Abstract
A number of MC29 mutants with deleted myc genes have been previously characterized. Many of these mutants have been found to be defective for transformation of chicken macrophages in vitro and for tumor induction in chickens. Such mutants are capable of transforming Japanese quail macrophages in vitro and inducing a high incidence of tumors in Japanese quail. Thus, Japanese quail may contain a factor(s) capable of complementing the defective transforming proteins encoded by some deleted v-myc genes.
Full text
PDF

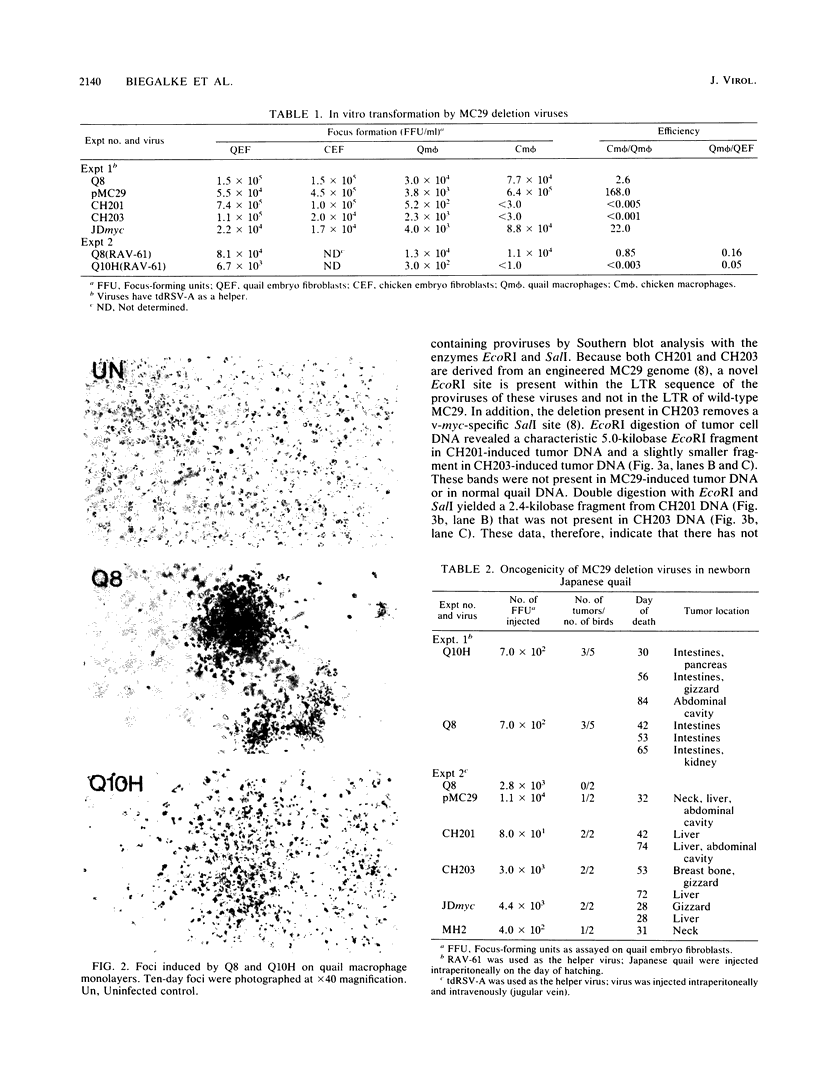


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrietto P. J., Hayman M. J., Ramsay G. M., Wyke J. A., Payne L. N. Altered pathogenicity of avian myelocytomatosis (MC29) viruses with mutations in the v-myc gene. Virology. 1983 Jan 15;124(1):164–172. doi: 10.1016/0042-6822(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Enrietto P. J., Hayman M. J. Restriction enzyme analysis of partially transformation-defective mutants of acute leukemia virus MC29. J Virol. 1982 Nov;44(2):711–715. doi: 10.1128/jvi.44.2.711-715.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin G. M., Parsons J. T. Identification of transcriptional elements within the long terminal repeat of Rous sarcoma virus. Mol Cell Biol. 1983 Oct;3(10):1834–1845. doi: 10.1128/mcb.3.10.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., von Weizsaecker F., Grieser S., Coll J., Stehelin D., Patschinsky T., Bister K., Bechade C., Calothy G., Leutz A. v-mil induces autocrine growth and enhanced tumorigenicity in v-myc-transformed avian macrophages. Cell. 1986 May 9;45(3):357–364. doi: 10.1016/0092-8674(86)90321-1. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney M. L., Pierce J., Parsons J. T. Site-directed mutagenesis of the gag-myc gene of avian myelocytomatosis virus 29: biological activity and intracellular localization of structurally altered proteins. J Virol. 1986 Oct;60(1):167–176. doi: 10.1128/jvi.60.1.167-176.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. W., Patschinsky T., Bister K. Avian oncovirus MH2: molecular cloning of proviral DNA and structural analysis of viral RNA and protein. J Virol. 1983 Oct;48(1):61–73. doi: 10.1128/jvi.48.1.61-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen H. W., Rückert B., Lurz R., Bister K. Two unrelated cell-derived sequences in the genome of avian leukemia and carcinoma inducing retrovirus MH2. EMBO J. 1983;2(11):1969–1975. doi: 10.1002/j.1460-2075.1983.tb01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Flordellis C. S., Mark G. E., Duesberg P. H., Papas T. S. A common onc gene sequence transduced by avian carcinoma virus MH2 and by murine sarcoma virus 3611. Science. 1984 Feb 24;223(4638):813–816. doi: 10.1126/science.6320371. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Schulz R. A., Garon C. F., Tsichlis P. N., Papas T. S. Molecular cloning of avian myelocytomatosis virus (MC29) transforming sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1518–1522. doi: 10.1073/pnas.78.3.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. Two retroviruses with similar transforming genes exhibit differences in transforming potential. Virology. 1982 Jun;119(2):382–391. doi: 10.1016/0042-6822(82)90097-6. [DOI] [PubMed] [Google Scholar]
- Mellon P., Pawson A., Bister K., Martin G. S., Duesberg P. H. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5874–5878. doi: 10.1073/pnas.75.12.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E. The structure and protein kinase activity of proteins encoded by nonconditional mutants and back mutants in the sec gene of avian sarcoma virus. Virology. 1981 Jan 15;108(1):47–70. doi: 10.1016/0042-6822(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Pachl C., Biegalke B., Linial M. RNA and protein encoded by MH2 virus: evidence for subgenomic expression of v-myc. J Virol. 1983 Jan;45(1):133–139. doi: 10.1128/jvi.45.1.133-139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Enrietto P. J., Graf T., Hayman M. J. Recovery of myc-specific sequences by a partially transformation-defective mutant of avian myelocytomatosis virus, MC29, correlates with the restoration of transforming activity. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6885–6889. doi: 10.1073/pnas.79.22.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Hayman M. J. Isolation and biochemical characterization of partially transformation-defective mutants of avian myelocytomatosis virus strain MC29: localization of the mutation to the myc domain of the 110,000-dalton gag-myc polyprotein. J Virol. 1982 Mar;41(3):745–753. doi: 10.1128/jvi.41.3.745-753.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Wyke J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology. 1981 Jan 15;108(1):28–46. doi: 10.1016/0042-6822(81)90525-0. [DOI] [PubMed] [Google Scholar]




