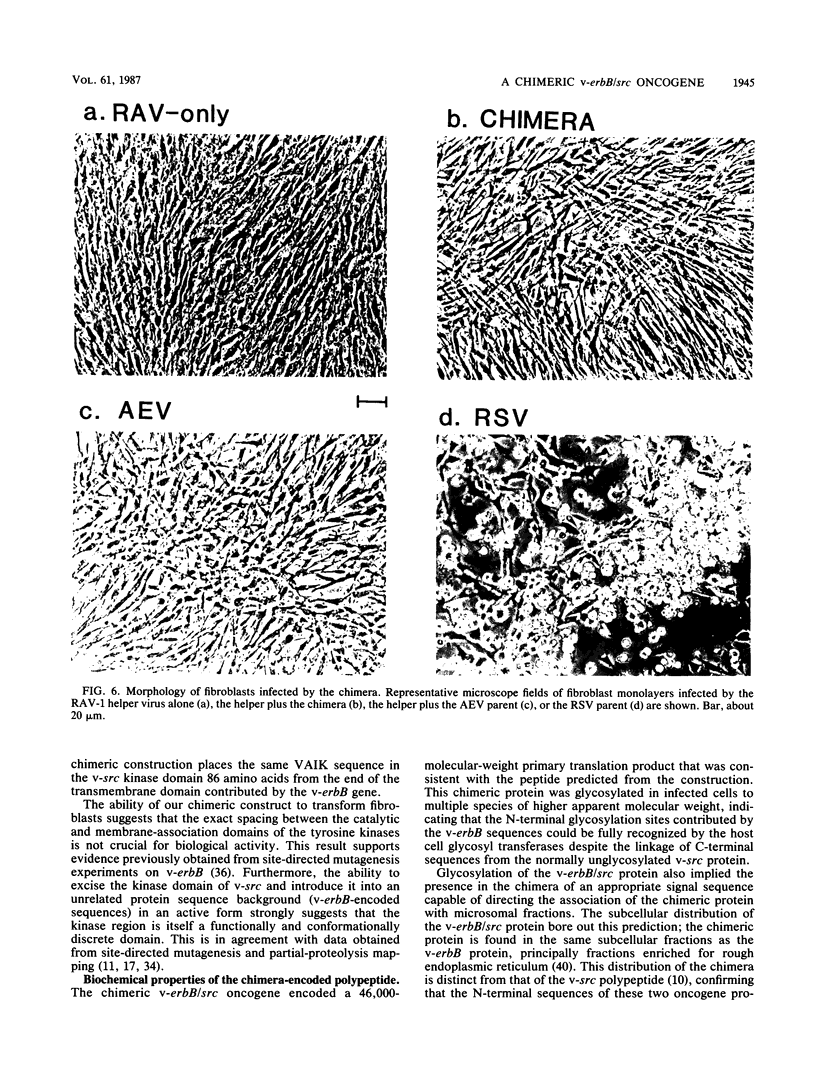
A novel gene was created that linked complementary portions of two different tyrosine kinase oncogenes: v-erB and v-src. The v-erbB/src chimera encoded a glycoprotein exhibiting the subcellular distribution of the v-erbB protein but containing the kinase catalytic domain of the v-src parent. Fibroblasts expressing the v-erbB/src gene product became transformed to an oncogenic state and closely resembled cells expressing the v-erbB parent oncogene. Our results indicated that v-erbB sequences can be functionally replaced by sequences derived from a different oncogene, v-src, and that important determinants of the transformed phenotype appear to be encoded in oncogene sequences distinct from those defining the kinase catalytic domain itself.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. D., Beckmann R. P., Harms E. H., Nakamura K., Weber M. J. Biological properties of "partial" transformation mutants of Rous sarcoma virus and characterization of their pp60src kinase. J Virol. 1981 Jan;37(1):445–458. doi: 10.1128/jvi.37.1.445-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Nothnagel E. A., Webb W. W. Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. Proc Natl Acad Sci U S A. 1980 Feb;77(2):980–984. doi: 10.1073/pnas.77.2.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker W. C., Dayhoff M. O. Viral src gene products are related to the catalytic chain of mammalian cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 May;79(9):2836–2839. doi: 10.1073/pnas.79.9.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Hayman M. J. Temperature-sensitive mutants of avian erythroblastosis virus: surface expression of the erbB product correlates with transformation. Cell. 1984 Apr;36(4):963–972. doi: 10.1016/0092-8674(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. Corrections to the nucleotide sequence of the src gene of Rous sarcoma virus. Nature. 1983 Feb 24;301(5902):736–738. doi: 10.1038/301736b0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerman M. H., Davis B. R., Pattengale P. K., Fan H. Generation of a recombinant Moloney murine leukemia virus carrying the v-src gene of avian sarcoma virus: transformation in vitro and pathogenesis in vivo. J Virol. 1985 Jun;54(3):804–816. doi: 10.1128/jvi.54.3.804-816.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Bechberger J., Nedic I. Four Rous sarcoma virus mutants which affect transformed cell morphology exhibit altered src gene products. Virology. 1981 Oct 15;114(1):256–260. doi: 10.1016/0042-6822(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L., Samarut J., Bouabdelli M., Blanchet J. P. Early precursors in the erythroid lineage are the specific target cells of avian erythroblastosis virus in vitro. Cell. 1980 Dec;22(3):683–691. doi: 10.1016/0092-8674(80)90544-9. [DOI] [PubMed] [Google Scholar]
- Gilmore T., DeClue J. E., Martin G. S. Protein phosphorylation at tyrosine is induced by the v-erbB gene product in vivo and in vitro. Cell. 1985 Mar;40(3):609–618. doi: 10.1016/0092-8674(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Graf T. In vitro transformation of chicken bone marrow cells with avian erythroblastosis virus. Z Naturforsch C. 1975 Nov-Dec;30(6):847–849. doi: 10.1515/znc-1975-11-1232. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Beug H. Identification of a form of the avian erythroblastosis virus erb-B gene product at the cell surface. 1984 May 31-Jun 6Nature. 309(5967):460–462. doi: 10.1038/309460a0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Shimizu T. Heterogeneity of strain R avian (erythroblastosis) virus. Cancer Res. 1970 Dec;30(12):2827–2831. [PubMed] [Google Scholar]
- Iwashita S., Kitamura N., Yoshida M. Molecular events leading to fusiform morphological transformation by partial src deletion mutant of Rous sarcoma virus. Virology. 1983 Mar;125(2):419–431. doi: 10.1016/0042-6822(83)90213-1. [DOI] [PubMed] [Google Scholar]
- Kahn P., Adkins B., Beug H., Graf T. src- and fps-containing avian sarcoma viruses transform chicken erythroid cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7122–7126. doi: 10.1073/pnas.81.22.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Kris R. M., Lax I., Gullick W., Waterfield M. D., Ullrich A., Fridkin M., Schlessinger J. Antibodies against a synthetic peptide as a probe for the kinase activity of the avian EGF receptor and v-erbB protein. Cell. 1985 Mar;40(3):619–625. doi: 10.1016/0092-8674(85)90210-7. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R. Subcellular localization of pp60src in RSV-transformed cells. Curr Top Microbiol Immunol. 1983;107:51–124. [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Ng M., Privalsky M. L. Structural domains of the avian erythroblastosis virus erbB protein required for fibroblast transformation: dissection by in-frame insertional mutagenesis. J Virol. 1986 May;58(2):542–553. doi: 10.1128/jvi.58.2.542-553.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E., Levintow L., Bishop J. M. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src). Proc Natl Acad Sci U S A. 1979 Apr;76(4):1804–1808. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri S., Beug H., Graf T. Isolation and characterization of four new temperature-sensitive mutants of avian erythroblastosis virus (AEV). Virology. 1982 Dec;123(2):296–311. doi: 10.1016/0042-6822(82)90263-x. [DOI] [PubMed] [Google Scholar]
- Palmieri S. Transformation of erythroid cells by Rous sarcoma virus (RSV). Virology. 1985 Jan 30;140(2):269–280. doi: 10.1016/0042-6822(85)90365-4. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Subcellular localization of the v-erb-B protein, the product of a transforming gene of avian erythroblastosis virus. Virology. 1984 Jun;135(2):356–368. doi: 10.1016/0042-6822(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Sealy L., Bishop J. M., McGrath J. P., Levinson A. D. The product of the avian erythroblastosis virus erbB locus is a glycoprotein. Cell. 1983 Apr;32(4):1257–1267. doi: 10.1016/0092-8674(83)90307-0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Jovanovich S., Erikson R. L. Sites of synthesis of viral proteins in avian sarcoma virus-infected chicken cells. J Virol. 1980 Sep;35(3):629–636. doi: 10.1128/jvi.35.3.629-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L., Reynolds S. Regulation of cellular morphology by the Rous sarcoma virus src gene: analysis of fusiform mutants. Mol Cell Biol. 1985 Nov;5(11):3097–3107. doi: 10.1128/mcb.5.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]