Abstract
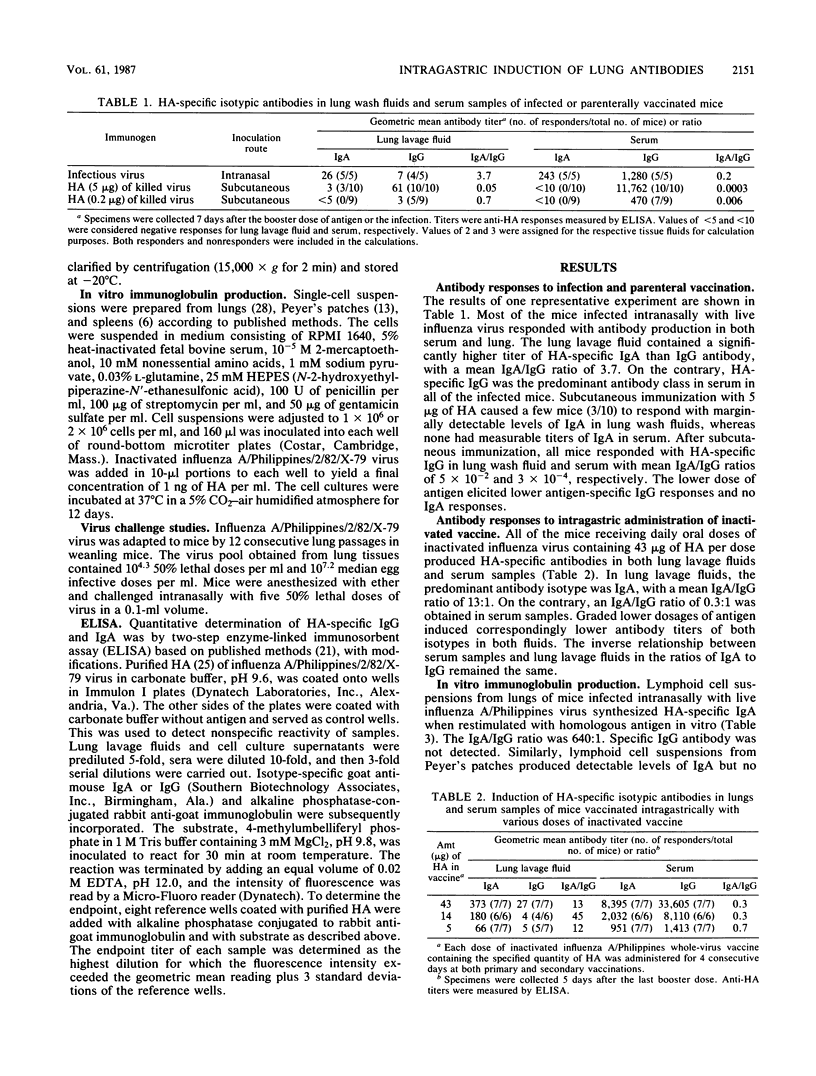
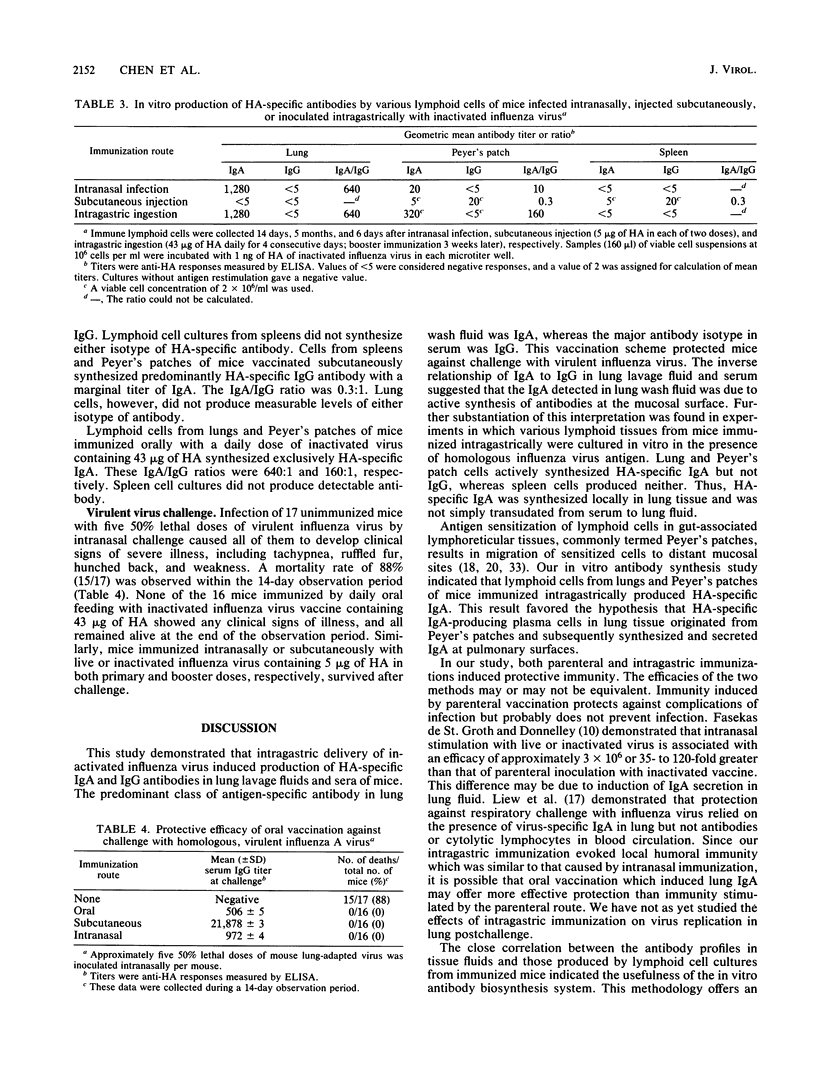
Intragastric inoculation with whole-virion vaccine of inactivated influenza virus resulted in production of hemagglutinin (HA)-specific immunoglobulin A (IgA) and IgG both in lung lavage fluids and in serum samples of mice. HA-specific IgA was the predominant isotypic antibody secreted in the lung lavage fluids (average IgA/IgG ratio, 13:1), whereas HA-specific IgG was the major antibody class in serum (average IgA/IgG ratio, 0.3:1). These responses were similar to the antibody responses stimulated by intranasal infection with live influenza virus. In vitro cultures of lymphoid cells from lungs and Peyer's patches, but not from spleens, in the presence of homologous antigen, from mice vaccinated intragastrically synthesized mostly HA-specific IgA. Mice immunized parenterally with inactivated influenza virus produced only IgG in lung lavage fluids and sera. Cultures of lymphoid cells from their spleens, but not their lungs, synthesized HA-specific IgG upon antigenic stimulation in vitro; neither synthesized IgA. These in vitro cell culture results, as well as the inverse relationship of IgA/IgG ratios in lung lavage fluids and sera, demonstrated that the IgA antibody in lung lavage fluids was actively synthesized locally in the lungs of intragastrically immunized mice. This finding was consistent with the migratory distribution of antigen-primed lymphoid cells from Peyer's patches to distant lymphoid tissue such as lung. Intragastric vaccination conferred protection against intranasal challenge with a lethal dose of virulent virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrova G. I., Smorodintsev A. A., Beljaeva N. M., Vasil'ev B. J., Geft R. A., Panteleev V. G., Sejfer M. A., Selivanov A. A. Testing the safety and effectiveness of oral administration of a live influenza vaccine. Bull World Health Organ. 1970;42(3):429–436. [PMC free article] [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H. Antibodies against influenza and stimulated macrophages in the lung lavage fluid of mice following oral immunization. Allerg Immunol (Leipz) 1983;29(4):215–222. [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H. Occurrence of influenza specific antibodies in the lung after oral immunization in mice. Poumon Coeur. 1982;38(5):289–292. [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H., Tischner H., Pohl W. D. Antibody in tears, saliva and nasal secretions following oral immunization of humans with inactivated influenza virus vaccine. Int Arch Allergy Appl Immunol. 1986;80(1):107–109. doi: 10.1159/000234034. [DOI] [PubMed] [Google Scholar]
- Clements M. L., Betts R. F., Tierney E. L., Murphy B. R. Resistance of adults to challenge with influenza A wild-type virus after receiving live or inactivated virus vaccine. J Clin Microbiol. 1986 Jan;23(1):73–76. doi: 10.1128/jcm.23.1.73-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Murphy B. R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986 Jan;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. C. The sequential appearance of antibody and immunoglobins in nasal secretion after immunization of volunteers with live and inactivated influenza B virus vaccines. J Hyg (Lond) 1973 Sep;71(3):433–445. doi: 10.1017/s0022172400046416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAZEKAS de ST GROTH S., DONNELLEY M. Studies in experimental immunology of influenza. IV. The protective value of active immunization. Aust J Exp Biol Med Sci. 1950 Jan;28(1):61–75. doi: 10.1038/icb.1950.5. [DOI] [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Smith A. J., Miller C. L., Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979 Jan 6;1(8106):33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Fulk R. V., Togo Y., Hornick R. B., Heiner G. G., Dawkins A. T., Jr, Mann J. J. Influenza antibody in human respiratory secretions after subcutaneous or respiratory immunization with inactivated virus. Nature. 1968 May 11;218(5141):594–595. doi: 10.1038/218594a0. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Characteristics and regulatory function of murine con A-induced, cloned T cells obtained from Peyer's patches and spleen: mechanisms regulating isotype-specific immunoglobulin production by Peyer's patch B cells. J Immunol. 1982 Aug;129(2):475–483. [PubMed] [Google Scholar]
- Lauteria S. F., Kantzler G. B., High P. C., Lee J. D., Waldman R. H. An attenuated influenza virus vaccine: Reactogenicity, transmissibility, immunogenicity, and protective efficacy. J Infect Dis. 1974 Oct;130(4):380–383. doi: 10.1093/infdis/130.4.380. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Lazzell V., Waldman R. H., Rose C., Khakoo R., Jacknowitz A., Howard S. Immunization against influenza in humans using an oral enteric-coated killed virus vaccine. J Biol Stand. 1984 Jul;12(3):315–321. doi: 10.1016/s0092-1157(84)80012-8. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Appleyard G., Brand C. M., Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984 Apr;14(4):350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- MEIKLEJOHN G., KEMPE C. H., THALMAN W. G., LENNETTE E. H. Evaluation of monovalent influenza vaccines. II. Observations during an influenza a-prime epidemic. Am J Hyg. 1952 Jan;55(1):12–21. doi: 10.1093/oxfordjournals.aje.a119500. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Arnold R. R., Michalek S. M., Prince S. J., Babb J. L. Induction of secretory antibodies in humans following ingestion of Streptococcus mutans. Adv Exp Med Biol. 1978;107:177–184. doi: 10.1007/978-1-4684-3369-2_21. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Arnold R. R., Mestecky J. Effective immunity to dental caries: selective induction of secretory immunity by oral administration of Streptococcus mutans in rodents. Adv Exp Med Biol. 1978;107:261–269. doi: 10.1007/978-1-4684-3369-2_31. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Rennels M. B., Douglas R. G., Jr, Betts R. F., Couch R. B., Cate T. R., Jr, Chanock R. M., Kendal A. P., Maassab H. F., Suwanagool S. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect Immun. 1980 Aug;29(2):348–355. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Perkins J. C., Tucker D. N., Knopf H. L., Wenzel R. P., Kapikian A. Z., Chanock R. M. Comparison of protective effect of neutralizing antibody in serum and nasal secretions in experimental rhinovirus type 13 illness. Am J Epidemiol. 1969 Dec;90(6):519–526. doi: 10.1093/oxfordjournals.aje.a121098. [DOI] [PubMed] [Google Scholar]
- Phelan M. A., Mayner R. E., Bucher D. J., Ennis F. A. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand. 1980;8(3):233–242. doi: 10.1016/s0092-1157(80)80039-4. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Cogliano R. C., Shands J. W., Jr, Small P. A., Jr Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect Immun. 1979 Sep;25(3):992–997. doi: 10.1128/iai.25.3.992-997.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. H., Sydiskis R. J. Responses of mice immunized with influenza virus by serosol and parenteral routes. Infect Immun. 1976 Mar;13(3):696–703. doi: 10.1128/iai.13.3.696-703.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. H., Walker J. S. Immunoglobulin-bearing cells in lungs of mice infected with influenza virus. Infect Immun. 1976 May;13(5):1525–1527. doi: 10.1128/iai.13.5.1525-1527.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. M., Dudding B. A., Romano S. V., Russell P. K. Enteric immunization with live adenovirus type 21 vaccine. II. Systemic and local immune responses following immunization. Infect Immun. 1972 Mar;5(3):300–304. doi: 10.1128/iai.5.3.300-304.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W. Vaccination in the control of influenza. Interim report to the Director of the Public Health Laboratory Service on a Collaborative Study with the Post Office. Lancet. 1974 Aug 10;2(7876):330–333. doi: 10.1016/s0140-6736(74)91704-8. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Clements M. L., Betts R. F., Dolin R., Buckler-White A. J., Tierney E. L., Murphy B. R. Evaluation of live avian-human reassortant influenza A H3N2 and H1N1 virus vaccines in seronegative adult volunteers. J Clin Microbiol. 1986 May;23(5):852–857. doi: 10.1128/jcm.23.5.852-857.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz-Carrington P., Roux M. E., McWilliams M., PHILLIPS-Quagliata J. M., Lamm M. E. Organ and isotype distribution of plasma cells producing specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979 Oct;123(4):1705–1708. [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Murphy B. R., Kervina M., Lawrence E. M., Phelan M. A., Karzon D. T. Secretory immunological response after intranasal inactivated influenza A virus vaccinations: evidence for immunoglobulin A memory. Infect Immun. 1983 Jun;40(3):1092–1095. doi: 10.1128/iai.40.3.1092-1095.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



