Abstract
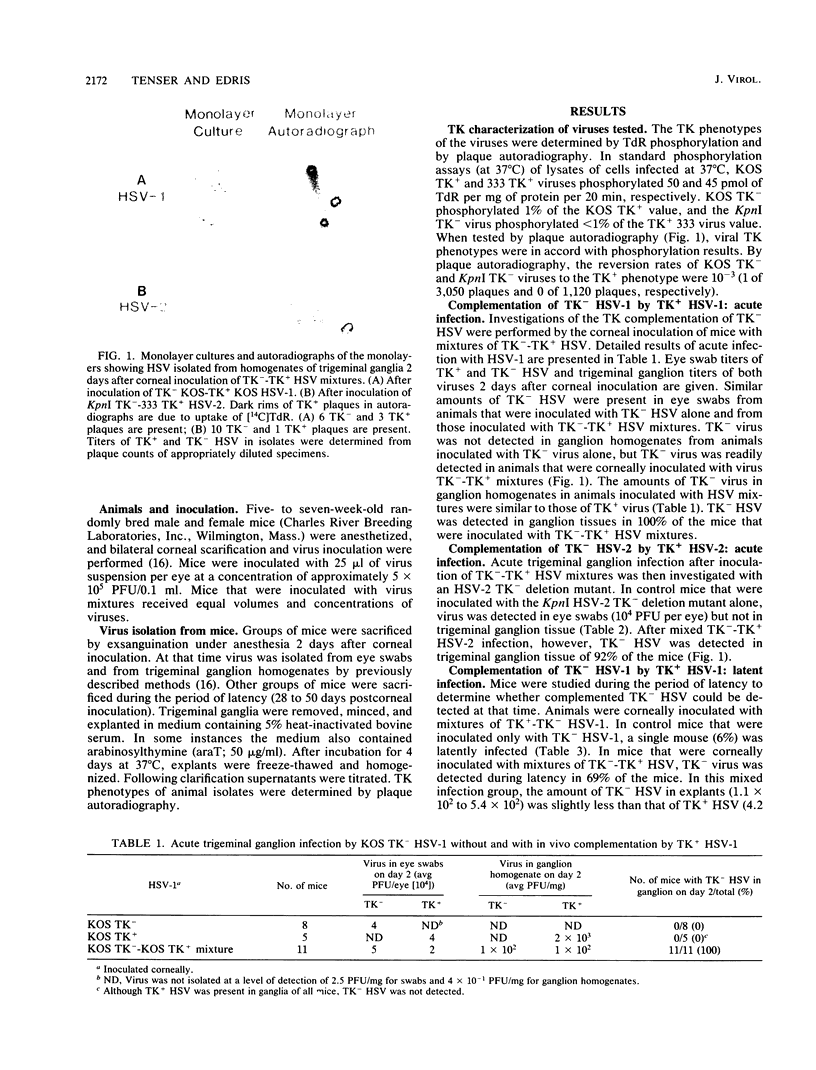
Infection of trigeminal ganglion by herpes simplex virus (HSV) thymidine kinase-negative (TK-) mutants was investigated in mixed infection studies in mice. Mice were corneally inoculated with TK- HSV alone or with mixtures of TK- HSV-TK+ HSV. When inoculated alone, an arabinosylthymine-selected HSV type 1 TK- mutant and a HSV type 2 TK- deletion mutant infected mouse ocular tissues but rarely infected ganglion tissues. However, both TK- mutants readily infected ganglion tissues when they were inoculated in mixtures with TK+ HSV. By means of mixed infection studies, it was demonstrated that TK- HSV could readily establish acute and latent ganglion infections. It was thought that the frequent infection of trigeminal ganglion tissue by both TK- mutants after mixed TK(-)-TK+ HSV infection was the result of in vivo complementation. After mixed TK(-)-TK+ HSV infection and subsequent cultivation of ganglion explants in arabinosylthymine, results supported the conclusion that when TK- was present in ganglia it was in the same neurons that contained TK+ HSV.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Gerdes J. C., Smith D. S. Recurrence phenotypes and establishment of latency following rabbit keratitis produced by multiple herpes simplex virus strains. J Gen Virol. 1983 Nov;64(Pt 11):2441–2454. doi: 10.1099/0022-1317-64-11-2441. [DOI] [PubMed] [Google Scholar]
- Gordon Y., Gilden D. H., Shtram Y., Asher Y., Tabor E., Wellish M., Devlin M., Snipper D., Hadar J., Becker Y. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76(1):39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Klein R. J., DeStefano E., Brady E., Friedman-Kien A. E. Experimental skin infection with an acyclovir resistant herpes simplex virus mutant: response to antiviral treatment and protection against reinfection. Arch Virol. 1980;65(3-4):237–246. doi: 10.1007/BF01314540. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Cheng Y. C., Darby G. Characterization of abnormal thymidine kinases induced by drug-resistant strains of herpes simplex virus type 1. J Gen Virol. 1983 Mar;64(Pt 3):523–532. doi: 10.1099/0022-1317-64-3-523. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Leung W. C., Jeffrey V. M., Warren K. G. Detection of multiple strains of latent herpes simplex virus type 1 within individual human hosts. J Virol. 1984 Oct;52(1):300–305. doi: 10.1128/jvi.52.1.300-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Smiley J. R., Leslie P., Brais J., Rudzroga H. E., Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984 Sep;51(3):747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignier B., Norrild B., Roizman B. Colonization of murine ganglia by a superinfecting strain of herpes simplex virus. Infect Immun. 1983 Aug;41(2):702–708. doi: 10.1128/iai.41.2.702-708.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Khan A. Resistance of peripheral autonomic neurons to in vivo productive infection by herpes simplex virus mutants deficient in thymidine kinase activity. Infect Immun. 1981 Nov;34(2):571–580. doi: 10.1128/iai.34.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., Meignier B., Roizman B. Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol. 1985 Aug;55(2):410–416. doi: 10.1128/jvi.55.2.410-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L. R., Kit S., Myers M. G. Thymidine kinase-deficient herpes simplex virus type 2 genital infection in guinea pigs. J Virol. 1985 Aug;55(2):322–328. doi: 10.1128/jvi.55.2.322-328.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Edris W. A. Thymidine kinase (TK) activity in herpes simplex virus type 1 recombinants that carry insertions affecting regulation of the TK gene. Virology. 1986 Nov;155(1):257–261. doi: 10.1016/0042-6822(86)90185-6. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Jones J. C., Ressel S. J. Acute and latent infection by thymidine kinase mutants of herpes simplex virus type 2. J Infect Dis. 1985 Mar;151(3):548–550. doi: 10.1093/infdis/151.3.548. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Jones J. C., Ressel S. J., Fralish F. A. Thymidine plaque autoradiography of thymidine kinase-positive and thymidine kinase-negative herpesviruses. J Clin Microbiol. 1983 Jan;17(1):122–127. doi: 10.1128/jcm.17.1.122-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S., Dunstan M. E. Herpes simplex virus thymidine kinase expression in trigeminal ganglion infection: correlation of enzyme activity with ganglion virus titer and evidence of in vivo complementation. Virology. 1981 Jul 15;112(1):328–341. doi: 10.1016/0042-6822(81)90638-3. [DOI] [PubMed] [Google Scholar]
- Thomas E., Lycke E., Vahlne A. Retrieval of latent herpes simplex virus type 1 genetic information from murine trigeminal ganglia by superinfection with heterotypic virus in vivo. J Gen Virol. 1985 Aug;66(Pt 8):1763–1770. doi: 10.1099/0022-1317-66-8-1763. [DOI] [PubMed] [Google Scholar]