Abstract
Synthetic oligonucleotides complementary to putative retroviral primer-binding sites were used as hybridization probes to detect novel retroviruslike sequences. An 8.1-kilobase element with structural features of a retroviral provirus was isolated from a human genomic library by this approach. Nucleotide sequence analysis of its 600-base-pair long terminal repeats revealed characteristic motifs known as regulatory signals for RNA polymerase II transcription: CCAAT, TATA, and ATTAAA. In addition, a putative pol gene displays apparent homologies to conserved regions of retroviral reverse transcriptase. The 5' long terminal repeat is flanked at its 3' end by a putative primer-binding site for reverse transcription with homology to tRNA(Pro). This element is therefore termed HuRRS-P (human retrovirus-related sequence-proline). There are 20 to 40 copies of HuRRS-P homologous sequences in DNAs of human and simian origin.
Full text
PDF

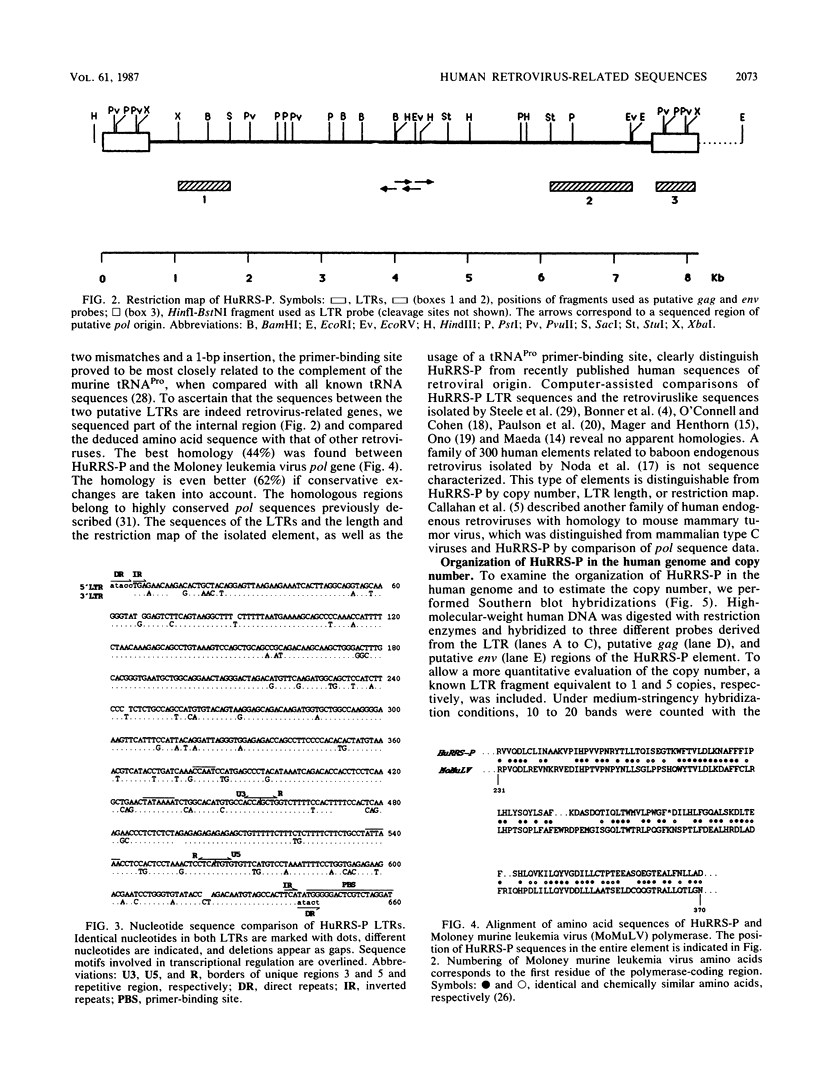
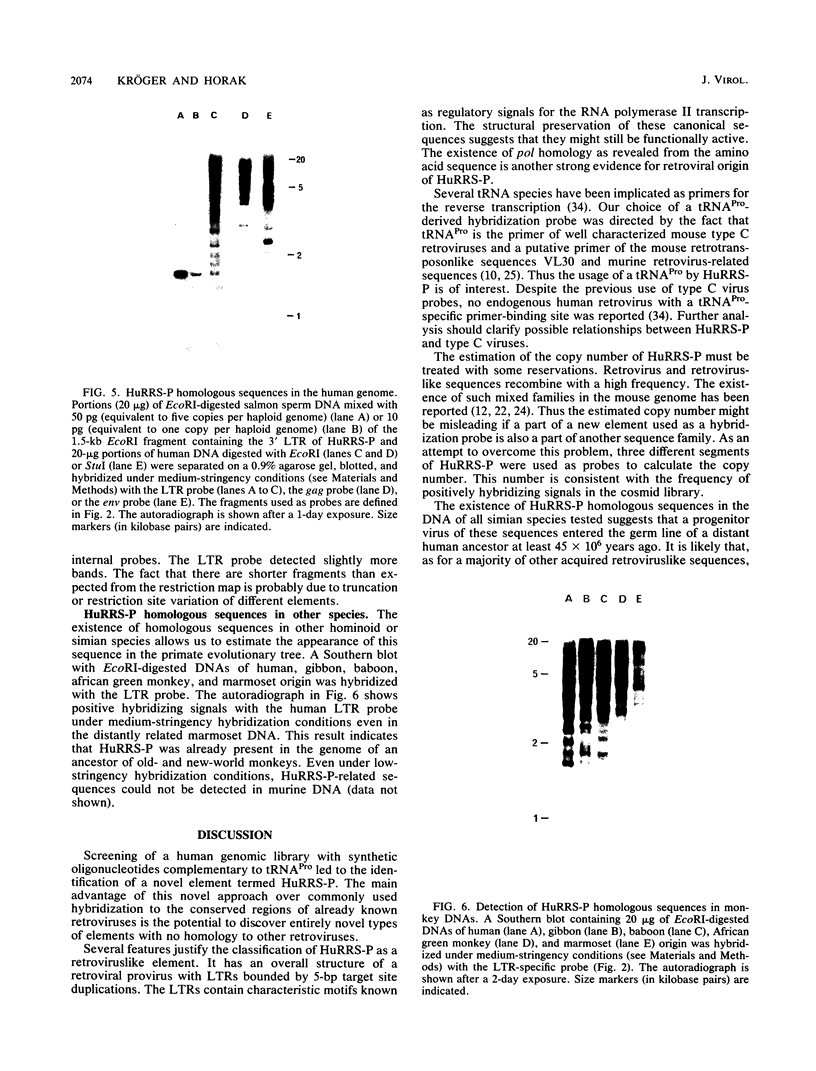

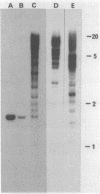
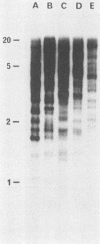
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Chiu I. M., Wong J. F., Tronick S. R., Roe B. A., Aaronson S. A., Schlom J. A new class of endogenous human retroviral genomes. Science. 1985 Jun 7;228(4704):1208–1211. doi: 10.1126/science.2408338. [DOI] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels G. R., Deininger P. L. Repeat sequence families derived from mammalian tRNA genes. 1985 Oct 31-Nov 6Nature. 317(6040):819–822. doi: 10.1038/317819a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Rechavi G., Givol D., Canaani E. Homology between an endogenous viral LTR and sequences inserted in an activated cellular oncogene. Nature. 1983 Apr 7;302(5908):547–548. doi: 10.1038/302547a0. [DOI] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Kurihara M., Takano T. Retrovirus-related sequences in human DNA: detection and cloning of sequences which hybridize with the long terminal repeat of baboon endogenous virus. Nucleic Acids Res. 1982 May 11;10(9):2865–2878. doi: 10.1093/nar/10.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell C. D., Cohen M. The long terminal repeat sequences of a novel human endogenous retrovirus. Science. 1984 Dec 7;226(4679):1204–1206. doi: 10.1126/science.6505687. [DOI] [PubMed] [Google Scholar]
- Ono M. Molecular cloning and long terminal repeat sequences of human endogenous retrovirus genes related to types A and B retrovirus genes. J Virol. 1986 Jun;58(3):937–944. doi: 10.1128/jvi.58.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson K. E., Deka N., Schmid C. W., Misra R., Schindler C. W., Rush M. G., Kadyk L., Leinwand L. A transposon-like element in human DNA. Nature. 1985 Jul 25;316(6026):359–361. doi: 10.1038/316359a0. [DOI] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst F., Vande Woude G. F. A novel transposon-like repeat interrupted by an LTR element occurs in a cluster of B1 repeats in the mouse c-mos locus. Nucleic Acids Res. 1984 Nov 26;12(22):8381–8392. doi: 10.1093/nar/12.22.8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Glöggler K., Wirth T., Horak I. Evidence that a major class of mouse endogenous long terminal repeats (LTRs) resulted from recombination between exogenous retroviral LTRs and similar LTR-like elements (LTR-IS). Proc Natl Acad Sci U S A. 1984 Nov;81(21):6696–6700. doi: 10.1073/pnas.81.21.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Wirth T., Kröger B., Horak I. Structure and genomic organization of a new family of murine retrovirus-related DNA sequences (MuRRS). Nucleic Acids Res. 1985 May 24;13(10):3461–3470. doi: 10.1093/nar/13.10.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Moll J., Meissner F., Hartmann T. Compilation of tRNA sequences. Nucleic Acids Res. 1985;13 (Suppl):r1–49. doi: 10.1093/nar/13.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele P. E., Rabson A. B., Bryan T., Martin M. A. Distinctive termini characterize two families of human endogenous retroviral sequences. Science. 1984 Aug 31;225(4665):943–947. doi: 10.1126/science.6089336. [DOI] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Ono M., Miyata T. Retroviral gag and DNA endonuclease coding sequences in IgE-binding factor gene. 1985 Nov 28-Dec 4Nature. 318(6044):388–389. doi: 10.1038/318388a0. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]