Abstract
Background. Obstructive nephropathy decreases renal blood flow (RBF) and glomerular filtration rate (GFR), causing tubular abnormalities, such as urinary concentrating defect, as well as increasing oxidative stress. This study aimed to evaluate the effects of N-acetylcysteine (NAC) on renal function, as well as on the protein expression of aquaporin 2 (AQP2) and endothelial nitric oxide synthase (eNOS), after the relief of bilateral ureteral obstruction (BUO).
Methods. Adult male Wistar rats were divided into four groups: sham (sham operated); sham operated + 440 mg/kg body weight (BW) of NAC daily in drinking water, started 2 days before and maintained until 48 h after the surgery; BUO (24-h BUO only); BUO + NAC-pre (24-h BUO plus 440 mg/kg BW of NAC daily in drinking water started 2 days before BUO); and BUO + NAC-post (24-h BUO plus 440 mg/kg BW of NAC daily in drinking water started on the day of BUO relief). Experiments were conducted 48 h after BUO relief.
Results. Serum levels of thiobarbituric reactive substances, which are markers of lipid peroxidation, were significantly lower in NAC-treated rats than in the BUO group rats. The administration of NAC provided significant protection against post-BUO GFR drops and reductions in RBF. Renal cortices and BUO rats presented decreased eNOS protein expression of eNOS in the renal cortex of BUO group rats, whereas it was partially recovered in BUO + NAC-pre group rats. Urine osmolality was significantly lower in BUO rats than in sham group rats or NAC-treated rats, the last also presenting less interstitial fibrosis. Post-BUO downregulation of AQP2 protein expression was averted in the BUO + NAC-pre group rats.
Conclusions. This study demonstrates that NAC administration ameliorates the renal function impairment observed 48 h after the relief of 24-h BUO. Oxidative stress is important for the suppression of GFR, RBF, tissue AQP2 and eNOS in the polyuric phase after the release of BUO.
Keywords: aquaporin 2, bilateral ureteral obstruction, endothelial nitric oxide synthase, inulin clearance, N-acetylcysteine
Introduction
Obstructive nephropathy decreases renal blood flow (RBF) and the glomerular filtration rate (GFR), thereby causing tubular abnormalities, such as urinary concentrating defect, as well as having the potential to increase oxidative stress [1]. Functional recovery typically occurs within the first 7–10 days after the obstruction is relieved, although some patients require dialysis for a period of weeks before recovering full renal function [2]. In animal studies, it has been demonstrated that there is long-term downregulation of aquaporin 2 (AQP2) protein expression after bilateral ureteral obstruction (BUO), suggesting that dysregulation of AQPs in the collecting duct plays a role in the long-term polyuria and impaired urinary concentrating ability that are associated with obstructive nephropathy [3–5]. A selective decrease in post-obstruction urinary AQP2 excretion has also been observed in patients undergoing pyeloplasty for the treatment of congenital unilateral hydronephrosis resulting from ureteropelvic junction obstruction [6].
Reactive oxygen metabolites are believed to be important mediators of various forms of acute kidney injury, such as ischaemic renal failure, radiocontrast nephrotoxicity and ureteral obstruction [7–10]. Acute loss of renal function and impaired urinary excretion lead to accumulation of advanced glycation end products, as well as oxidation and high levels of nitration-free adducts, in the plasma of rats subjected to BUO [11].
Previous studies have shown that, in rats with ischaemia/reperfusion-induced acute kidney injury, treatment with the antioxidant N-acetylcysteine (NAC) improves renal haemodynamics, upregulating the protein expression of endothelial nitric oxide synthase (eNOS) and AQP2, thereby protecting against urinary concentrating defect [7].
Although the mechanism by which NAC protects organs primarily involves the scavenging of oxygen-free radicals (either directly or by increasing intracellular concentrations of glutathione), the greater NO viability resulting from increased eNOS protein expression also plays a role [12].
The purpose of the present study was to evaluate a protective effect on the renal function of rats after the relief of BUO.
Methods
Experimental animals
Adult male Wistar rats weighing 150–200 g were obtained from the animal facilities of the University of São Paulo School of Medicine. Selected rats were treated with NAC [440 mg/kg body weight (BW) in drinking water] [7], initiated either 2 days before BUO or on the day of the relief of the obstruction.
Induction of bilateral ureteral obstruction
Animals were anaesthetized with 50 mg/kg BW of sodium pentobarbital, administered intraperitoneally. Through a midline abdominal incision, both ureters were exposed. We encased each ureter in a section of polyethylene tubing (PE-50), after which we placed two ligatures, 5 mm apart, in the upper two-thirds of each, using 5-0 silk to tighten the tubing and occlude the ureter. After having recovered from the anaesthesia, the animals were returned to their original cages and given free access to water and standard rat chow (Nuvilab, Colombo, Brazil). The ligation, together with the tubing, was removed after 24 h.
The following groups were studied:
sham operated and untreated (n = 6);
sham operated and treated with 440 mg/kg BW of NAC daily in drinking water, started 2 days before BUO and maintained until 48 h after the surgery (n = 7);
24-h BUO and untreated (n = 6);
24-h BUO plus 440 mg/kg BW of NAC daily in drinking water, started 2 days before BUO and maintained until 48 h after the relief of the obstruction (n = 7);
24-h BUO plus 440 mg/kg BW of NAC daily in drinking water, started on the day on which the obstruction was relieved and maintained for an additional 48 h thereafter (n = 6).
Clearance studies and collection of blood/urine samples
To determine GFRs, inulin clearance studies were conducted. On the day of the experiment, the animals were anaesthetized intraperitoneally with sodium pentobarbital (50 mg/kg BW). The trachea was cannulated with a PE-240 catheter, and spontaneous breathing was maintained. To control mean arterial pressure and allow blood sampling, a PE-60 catheter was inserted into the right carotid artery. For the infusion of inulin and fluids, another PE-60 catheter was inserted into the left jugular vein. In order to collect urine samples, a suprapubic incision was made, and the urinary bladder was cannulated with a PE-240 catheter. After the surgical procedure had been completed, a loading dose of inulin (100 mg/kg BW diluted in 0.9% saline) was administered through the jugular vein. Subsequently, a constant infusion of inulin (10 mg/kg BW in 0.9% saline) was started and was continued at 0.04 ml/min throughout the experiment. Three urine samples were collected at 30-min intervals. Blood samples were obtained at the beginning and at the end of the experiment. Blood and urine inulin were determined using the anthrone method. GFRs are expressed as ml/min/100 g.
Renal blood flow
To measure RBF, a midline incision was made. We then carefully dissected the left renal pedicle and isolated the renal artery, taking precautions to avoid disturbing the renal nerves. An ultrasonic flow probe (T110; Transonic Systems, Bethesda, MD, USA) was placed around the exposed renal artery. RBF was measured using an ultrasonic flowmeter (T106; Transonic Systems) and is expressed as ml/min. Renal vascular resistance was calculated by dividing the blood pressure by RBF and is expressed in mmHg/ml/min/100 g BW.
Tissue sample collection/preparation
At the end of the experiments, the left kidney was flushed with saline and perfused in situ with the Duboscq-Brazil solution. The renal tissue was then fixed, after which it was weighed, and two sections were postfixed in a buffered 10% formaldehyde solution. The material was embedded in paraffin for the assessment of glomerular injury, as well as of interstitial injury in the renal cortex. The right kidney was removed and was divided into cortex, outer medulla and inner medulla. The kidney sections were frozen in liquid nitrogen and stored at −70°C.
Urine osmolality
Urine osmolality was determined using a freezing point osmometer (3D3; Advanced Instruments, Norwood, MA, USA) and is expressed as mOsm/kg H2O.
Reactive oxygen metabolites
Serum levels of thiobarbituric acid reactive substances (TBARS), which are markers of lipid peroxidation, were determined using the thiobarbituric acid assay. In brief, a 0.2-ml serum sample was diluted in 0.8 ml of distilled water. Immediately thereafter, 1 ml of 17.5% trichloroacetic acid was added. Following the addition of 1 ml of 0.6% thiobarbituric acid, pH 2, the sample was placed in a boiling water bath for 15 min, after which it was allowed to cool. Subsequently, 1 ml of 70% trichloroacetic acid was added, and the mixture was incubated for 20 min. The sample was then centrifuged for 15 min at 2000 rpm. The optical density of the supernatant was read at 534 nm against a reagent blank using a spectrophotometer. The quantity of TBARS was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1. Serum levels of TBARS are expressed as nmol/ml [13]. In order to validate that the used anaesthetic, which is also a barbituric acid compound, does not interfere with the assay, an additional group of six rats was anaesthetized with ether and blood was obtained by cardiac puncture to TBARS determination.
Preparation of membrane fractions for the determination of aquaporin 2 protein expression
In order to quantify AQP2 protein expression, medulla samples were prepared. The samples were homogenized using a Teflon pestle glass homogenizer (Schmidt and Co., Frankfurt am Main, Germany) in an ice-cold isolation solution of 200 mM mannitol, 80 mM HEPES and 41 mM KOH, pH 7.5, also containing a protease inhibitor cocktail (Sigma Chemical Company, St. Louis, MO, USA).
The homogenates were centrifuged at a low speed (2000 × g) for 15 min at 4°C to remove nuclei and cell debris. Subsequently, the supernatants were centrifuged at 100 000 × g for 1 h at 4°C using a Ti70i rotor (Beckman Coulter, Fullerton, CA, USA) to produce a pellet that contained membrane fractions enriched with plasma membranes and intracellular vesicles. The pellets were suspended in an isolation solution.
Preparation of samples for the determination of endothelial nitric oxide synthase protein expression
In order to quantify eNOS protein expression, kidney sections were prepared. The sections were homogenized using a Teflon pestle glass homogenizer (Schmidt and Co.) in an ice-cold isolation solution of 200 mM mannitol, 80 mM HEPES and 41 mM KOH, pH 7.5, also containing a protease inhibitor cocktail (Sigma). The homogenates were centrifuged at a low speed (3000 × g) for 15 min at 4°C to remove nuclei and cell debris. The pellets were suspended in an isolation solution with protease inhibitors. Protein quantities were determined using the Bradford assay method.
Electrophoresis and immunoblotting
To determine AQP2 protein expression, samples of membrane fractions were run on 12.5% polyacrylamide minigels. After transfer by electroelution to polyvinylidene difluoride membranes (Poly-Screen, PVDF Transfer; Amersham Bioscience, Little Chalfont, UK), these blots were blocked for 1 h with 5% milk and 0.1% Tween 20 in phosphate-buffered saline (8.7 g/l of NaCl, 7.2 mM dibasic phosphate and 2.8 mM monobasic phosphate). The blots were then incubated with an anti-AQP2 antibody (1:2000). The labelling was visualized using a horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, diluted 1:2000, or anti-goat IgG, diluted 1:5000; Sigma) using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
For the evaluation of the eNOS isoform, 100 μg of total protein from each sample was separated on an 8% polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane. Immunoblotting was performed using an anti-eNOS antibody diluted 1:2000 in Tris-buffered saline and Tween 0.1%. Immunodetection was accomplished using the appropriate anti-mouse horseradish peroxidase-conjugated secondary antibody (diluted 1:2000 in Tris-buffered saline and 0.1% Tween) and the enhanced chemiluminescence kit (Amersham).
As a loading control, blots were incubated with an actin antibody (1:3000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The labelling was visualized using a horseradish peroxidase-conjugated anti-goat antibody (1:5000; Sigma).
For AQP2 and eNOS, values are expressed as a percentage of normal protein expression.
Quantification of protein levels
In order to determine the levels of AQP2 and eNOS protein, the enhanced chemiluminescence films presenting bands within the linear range were scanned using an image analysis system (ImageMaster VDS; Pharmacia Biotech, Uppsala, Sweden). Through densitometric analysis, these bands were normalized to actin protein abundance.
Preparation of histological sections
Paraffin sections of perfused/fixed kidneys were stained with haematoxylin and eosin for histological analysis under light microscopy.
Fractional interstitial area
The readers of the interstitial area were blinded to study group. The fractional interstitial area of the renal cortex was determined through morphometry using a light camera connected to an image analyser (Kontron Electronic System KS 300, Eching, Germany). In each renal cortex section, 20 grid fields (measuring 0.174 mm2 each) were evaluated. Interstitial areas were first manually encircled on a video screen and then determined by computerized morphometry in control rats, in rats subjected to BUO and receiving NAC on the day of BUO relief, as well as in rats subjected to BUO only (n = 6 in each group).
Statistical analysis
Results are presented as mean ± SEM. In order to determine the degree of injury resulting from the BUO, the untreated group was compared with the NAC-treated group using analysis of variance (ANOVA) and the Student–Newman–Keuls post hoc test using GraphPad Prism (version 3.0) and Stata (version 8.0) statistical software. The comparisons between NAC-treated and untreated rats were made using unpaired t-tests. The interstitial fibrosis area was analysed using the Mann–Whitney test. A P value <0.05 was considered statistically significant.
Results
Bilateral ureteral obstruction is associated with reductions in glomerular filtration rate and renal blood flow that improve after N-acetylcysteine administration
The results of the clearance studies are presented in Table 1. As can be seen, although 24-h BUO provoked an increase in serum levels of TBARS, this shows that NAC prevented (pre-BOU) and reversed (post-BUO) the increase in TBARS. The mean serum TBARS of rats that were anaesthetized with ether was 2.67 ± 0.15 nmol/ml, not significantly different from the sham rats anaesthetized with sodium pentobarbital (a barbituric acid compound), indicating that this last anaesthetic drug does not interfere with TBARS determinations. At 48 h after the relief of the obstruction, rats subjected to BUO and left untreated presented significant reductions in inulin clearance and RBF and increase in blood pressure in comparison with sham-operated rats and sham + NAC group. However, in comparison with the rats subjected to BUO only, those subjected to BUO and receiving NAC presented less impairment of renal function. Also, we observed no significant changes in blood pressure and a normal RBF associated with a lower renal vascular resistance (Table 1). It is noteworthy that the inulin clearance was higher in sham rats treated with NAC than in the untreated sham rats.
Table 1.
Renal function and haemodynamics at 48 h after the relief of bilateral ureteral obstruction
| GFR | Uosm | RVR | Serum TBARS | |||
|---|---|---|---|---|---|---|
| (ml/min/100 g) | (mOsm/kg H2O) | BP (mmHg) | RBF (ml/min) | (mmHg/ml/ min/100g) | (nmol/ml) | |
| Sham | 0.83 ± 0.05a | 919 ± 65a | 109 ± 3a | 2.93 ± 0.12b | 15.34 ± 0.44a | 2.80 ± 0.14d |
| Sham + NAC | 1.02 ± 0.03a,c | 851 ± 34a | 113 ± 4a | 2.94 ± 0.02b | 18.52 ± 0.80a | 2.00 ± 0.06a |
| BUO | 0.41 ± 0.09 | 409 ± 19 | 141 ± 5 | 2.25 ± 0.11 | 53.07 ± 9.51 | 3.74 ± 0.31 |
| BUO + NAC-pre | 0.81 ± 0.06a | 763 ± 60a | 131 ± 4 | 3.09 ± 0.16a | 23.00 ± 0.85a | 2.17 ± 0.39b |
| BUO + NAC-post | 0.67 ± 0.06b | 737 ± 30b | 128 ± 5 | 2.76 ± 0.13b | 20.14 ± 0.81a | 1.75 ± 0.31a |
GFR, glomerular filtration rate; Uosm, urine osmolality; BP, blood pressure; RBF, renal blood flow; RVR, renal vascular resistance; TBARS, thiobarbituric acid reactive substances; Sham, sham-operated group; Sham plus NAC started 2 days prior to surgery; BUO, 24-h bilateral ureteral obstruction (BUO) only group; BUO + NAC-pre, 24-h BUO plus NAC started 2 days prior to obstruction; BUO + NAC-post, 24-h BUO plus NAC started on the day of the relief of the obstruction.
aP < 0.001 versus BUO; bP < 0.01 versus BUO; cP < 0.05 versus sham; dP < 0.05 versus BUO.
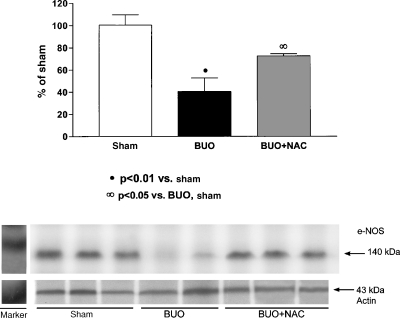
As can be seen in Figure 1, mean eNOS protein expression was markedly lower in the rats subjected to BUO and left untreated than in the sham-operated rats (40.7 ± 12.2% versus 100.5 ± 9.2%, P < 0.01). However, the mean eNOS protein expression seen in the rats in which NAC was started prior to BUO (72.5 ± 2.05%) was significantly higher than that observed in the rats subjected to BUO only (P < 0.05).
Fig. 1.
Semiquantitative immunoblotting of kidney samples 48 h after the relief of BUO. (A) Densitometric analysis of all samples from sham-operated, untreated rats, rats subjected to BUO and left untreated, and rats in which NAC was started prior to BUO. The rats subjected to BUO and left untreated presented decreased eNOS protein expression. Levels of eNOS protein expression were partially restored in the rats in which NAC was started prior to BUO. (B) Immunoblots that reacted with anti-eNOS revealed 140-kDa bands.
Bilateral ureteral obstruction is associated with reduced urine osmolality and downregulation of aquaporin 2 protein expression
Although urine osmolality was lower in all of the rats submitted to BUO than in the sham-operated untreated rats and in sham rats that received NAC, it was higher in those receiving NAC than in those that were left untreated.
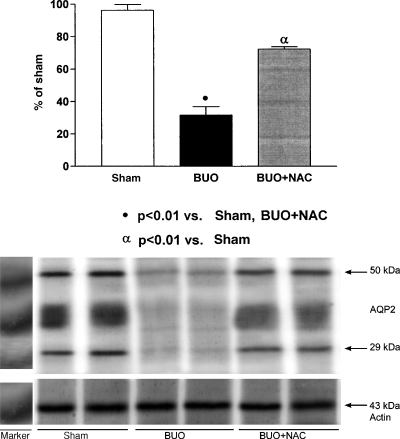
It is well known that AQP2 protein expression decreases after BUO. As shown in Figure 2, the mean AQP2 protein expression at 48 h after BUO relief was significantly downregulated in the rats subjected to BUO and left untreated in comparison with the sham-operated rats (31.7 ± 5.2 versus 96.3 ± 3.6%, P < 0.01). However, this effect was less pronounced in the rats in which NAC was started prior to BUO (72.3 ± 1.4%).
Fig. 2.
Semiquantitative immunoblotting of medullary membrane fractions 48 h after the relief of BUO. (A) Densitometric analysis of all samples from sham-operated, untreated rats, rats subjected to BUO and left untreated, and rats in which NAC was started prior to BUO. The rats subjected to BUO and left untreated presented decreased AQP2 protein expression. Levels of AQP2 protein expression were partially restored in rats in which NAC was started prior to BUO. (B) Immunoblots that reacted with anti-AQP2 revealed 29-kDa bands and 35- to 50-kDa AQP2 bands, representing the non-glycosylated and glycosylated forms of AQP2, respectively.
N-acetylcysteine administration decreases interstitial area in the bilateral ureteral obstruction model
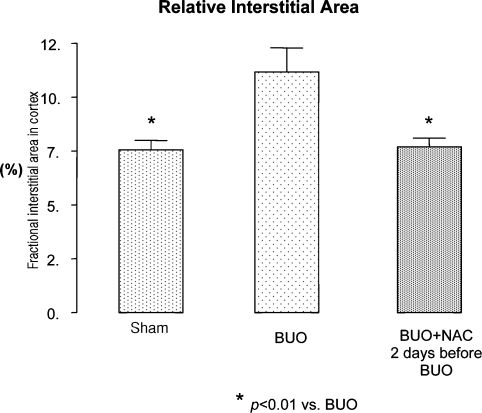
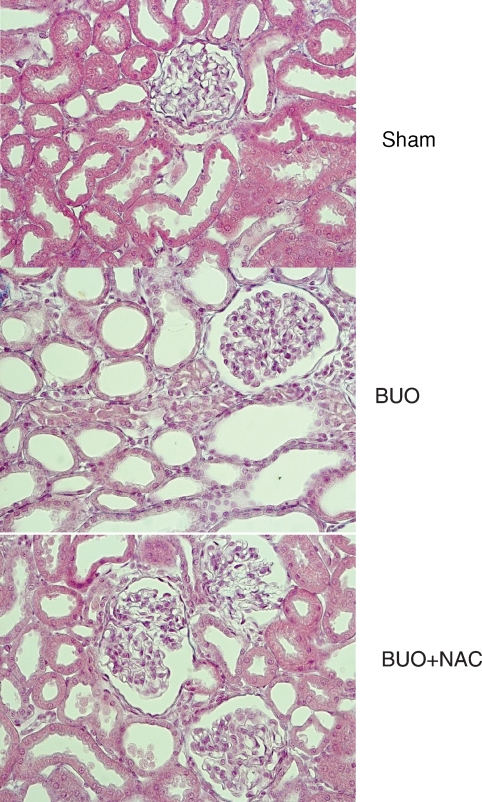
As can be seen in Figure 3, the mean relative interstitial area in the group of rats subjected to BUO and left untreated was 11.17 ± 1.11%, which was significantly higher than the 7.69 ± 0.41% seen in rats in which NAC was started prior to BUO (P < 0.01), and also higher than the sham group, 7.55 ± 0.44 (P < 0.01). Figure 4 shows renal cortex sections obtained from rats subjected to BUO only. As can be seen, all of the tubules were dilated, and the tubular cells were atrophied. In addition, an increase in interstitial area was observed. All of these histological alterations were reversed in the rats in which NAC was started prior to BUO.
Fig. 3.
Photomicrograph of a renal cortex section 48 h after the relief of the obstruction. The middle panel illustrates the renal cortex of a rat subjected to BUO and left untreated: all tubules are dilated, tubular cells are atrophied and the interstitial area is increased. None of these alterations were observed in sham (upper panel) and in NAC-treated rats (lower panel). Magnification ×280.
Fig. 4.
Fractional interstitial area in cortex 48 h after the relief of the obstruction of sham, sham-operated group; BUO, 24-h bilateral ureteral obstruction (BUO) group; BUO + NAC-pre, 24-h BUO plus NAC started 2 days prior to obstruction; data are expressed as mean ± SEM.
Discussion
The results of our study demonstrate that NAC administration ameliorates the renal function impairment observed 48 h after the relief of 24-h BUO. The effects of NAC included lower serum TBARS concentrations and histological improvement. Most importantly, these beneficial effects were also observed in the rats treated with NAC on the day of the relief of obstruction, indicating that patients suffering from obstructive nephropathies might benefit from NAC treatment as an adjunct to surgery.
Various mechanisms might be responsible for the beneficial haemodynamic effects of NAC in rats subjected to BUO. One previous study showed that rats subjected to 24-h BUO presented post-obstruction plasma levels of l-arginine that were significantly lower than those observed at baseline [14]. Ito et al. studied rats subjected to a 3-day unilateral ureteral obstruction and examined 7 days after the relief of the obstruction [15]. The authors found that dietary supplementation with l-arginine attenuated the renal damage resulting from the obstruction. Supplementation with l-arginine also increased NO production, as evidenced by an increase in the urinary excretion of NO2/NO3. In addition, fibrosis, apoptosis and macrophage infiltration were found to be significantly reduced after l-arginine treatment, although GFR and RBF were unaltered, as was eNOS protein expression in renal tissue.
In our study, NAC administration attenuated the BUO-induced decrease in renal protein expression of eNOS. This finding indicates that, at least in part, the normalization of GFR and RBF seen in the rats treated with NAC can be attributed to an increase in NO synthesis. This also suggests that other mechanisms modulate the protective effects of NAC.
Ullian et al. showed that antioxidants with SH groups are strong reducers of disulfide bonds and inhibit the binding of angiotensin II to its surface receptors, consequently attenuating signal transduction and cellular activity [16]. The authors found that when cultured vascular smooth muscle cells, which express angiotensin II type 1a (AT1a) receptors, were incubated with NAC for 1 h at 37°C, angiotensin II radioligand binding decreased in a concentration-dependent manner. They also found that NAC lowered angiotensin II receptor binding, and that angiotensin II-stimulated signal transduction was reduced in proportion to decreased receptor binding, suggesting that NAC ameliorates renal and vascular diseases mediated by angiotensin II. This represents another potential mechanism of the beneficial haemodynamic effect of NAC in BUO. A possible decrease of angiotensin II binding to glomerular mesangial cells induced by NAC may explain the increase in inulin clearance observed in sham plus NAC-treated rats in comparison to sham rats.
Various studies have demonstrated that angiotensin II plays a pivotal role in the pathogenesis of obstructive nephropathy. Jensen et al. demonstrated that the administration of candesartan, an AT1 receptor antagonist, at the onset of obstruction attenuates the reduction in GFR, as well as partially preventing the downregulation of AQP2 and NKCC2 protein expression [17]. Other authors have suggested that the downregulation of AQP channels is mediated via angiotensin II induction of cyclooxygenase 2 [18,19].
In the present study, we demonstrated that NAC treatment improves urine osmolality by reversing BUO-induced downregulation of AQP2. Our findings suggest that multiple pathways are involved in AQP downregulation. We speculate that BUO-related oxidative stress triggers angiotensin II induction of cyclooxygenase 2, thereby downregulating AQP2 protein expression and leading to polyuria.
We observed histological improvement in rats subjected to BUO and treated with NAC, all of which also presented a reduction in interstitial area, as well as normalization of tubular dilation and tubular cell atrophy. It is well known that angiotensin II activates nuclear factor kappa B (NF-κB) via AT1 and AT2 receptors. In turn, NF-κB activation leads to fibroblast proliferation and to the subsequent differentiation of fibroblasts into myofibroblasts. In addition, NF-κB stimulates tubular epithelial cells to produce chemoattractants and adhesion proteins that cause inflammation and produce profibrotic cytokines, thereby increasing the production of extracellular matrix proteins. Working in concert, these mechanisms lead to tubulointerstitial fibrosis [20]. The inhibition of NF-κB resulting from NAC administration might constitute the mechanism by which the interstitial area of the renal cortex was reduced in the NAC-treated rats evaluated in the present study.
Overall, our results are in agreement with the possibility of a pharmacological intervention in cases of urinary tract obstruction [21].
The present study describes a novel application of NAC, an inexpensive drug that produces no major side effects. Clinical studies are needed in order to determine the effects of NAC on the recovery of renal function after the relief of obstruction, especially in patients with severe renal failure. The practical application of such knowledge could effectively shorten the duration of dialysis treatment in such patients.
Acknowledgments
This study received financial support in the form of a grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo, proc. n° 2006/61703-5). Dr Antonio C. Seguro is a Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council for Scientific and Technological Development) scholar. The authors would like to thank Jefferson D. Boyles for the editorial assistance provided. A portion of this paper was presented in abstract form at the annual meeting of the American Society of Nephrology, San Diego, 2006 (published in J Am Soc Nephrol 17: 413A, 2006) and at the XLIV Congress of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA), Barcelona, 2007 (published in NDT 22, supplement 6: vi5–6: 456A, 2007).
Conflict of interest statement. None declared.
References
- 1.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 2.Cohen EP, Sobrero M, Roxe DM, et al. Reversibility of long-standing urinary tract obstruction requiring long-term dialysis. Arch Int Med. 1992;152:177–179. [PubMed] [Google Scholar]
- 3.Frøkiaer J, Marples D, Knepper MA, et al. Bilateral ureteral obstruction downregulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am J Physiol. 1996;270:F657–F668. doi: 10.1152/ajprenal.1996.270.4.F657. [DOI] [PubMed] [Google Scholar]
- 4.Kim SW, Cho SH, Oh BS, et al. Diminished renal expression of aquaporin water channels in rats with experimental bilateral ureteral obstruction. J Am Soc Nephrol. 2001;12:2019–2028. doi: 10.1681/ASN.V12102019. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Wang W, Kwon TH, et al. Downregulation of AQP1, -2, and -3 after ureteral obstructions is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol. 2001;281:F163–F171. doi: 10.1152/ajprenal.2001.281.1.F163. [DOI] [PubMed] [Google Scholar]
- 6.Murer L, Addabbo F, Carmosina M, et al. Selective decrease in urinary aquaporin 2 and increase in prostaglandin E2 excretion is associated with postobstructive polyuria in human congenital hydronephrosis. J Am Soc Nephrol. 2004;15:2705–2712. doi: 10.1097/01.ASN.0000139689.94776.7A. [DOI] [PubMed] [Google Scholar]
- 7.de Araújo M, Andrade L, Coimbra TM, et al. Magnesium supplementation combined with N-acetylcysteine protects against postischemic acute renal failure. J Am Soc Nephrol. 2005;16:3339–3349. doi: 10.1681/ASN.2004100832. [DOI] [PubMed] [Google Scholar]
- 8.Tepel M, Van Der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 9.Drager LF, Andrade L, Toledo JF, et al. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803–1807. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 10.Modi KS, Morrissey J, Shah SV, et al. Effects of probucol on renal function in rats with bilateral ureteral obstruction. Kidney Int. 1990;38:843–850. doi: 10.1038/ki.1990.280. [DOI] [PubMed] [Google Scholar]
- 11.Rabbani N, Sebekova K, Sebekova K, Jr, et al. Accumulation of free adduct glycation, oxidation and nitration products follows acute loss of renal function. Kidney Int. 2007;72:1113–1121. doi: 10.1038/sj.ki.5002513. [DOI] [PubMed] [Google Scholar]
- 12.Zafarullah M, Li WQ, Sylvester J, et al. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Reyes AA, Karl IE, Yates J, et al. Low plasma and renal tissue levels of l-arginine in rats with obstructive nephropathy. Kidney Int. 1994;45:782–787. doi: 10.1038/ki.1994.103. [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Chen J, Seshan SV, et al. Dietary arginine supplementation attenuates renal damage after relief of unilateral ureteral obstruction. Kidney Int. 2005;68:515–528. doi: 10.1111/j.1523-1755.2005.00429.x. [DOI] [PubMed] [Google Scholar]
- 16.Ullian ME, Gelasco AK, Fitzgibbon WR, et al. N-Acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J Am Soc Nephrol. 2005;16:2346–2353. doi: 10.1681/ASN.2004060458. [DOI] [PubMed] [Google Scholar]
- 17.Jensen AM, Li C, Praetorius HA, et al. Angiotensin II mediates downregulation of aquaporin water channels and key renal sodium transporters in response to urinary tract obstruction. Am J Physiol Renal Physiol. 2006;291:F1021–F1032. doi: 10.1152/ajprenal.00387.2005. [DOI] [PubMed] [Google Scholar]
- 18.Nørregaard R, Jensen BL, Li C, et al. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2005;289:F322–F333. doi: 10.1152/ajprenal.00061.2005. [DOI] [PubMed] [Google Scholar]
- 19.Nørregaard R, Jensen BL, Topcu SO, et al. COX-2 activity transiently contributes to increased water and NaCl excretion in the polyuric phase after release of ureteral obstruction. Am J Physiol Renal Physiol. 2007;292:F1322–F1333. doi: 10.1152/ajprenal.00394.2006. [DOI] [PubMed] [Google Scholar]
- 20.Klahr S. Urinary tract obstruction. Sem Nephrol. 2001;21:133–145. doi: 10.1053/snep.2001.20942. [DOI] [PubMed] [Google Scholar]
- 21.Frøkiaer J. Pharmacologic intervention in urinary tract obstruction—is it possible? Kidney Int. 2005;68:894–895. doi: 10.1111/j.1523-1755.2005.00474.x. [DOI] [PubMed] [Google Scholar]