Abstract
In eukaryotes, diploid cells give rise to haploid cells via meiosis, a program of two cell divisions preceded by one round of DNA replication. Although key molecular components of the meiotic apparatus are highly conserved among eukaryotes, the mechanisms responsible for initiating the meiotic program have diverged substantially among eukaryotes. This raises a related question in animals with two distinct sexes: Within a given species, are similar or different mechanisms of meiotic initiation used in the male and female germ lines? In mammals, this question is underscored by dramatic differences in the timing of meiotic initiation in males and females. Stra8 is a vertebrate-specific, cytoplasmic factor expressed by germ cells in response to retinoic acid. We previously demonstrated that Stra8 gene function is required for meiotic initiation in mouse embryonic ovaries. Here we report that, on an inbred C57BL/6 genetic background, the same factor is also required for meiotic initiation in germ cells of juvenile mouse testes. In juvenile C57BL/6 males lacking Stra8 gene function, the early mitotic development of germ cells appears to be undisturbed. However, these cells then fail to undergo the morphological changes that define meiotic prophase, and they do not display the molecular hallmarks of meiotic chromosome cohesion, synapsis and recombination. We conclude that, in mice, Stra8 regulates meiotic initiation in both spermatogenesis and oogenesis. Taken together with previous observations, our present findings indicate that, in both the male and female germ lines, meiosis is initiated through retinoic acid induction of Stra8.
Keywords: meiosis, spermatocyte
In mammals and other animals, both male and female germ cells undergo meiosis, a program of two cell divisions preceded by one round of DNA replication, resulting in halving of the chromosome number. Many structural and enzymatic components of the mammalian meiotic apparatus are shared between males and females, and several of these components are conserved across the breadth of the eukaryotic world (1, 2). The mechanical elements of meiosis are remarkably conserved between the sexes and among species.
The same cannot be said for the regulatory pathways that govern the transition of cells from mitosis to meiosis. These pathways have been studied intensively in the budding yeast Saccharomyces cerevisiae and in the fission yeast Schizosaccharomyces pombe (3). In these two species, the molecular mechanisms regulating meiotic initiation appear to share no components, and these initiation pathways do not appear to be conserved in multicellular organisms.
In mammals, moreover, the timing and regulation of meiosis differ dramatically between the sexes (4, 5). For example, all meioses in mammalian females initiate during a brief window in embryonic development, whereas meiotic initiation in males is first observed at puberty and then recurs repeatedly and continuously throughout adulthood. Do these sexual differences in timing reflect distinct mechanisms of meiotic initiation in females and males?
An opportunity to explore this question experimentally arises from recent studies of Stra8 (Stimulated by retinoic acid gene 8), a vertebrate-specific gene that encodes a cytoplasmic protein and whose expression is induced by retinoic acid (6–11). Our laboratory previously reported that, in female mice, Stra8 is expressed in embryonic ovarian germ cells shortly before they enter meiotic prophase (12). We further demonstrated that, in female embryonic germ cells, initiation of the meiotic program depends on Stra8 function. Stra8 plays no role in the mitotic phases of embryonic germ-cell development, but in females it is required for premeiotic DNA replication and the subsequent events of meiotic prophase, including chromosome condensation, cohesion, synapsis, and recombination (13). These findings established that Stra8 is a regulator of meiotic initiation in females.
In the present study, we address whether Stra8 serves an analogous function in males. In male mice, Stra8 is expressed postnatally, in the mitotically active cells of the spermatogenic lineage (spermatogonia) and their immediate descendants (preleptotene spermatocytes), the most advanced cell type before meiotic prophase (6, 10, 14). We previously reported that male mice lacking Stra8 function produced no sperm; most spermatogenic cells underwent apoptosis at a developmental stage when they normally would have progressed through meiotic prophase (13). While attempting to refine these preliminary observations, we detected variability in the spermatogenic phenotypes of Stra8-deficient males. Suspecting that this confounding phenotypic variability might derive from genetic (strain background) variation unlinked to the Stra8 locus, we extensively backcrossed the Stra8 mutation onto an inbred strain. Having neutralized unlinked genetic modifiers, we then proceeded to explore experimentally whether Stra8 is required for meiotic initiation in male mice.
Results
Backcrossing to C57BL/6 in Response to Inconsistent Spermatogenic Defects in Stra8-Deficient Males of Mixed Genetic Background.
Our laboratory previously generated the Stra8 mutant allele (13) by homologous recombination in v6.5 embryonic stem (ES) cells, which had been derived from an F1 hybrid (C57BL/6 × 129) embryo (15). Thus, our earlier studies of Stra8 function were conducted on animals of mixed rather than inbred genetic backgrounds (13). All mutant animals were infertile, with no evidence of mature sperm. Nonetheless, as we continued to characterize Stra8-deficient testes by using histological and immunocytochemical methods, we observed that meiotic progression and spermatocyte survival varied considerably between and within animals of the same Stra8 genotype. Although these phenotypes are potentially of interest, the mixed genetic backgrounds on which they had been observed would be difficult to reproduce. Accordingly, we decided to forego further characterization of the Stra8-deficient testicular phenotype until we had backcrossed the mutant allele onto an inbred strain background, in this case C57BL/6.
All experiments reported here were conducted on mice backcrossed for at least 15 generations, when >99.9% of the genome is expected to be of C57BL/6 origin. As previously reported for Stra8-deficient mice on a mixed genetic background (13), Stra8-deficient C57BL/6 males and females were infertile, and we detected no phenotypic abnormalities apart from the gonads in either sex. We observed no gonadal or extragonadal abnormalities in Stra8-heterozygous C57BL/6 animals of either sex, again consistent with our observations on a mixed genetic background (13).
Absence of Leptotene, Zygotene, or Pachytene Cells in Testes of Stra8-Deficient C57BL/6 Males.
Spermatogenesis in mice is a lengthy (>35 days), multifaceted process by which diploid spermatogonial stem cells give rise to haploid spermatozoa. Spermatogenesis involves two meiotic divisions, which modify the nucleus and its chromosomes, and also an elaborate program of cellular differentiation. This differentiation program includes dramatic changes in cellular morphology and function, including postmeiotic acquisition of the ability to swim. Many distinct steps in cellular differentiation, and in meiotic progression, have been defined through microscopic studies of spermatogenesis in mice (14). In the testis of an adult mouse, one finds spermatogenic cells at all stages of differentiation and meiotic progression. To study meiotic initiation in a simpler and more nearly synchronous setting, we focused instead on the testes of juvenile males at 10–21 days after birth, when the first and second cohorts of spermatogenic cells normally enter prophase of the first meiotic division. This allowed us to assay male meiotic initiation in the absence of later meiotic and postmeiotic cells.
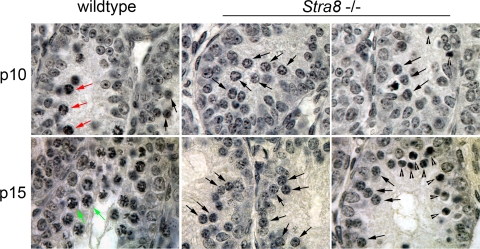
We began by comparing the histologies of wild-type and Stra8-deficient testes at 10 and 15 days after birth. We paid particular attention to the spermatogenic cells' chromatin, whose changing microscopic appearance serves to define the stages of meiotic prophase. As expected, by p10 (10 days of age) in wild-type C57BL/6 males, pioneer cohorts of spermatogenic cells have initiated meiosis and progressed to leptotene, the first stage of meiotic prophase (see red arrows in Fig. 1). By p15 in wild-type C57BL/6 males, the most advanced cohorts have transited zygotene and progressed to pachytene of meiotic prophase (see green arrows in Fig. 1). By contrast, in Stra8-deficient C57BL/6 testes, we found no leptotene, zygotene, or pachytene spermatocytes at either p10 or p15 despite extensive searching. Instead, the most advanced spermatogenic cells that we observed in Stra8-deficient animals were at the preleptotene stage—the last microscopically defined stage before meiotic prophase (see black arrows in Fig. 1). As expected, preleptotene cells are also present in wild-type testes (Fig. 1). These findings suggested that, in C57BL/6 males, Stra8 function is required for spermatogenic cells to transition from preleptotene to leptotene, and thus to enter meiotic prophase. We also note that apoptotic cells were observed in some Stra8-deficient testicular tubules but were rarely seen in wild-type testes (Fig. 1). In summary, histological examination suggested that germ-cell development in juvenile Stra8-deficient C57BL/6 males proceeded normally to the preleptotene stage, but stalled there without progressing into meiotic prophase.
Fig. 1.
Photomicrographs of hematoxylin-stained sections from wild-type and Stra8-deficient testes at p10 or p15 (10 or 15 days after birth, respectively). Black arrows indicate representative preleptotene cells; red arrows, leptotene spermatocytes; green arrows, pachytene spermatocytes; arrowheads, apoptotic cells.
To examine this working hypothesis, we then tested juvenile Stra8-deficient testes for key molecular hallmarks of meiotic prophase.
Absence of Meiotic Recombination in Testes of Stra8-Deficient C57BL/6 Males.
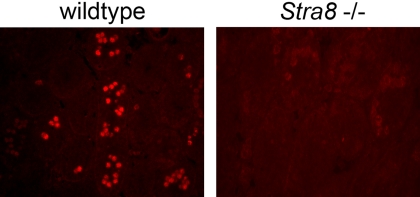
Recombination between homologous chromosomes occurs during prophase of the first meiotic division. If Stra8 is required for preleptotene cells to progress into meiotic prophase, as indicated by our histological studies (Fig. 1), then spermatogenic cells in Stra8-deficient testes should not engage in meiotic recombination. We first tested this prediction by assaying whether Stra8-deficient testicular germ cells form DNA double-strand breaks (DSBs), which initiate meiotic recombination. When DNA DSBs are formed, cells respond by phosphorylating H2AX, an isoform of histone H2A, to generate γ-H2AX (16). We tested for γ-H2AX by immunostaining sections from p10 wild-type and Stra8-deficient testes. As expected, γ-H2AX staining demonstrated the presence of DNA DSBs in many cells of wild-type testes (Fig. 2). By contrast, γ-H2AX staining is absent in Stra8-deficient testes, indicating that DNA DSBs have not formed (Fig. 2).
Fig. 2.
Immunohistochemical staining for γ-H2AX protein in sections of wild-type and Stra8-deficient testes 10 days after birth.
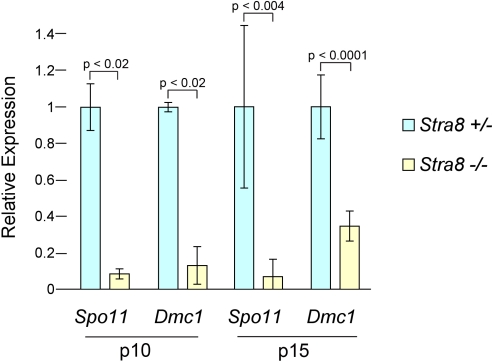
To further examine their capacity for meiotic recombination, we assayed whether spermatogenic cells in Stra8-deficient testes express Spo11, encoding a topoisomerase required to form meiotic DSBs (17, 18), and Dmc1, which encodes a recombinase functioning in meiotic DSB repair (19, 20). We measured mRNA expression of Spo11 and Dmc1 in control and Stra8-deficient testes at p10 and p15 by using quantitative RT-PCR (Fig. 3). We found that mRNA expression of both genes was dramatically reduced in Stra8-deficient testes at both time points. Taken together, our γ-H2AX, Spo11, and Dmc1 findings provide strong evidence that spermatogenic cells in Stra8-deficient testes do not form or repair meiotic DSBs, and thus do not undertake meiotic recombination.
Fig. 3.
Quantitative RT-PCR analysis of Spo11 and Dmc1 mRNA levels in Stra8-heterozygous and Stra8-deficient testes, 10 or 15 days after birth. Plotted here are average fold changes, normalized to Hprt, in independent biological replicates (two replicates at p10, and four at p15). Error bars represent standard deviations among biological replicates; P values are from the Smith–Satterthwaite test, one-tailed.
Absence of Meiotic Cohesion and Synapsis in Testes of Stra8-Deficient C57BL/6 Males.
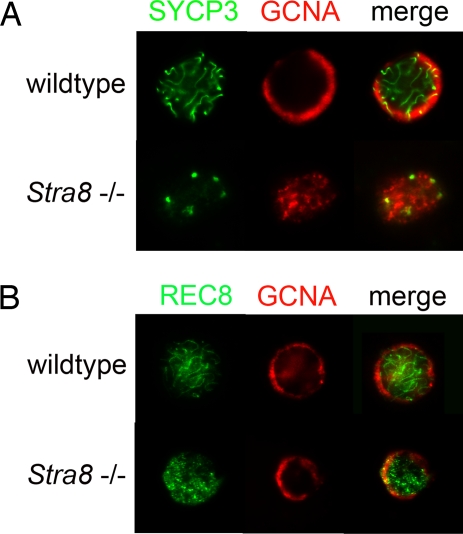
Like meiotic recombination, meiotic chromosome cohesion and synapsis are molecularly defined processes that underpin proper chromosome segregation, and both are hallmark features of meiotic prophase. If Stra8 is required for preleptotene cells to enter meiotic prophase, then chromosomal cohesion and synapsis should not occur in spermatogenic cells of Stra8-deficient testes. To test this prediction, we immunostained cell spreads from wild-type and Stra8-deficient testes at p15 by using antibodies against either REC8, a meiosis-specific cohesin (21–27), or SYCP3, a synaptonemal complex protein (28). We simultaneously immunostained for GCNA, a germ-cell specific marker (29), to distinguish between spermatogenic and somatic cells in these populations of dispersed cells. As expected, SYCP3 and REC8 decorated the lengths of the chromosomes in most wild-type spermatogenic cells at p15, demonstrating the presence, respectively, of synaptonemal and meiotic cohesin complexes (Fig. 4). By contrast, in Stra8-deficient spermatogenic cells, the SYCP3 and REC8 proteins, although present, did not appear to be loaded onto chromosomes (Fig. 4), but instead were localized in patterns reminiscent of those previously reported in premeiotic germ cells, including germ cells of Stra8-deficient embryonic ovaries (13, 30). We concluded that, in spermatogenic cells of juvenile C57BL/6 males, Stra8 function is required for meiotic cohesion and synapsis to occur.
Fig. 4.
Immunohistochemical staining for SYCP3 protein (A) or REC8 protein (B) in germ cells from wild-type and Stra8-deficient p15 testes. Costaining for GCNA confirms the germ-cell identity of these nuclei.
Abundant DNA Replication in Preleptotene Cells of Stra8-Deficient C57BL/6 Males.
Taken together, our results lead us to conclude that in germ cells of juvenile C57BL/6 males, Stra8 is required for both the histological and molecular manifestations of meiotic prophase, including chromosomal cohesion, synaptonemal complex formation, and recombination. Thus, in the absence of Stra8 function, spermatogenic cells in juvenile C57BL/6 males progress to the preleptotene stage but do not enter meiotic prophase. In wild-type testes, it is thought that preleptotene cells replicate their DNA immediately before they advance into meiotic prophase (14). The question then arises whether Stra8-deficient preleptotene cells also replicate their DNA.
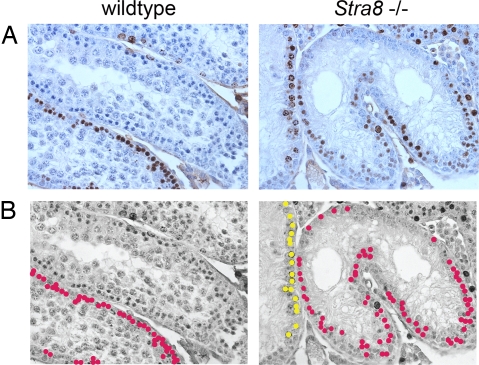
We explored this question at p21, when, in wild-type testes, a second round of preleptotene cells form and replicate their DNA. At that time, the neighboring somatic (Sertoli) cells have stopped dividing, effectively eliminating the noise that these somatic cells might otherwise contribute to an analysis of germ-cell replication (31). We injected bromodeoxyuridine (BrdU) into wild-type and Stra8-deficient male mice and two hours later harvested the testes. We immunostained testicular sections to detect incorporation of BrdU into newly replicated DNA in preleptotene and other spermatogenic cell types. Within these sections, we identified preleptotene and other spermatogenic cells by their location, nuclear size, and chromatin pattern (14). In both wild-type and Stra8-deficient testes, we found many BrdU-positive preleptotene cells, indicating that DNA replication had occurred (Fig. 5). These results demonstrate that Stra8 is not required for preleptotene cells to replicate their DNA.
Fig. 5.
Analysis of BrdU incorporation in sections of wild-type and Stra8-deficient testes 21 days after birth. (A) Immunohistochemical staining for BrdU counterstained with hematoxylin. (B) Interpretation of images in A. On these grayscale versions of the images in A, magenta dots indicate BrdU-positive preleptotene cells, and yellow dots indicate BrdU-positive type B spermatogonia.
Discussion
Here we have demonstrated that, on a highly inbred C57BL/6 genetic background, Stra8 is required for male meiotic initiation—for preleptotene cells in juvenile mouse testes to transition into meiotic prophase. Specifically, we have found that Stra8 is required for spermatogenic cells to undergo the morphological changes that define meiotic prophase, and for these cells to exhibit the molecular hallmarks of meiotic chromosome cohesion, synapsis, and recombination. However, Stra8 is not required for preleptotene cells of juvenile testes to undergo DNA replication. We will now discuss the implications of these findings for our understanding of meiotic initiation in mice and other multicellular organisms.
Although many genes in addition to Stra8 have been shown to be required for meiosis in male mice, these genes function during or after meiotic prophase and are not needed for preleptotene cells to transition into meiotic prophase. For example, spermatogenic cells deficient in either Rec8, Smc1b, Sycp3, Spo11, or Dmc1 readily progress into meiotic prophase (17–28, 32). To our knowledge, Stra8 displays the earliest meiotic phenotype among characterized mutants affecting the male mouse germ line. Remarkably, all of these statements also hold true for Stra8's function in the female germ line (13). Thus, Stra8 appears to function upstream of all other known meiotic mutants in both spermatogenesis and oogenesis, yet it is not required in embryos of either sex, or in juvenile males, for the early mitotic development of the germ line.
Our present findings, together with previous work from our and other laboratories, lead us to hypothesize a common molecular pathway for meiotic initiation in the mammalian male and female germ lines in vivo. Specifically, we postulate that, in both sexes, retinoic acid (RA) produced in somatic cells acts directly on germ cells to induce expression of Stra8, which in turn is required for initiation of the meiotic program. There is now much data in vivo and in vitro, in one or both sexes, to support this model. First, the model rests on the evidence, presented here and in our previous study (13), that Stra8 function is required, in vivo, for meiotic initiation in the male and female germ lines. Second, there is a wealth of evidence that in vivo expression of Stra8 is germ-line-specific in both sexes, and that Stra8 expression in germ cells of embryonic ovaries and juvenile and adult testes is induced by, and requires, RA signaling (6–8, 10–12). Finally, in vitro studies of isolated spermatogenic cells suggest that RA acts directly on germ cells to induce Stra8 expression (9). Combined with previous reports, our present findings suggest that the meiotic initiation pathway in which Stra8 and its inducer, RA, figure so prominently is shared between the male and female germ lines in vivo. This male–female commonality in the regulation of meiotic initiation provides a counterpoint to profound sexual dimorphisms in regulatory checkpoints during meiotic prophase (4, 5).
This model and our findings also raise fundamental questions for future study. First, it remains to be determined whether mammalian regulators of meiotic initiation, aside from Stra8 and RA, are sex-specific or are shared between the sexes. Second, apart from mammals, it will be of great interest to learn whether spermatogenesis and oogenesis within a given species employ common regulators of meiotic initiation. This is the case in the nematode Caenorhabditis elegans, where gld-1 and gld-2 appear to play similar roles in regulating meiotic initiation in spermatogenesis and oogenesis (33, 34). Finally, it is intriguing that Stra8-deficient preleptotene cells replicate their DNA but fail to enter meiotic prophase. One possible explanation is that these cells, despite having taken on the morphological appearance of preleptotene spermatocytes, retain the proliferative character of spermatogonia. This would account for the apparent failure of REC8 loading in spermatogenic cells of Stra8-deficient testes (Fig. 4), as this loading is a hallmark of premeiotic but not mitotic DNA replication (23). By this model, Stra8 regulates meiotic initiation upstream of premeiotic DNA replication in both sexes (13). This model also aligns with evidence from C. elegans, where failure to initiate meiosis may result in continued mitotic proliferation of germ cells (33, 34). Experiments to explore these questions can now be envisioned.
Materials and Methods
Mice.
Stra8 heterozygous mice (13) were crossed to C57BL/6NtacfBR mice (Taconic Farms). All experiments were carried out on mice backcrossed to C57BL/6NtacfBR between 15 and 17 generations, when >99.9% of the genome is expected to be of C57BL/6NtacfBR origin; all Y chromosomes and mitochondria are of C57BL/6NtacfBR origin. Stra8-deficient males were generated by mating heterozygotes. Stra8 genotypes were assayed by PCR as described (13). All experiments involving mice were approved by the Committee on Animal Care at the Massachusetts Institute of Technology.
Histology.
Testes were fixed overnight in Bouin's solution, embedded in paraffin, sectioned, and stained with hematoxylin.
γ-H2AX Immunohistochemistry.
Testes were fixed overnight in Bouin's solution, embedded in paraffin and sectioned. Slides were dewaxed, rehydrated, and microwaved in 10 mM sodium citrate buffer, pH 6.0, for 10 min. Slides were then treated with 2% donkey serum for 30 min and washed with PBS. Slides were then incubated for 60 min in a 1:100 dilution of rabbit polyclonal anti-γ-H2AX (Upstate Biotech). Slides were washed with PBS and incubated for 60 min at room temperature with donkey anti-rabbit secondary antibody, conjugated with Texas Red (Jackson ImmunoResearch Laboratories), at 1:200 dilution.
Quantitative RT-PCR.
Testes were stripped of the tunica albuginea, placed in TRIzol (Invitrogen), and stored at −20°C. Total RNAs were prepared according to the manufacturer's protocol. Total RNAs were then DNase-treated by using DNA Free Turbo (Ambion). One microgram of total RNA was reverse transcribed by using a RETROscript kit (Ambion). Quantitative PCR was performed by using SYBR Green Core PCR Reagents (Applied Biosystems) on an ABI9700 Fast Real-time PCR machine (Applied Biosystems). Results were analyzed by using the delta-delta Ct method with use of Hprt (hypoxanthine-guanine phosphoribosyltransferase) as a normalization control.
RT-PCR primer sequences were as follows:
Spo11:
5′ CGTGGCCTCTAGTTCTGAGGT 3′ and 5′ GCTCGATCTGTTGTCTATTGTGA 3′
Dmc1:
5′ CCCTCTGTGTGACAGCTCAAC 3′ and 5′ GGTCAGCAATGTCCCGAAG 3′
Hprt:
5′ TCAGTCAACGGGGGACATAAA 3′ and 5′ GGGGCTGTACTGCTTAACCAG 3′
SYCP3 and REC8 Immunocytochemistry.
Testes were dissected from p15 male mice. To obtain single cells, tubules were teased apart with forceps, minced, and pipetted repeatedly in PBS. Cells were pelletted and resuspended once in PBS and twice in hypotonic solution (0.5% sodium chloride in H2O). Cell suspensions were then placed on poly-l-lysine-coated slides and kept in a humid chamber at room temperature (22°C) for 60 min. The slides were then fixed in 2% paraformaldehyde and 0.03% SDS for 15 min at 4°C, washed three times in 0.4% Photoflo (Kodak) for 1 min and air dried. These slides were stored at −80°C before use.
Before fluorescence immunostaining, slides were brought to room temperature, washed twice in PBS, and treated with blocking buffer (10% donkey serum, 10% goat serum, 0.05% Triton X-100 in PBS). Slides were then incubated with anti-GCNA IgM (courtesy of G. Enders, University of Kansas, Kansas City, KS; undiluted supernatant) and a 1:1000 dilution of rabbit anti-SCP3 IgG or anti-REC8 IgG (courtesy of C. Heyting, Agricultural University, Wageningen, The Netherlands) overnight at 4°C. Slides were then washed with PBS and incubated for 60 min at room temperature with donkey anti-rabbit secondary antibody, conjugated with either Texas Red or fluorescein isothiocyanate (Jackson ImmunoResearch Laboratories), at 1:200 dilution.
5-Bromo-2-deoxyuridine (BrdU) Incorporation.
Twenty-one-day-old male mice were injected i.p. with 10 μl/g body weight of 20 g/liter BrdU in PBS. The mice were euthanized 2 h later. Testes were fixed overnight in Bouin's solution, embedded in paraffin, and sectioned. Slides were dewaxed, rehydrated, and pretreated with 1% periodic acid at 60°C for 30 min. Slides were then incubated in 5% BSA, followed by a 30-min incubation with mouse anti-BrdU sera (BD Bioscience) at a dilution of 1:80. Slides were then washed with PBS and incubated with anti-mouse secondary antibody conjugated with horseradish peroxidase (ImmunoVision Technologies). Peroxidase activity was visualized by using 3,3-diaminobenzidine-tetrahydrochloride (Sigma) as substrate. Sections were counterstained with hematoxylin.
Acknowledgments.
We thank G. Enders for GCNA antisera; C. Heyting for REC8 and SYCP3 antisera; H. Skaletsky for statistical advice; and M. Carmell, G. Dokshin, M. Gill, M. Griswold, Y.-C. Hu, J. Koubova, D. Menke, and J. Mueller for critical reading of the manuscript. This work was supported by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Richardson C, Horikoshi N, Pandita TK. The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst) 2004;3:1149–1164. doi: 10.1016/j.dnarep.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol. 2001;52:1–53. doi: 10.1016/s0070-2153(01)52008-6. [DOI] [PubMed] [Google Scholar]
- 3.Marston AL, Amon A. Meiosis: Cell-cycle controls shuffle and deal. Nat Rev Mol Cell Biol. 2004;5:983–997. doi: 10.1038/nrm1526. [DOI] [PubMed] [Google Scholar]
- 4.Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- 5.Morelli MA, Cohen PE. Not all germ cells are created equal: Aspects of sexual dimorphism in mammalian meiosis. Reproduction. 2005;130:761–781. doi: 10.1530/rep.1.00865. [DOI] [PubMed] [Google Scholar]
- 6.Oulad-Abdelghani M, et al. Characterization of a premeiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koubova J, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, et al. Expression of stimulated by retinoic Acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: An in vivo study in vitamin a-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghyselinck NB, et al. Retinoids and spermatogenesis: Lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- 12.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 13.Baltus AE, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 14.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 15.Rideout WM, III, et al. Generation of mice from wild-type and targeted ES cells by nuclear cloning. Nat Genet. 2000;24:109–110. doi: 10.1038/72753. [DOI] [PubMed] [Google Scholar]
- 16.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 17.Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 18.Romanienko PJ, Camerini-Otero RD. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol Cell. 2000;6:975–987. doi: 10.1016/s1097-2765(00)00097-6. [DOI] [PubMed] [Google Scholar]
- 19.Pittman DL, et al. Meiotic prophase arrest with failure of chromosome synapsis in mice deficient for Dmc1, a germline-specific RecA homolog. Mol Cell. 1998;1:697–705. doi: 10.1016/s1097-2765(00)80069-6. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida K, et al. The mouse RecA-like gene Dmc1 is required for homologous chromosome synapsis during meiosis. Mol Cell. 1998;1:707–718. doi: 10.1016/s1097-2765(00)80070-2. [DOI] [PubMed] [Google Scholar]
- 21.Klein F, et al. A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell. 1999;98:91–103. doi: 10.1016/S0092-8674(00)80609-1. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe Y, Yokobayashi S, Yamamoto M, Nurse P. Pre-meiotic S phase is linked to reductional chromosome segregation and recombination. Nature. 2001;409:359–363. doi: 10.1038/35053103. [DOI] [PubMed] [Google Scholar]
- 24.Eijpe M, Offenberg H, Jessberger R, Revenkova E, Heyting C. Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3. J Cell Biol. 2003;160:657–670. doi: 10.1083/jcb.200212080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Iwai T, Yokota T, Yamashita M. Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci. 2003;116:2781–2790. doi: 10.1242/jcs.00495. [DOI] [PubMed] [Google Scholar]
- 26.Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis. 2004;40:184–194. doi: 10.1002/gene.20085. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell. 2005;8:949–961. doi: 10.1016/j.devcel.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Yuan L, et al. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol Cell. 2000;5:73–83. doi: 10.1016/s1097-2765(00)80404-9. [DOI] [PubMed] [Google Scholar]
- 29.Enders GC, May JJ., II Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- 30.Prieto I, et al. Cohesin component dynamics during meiotic prophase I in mammalian oocytes. Chromosome Res. 2004;12:197–213. doi: 10.1023/b:chro.0000021945.83198.0e. [DOI] [PubMed] [Google Scholar]
- 31.Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. doi: 10.1530/jrf.0.0930233. [DOI] [PubMed] [Google Scholar]
- 32.Revenkova E, et al. Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol. 2004;6:555–562. doi: 10.1038/ncb1135. [DOI] [PubMed] [Google Scholar]
- 33.Hansen D, Schedl T. The regulatory network controlling the proliferation-meiotic entry decision in the Caenorhabditis elegans germ line. Curr Top Dev Biol. 2006;76:185–215. doi: 10.1016/S0070-2153(06)76006-9. [DOI] [PubMed] [Google Scholar]
- 34.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]