Abstract
Background. A well-functioning vascular access (VA) is essential to efficient dialysis therapy. Guidelines have been implemented improving care, yet access use varies widely across countries and VA complications remain a problem. This study took advantage of the unique opportunity to utilize data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) to examine international trends in VA use and trends in patient characteristics and practices associated with VA use from 1996 to 2007. DOPPS is a prospective, observational study of haemodialysis (HD) practices and patient outcomes at >300 HD units from 12 countries and has collected data thus far from >35 000 randomly selected patients.
Methods. VA data were collected for each patient at study entry (1996–2007). Practice pattern data from the facility medical director, nurse manager and VA surgeon were also analysed.
Results. Since 2005, a native arteriovenous fistula (AVF) was used by 67–91% of prevalent patients in Japan, Italy, Germany, France, Spain, the UK, Australia and New Zealand, and 50–59% in Belgium, Sweden and Canada. From 1996 to 2007, AVF use rose from 24% to 47% in the USA but declined in Italy, Germany and Spain. Moreover, graft use fell by 50% in the USA from 58% use in 1996 to 28% by 2007. Across three phases of data collection, patients consistently were less likely to use an AVF versus other VA types if female, of older age, having greater body mass index, diabetes, peripheral vascular disease or recurrent cellulitis/gangrene. In addition, countries with a greater prevalence of diabetes in HD patients had a significantly lower percentage of patients using an AVF. Despite poorer outcomes for central vein catheters, catheter use rose 1.5- to 3-fold among prevalent patients in many countries from 1996 to 2007, even among non-diabetic patients 18–70 years old. Furthermore, 58–73% of patients new to end-stage renal disease (ESRD) used a catheter for the initiation of HD in five countries despite 60–79% of patients having been seen by a nephrologist >4 months prior to ESRD. Patients were significantly (P < 0.05) less likely to start dialysis with a permanent VA if treated in a faciity that (1) had a longer time from referral to access surgery evaluation or from evaluation to access creation and (2) had longer time from access creation until first AVF cannulation. The median time from referral until access creation varied from 5–6 days in Italy, Japan and Germany to 40–43 days in the UK and Canada. Compared to patients using an AVF, patients with a catheter displayed significantly lower mean Kt/V levels.
Conclusions. Most countries meet the contemporary National Kidney Foundation's Kidney Disease Outcomes Quality Initiative goal for AVF use; however, there is still a wide variation in VA preference. Delays between the creation and cannulation must be improved to enhance the chances of a future permanent VA. Native arteriovenous fistula is the VA of choice ensuring dialysis adequacy and better patient outcomes. Graft is, however, a better alternative than catheter for patients where the creation of an attempted AVF failed or could not be created for different reasons.
Keywords: arteriovenous fistulae, catheter, DOPPS, haemodialysis, vascular access
Introduction
A well-functioning vascular access (VA) remains the Achilles’ heel of haemodialysis (HD) and is essential to providing efficient dialysis therapy. VA complications remain the leading cause of morbidity in the HD population and account for high healthcare costs. There are three main types of access: native arteriovenous fistula (AVF), AVF graft (AVG) and central vein catheter. Catheter use is linked to higher rates of infection and could compromise dialysis adequacy [1,2]. However, AVF provides the best access for longevity and lowest association with morbidity and mortality [3–6]. Guidelines from different countries strongly recommend AVF use [7–10].
VA use varies widely across countries [6,11]. AVF use is linked to patient characteristics, with the use of AVF versus a graft being lower in patients who are elderly, female, have peripheral vascular disease, ischaemic cardiac disease, diabetes mellitus (DM), obesity, are unable to walk or have less education [6,12]. However, after accounting for these differences, HD patients in Europe were 3-fold more likely to have an AVF in DOPPS I [6]. Surgical practice patterns might explain this difference in treatment. Instances where the medical director or the nurse manager expressed a preference for grafts meant that their patients were more likely to have a graft over an AVF [13]. The surgeon's practice pattern clearly has an impact on the type of VA created [14]. In new dialysis patients, early referral to a nephrologist and early patient education strongly predict a successful functioning permanent VA at dialysis initiation [12,15–17]. AVF survival is also better when used for the first HD treatment compared to starting HD with a catheter and then subsequently using an AVF [6,18,19].
Since VA guidelines have changed during the last 10 years, the present study has used DOPPS data spanning this time period to describe the implementation of change in practice patterns and outcomes following these guidelines.
Patients and methods
The DOPPS is a prospective, observational study of HD treatment and patient outcomes. The DOPPS study design and methodology have been described in detail previously [20,21]. Briefly, DOPPS I (1996–2001) included data from randomly selected nationally representative samples of 308 HD units in France (20), Germany (21), Italy (20), Japan (65), Spain (20), the USA (142) and the UK (20). DOPPS II (2002–2004) and DOPPS III (2005–2007) included the additional participation of 20 HD units each from Australia, Belgium, Canada and Sweden, and two from New Zealand. By design, a smaller number of facilities contributed data for DOPPS II, to provide representative data in the countries of Japan (n = 60) and the USA (n = 79). Although the majority of sites have provided data in the USA (n = 64) and the UK (n = 14) in DOPPS III, data are still being collected in these two countries to fulfil the target of country facilities. Consequently, DOPPS III results for the UK in particular should be considered preliminary whereas DOPPS III VA use for the USA are very similar with US clinical performance measures data for the year 2006 [22]. Current analyses were based upon data from 16 402 randomly selected patients from DOPPS I; 320 HD units and 12 839 patients from DOPPS II; and 288 HD units and 7921 patients from DOPPS III.
VA data were collected for each patient at study entry. The medical director at each facility completed a questionnaire about local practice patterns, including VA preferences and practices. Finally, a VA surgery questionnaire was completed by the primary access surgeon at each facility, assessing VA practices, training and opinions (DOPPS II).
VAs were classified as one of six types: native AVF, synthetic graft, bovine graft, tunnelled central vein catheter, untunnelled temporary catheter or other. Temporary catheters were defined as any type or brand of uncuffed, percutaneously placed central vein catheter. Analyses that compared AVFs with grafts included both synthetic and bovine grafts. Some analyses were adjusted for baseline patient characteristics collected at study entry; age, sex, race, number of years with end-stage renal disease (ESRD), body weight and 14 summary comorbid conditions (listed in Table 2).
Table 2.
Predictors of fistula versus other access use: consistency across three study cohorts spanning an 11-year time period
| Fistula use (versus other access) | ||||||
|---|---|---|---|---|---|---|
| DOPPS I (n = 7575) | DOPPS II (n = 7995) | DOPPS III (n = 6266) | ||||
| Predictor | AOR | P-value | AOR | P-value | AOR | P-value |
| Age (per 10 years) | 0.92 | <0.0001 | 0.93 | 0.0001 | 0.93 | 0.005 |
| Male (versus female) | 1.72 | <0.0001 | 1.95 | <0.0001 | 2.15 | <0.0001 |
| BMI (per 1 kg/m2) | 0.52 | <0.0001 | 0.53 | 0.0002 | 0.53 | 0.001 |
| Time with ESRD (per year) | 1.00 | 0.34 | 1.00 | 0.44 | 0.98 | 0.005 |
| CAD | 0.87 | 0.008 | 0.96 | 0.45 | 1.00 | 0.95 |
| Cancer | 0.99 | 0.95 | 0.79 | 0.0003 | 0.84 | 0.03 |
| Other cardiovascular | 1.05 | 0.39 | 1.06 | 0.24 | 1.01 | 0.84 |
| Cerebrovascular disease | 1.00 | 0.97 | 0.83 | 0.003 | 0.93 | 0.35 |
| CHF | 0.90 | 0.07 | 0.83 | 0.002 | 0.93 | 0.32 |
| Diabetes | 0.85 | 0.004 | 0.87 | 0.007 | 0.75 | <0.0001 |
| GI bleeding | 0.79 | 0.01 | 1.02 | 0.80 | 0.94 | 0.63 |
| HIV | 0.90 | 0.74 | 0.96 | 0.88 | 0.71 | 0.51 |
| Hypertension | 0.98 | 0.71 | 1.13 | 0.03 | 1.27 | 0.0008 |
| Lung disease | 0.92 | 0.37 | 0.86 | 0.04 | 0.92 | 0.34 |
| Neurologic disease | 0.79 | 0.003 | 0.82 | 0.002 | 0.88 | 0.15 |
| Psych disorder | 1.00 | 0.96 | 0.89 | 0.08 | 0.79 | 0.01 |
| PVD | 0.81 | 0.002 | 0.83 | 0.003 | 0.85 | 0.02 |
| Recurrent cellulitis | 0.81 | 0.02 | 0.85 | 0.05 | 0.75 | 0.003 |
Statistically significant values (p < 0.05) are highlighted in bold.
Based upon the access in use at study entry for prevalent cross-sections of patients on dialysis >180 days in DOPPS I (1996–2001), DOPPS II (2002–2004) and DOPPS III (2005–2007); accounted for facility clustering effects.
Analyses describing access use among prevalent patients were based upon the access in use at study entry for a point-prevalent cross-section of patients in each dialysis unit at the time the unit began participation in the given study phase. Analyses describing access use among incident patients were based upon the access in use at study entry for patients who were new to ESRD and who had their first HD treatment for ESRD within 7 days of enrolment (n = 1131) into the DOPPS.
Sample means and distributions were calculated by the country for each characteristic. Statistical comparisons were made between means of country groups using mixed linear regression for continuous variables and logistic regression for dichotomous variables. Mixed linear regression models accounted for facility-clustering effects using a random effects model. An exchangeable correlation matrix was applied in logistic models using generalized estimating equations.
All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA).
Results
Vascular access use in prevalent patients
AVF and AVG use.
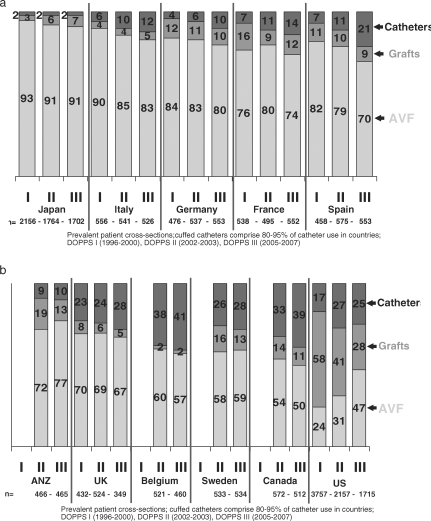
Trends in VA use were observed across three separate phases of DOPPS data from 1996 to 2006. VA use varied widely across the 12 DOPPS countries (Figure 1(a) and (b)), with a high proportion of AVF (>70%) in Japan, Australia, New Zealand and in the European countries except for Belgium, the UK and Sweden. Although AVF use had been much lower in the USA compared with other countries in DOPPS I (1996–2001), the USA has shown a large improvement in AVF use, nearly doubling from 24% in DOPPS I to 47% by DOPPS III (2005–2007). A trend towards greater AVF use was also observed in Australia, New Zealand and the UK. In contrast, AVF use has declined, especially in Spain, Italy and Germany. DOPPS III data (2005–2007) indicate 4–13% of HD patients using synthetic or bovine grafts in all countries except the USA. In all countries studied, graft use has either remained the same or has declined over the 10 years of DOPPS data collection, with the largest reduction observed in the USA declining from 58% in DOPPS I (1996–1999) to 29% in DOPPS III.
Fig. 1.
(a) Trends in vascular access use (arteriovenous fistula, catheter or graft) at study entry in DOPPS I, II and III (1996–2007) among prevalent patient cross-sections in Japan, Italy, Germany, France and Spain. (b) Trends in vascular access use (arteriovenous fistula, catheter or graft) at study entry in DOPPS I, II and III (1996–2007) among prevalent patient cross-sections in Australia and New Zealand (ANZ) the UK, Belgium, Sweden, Canada and the United States.
Central vein catheter use.
Despite higher risks associated with catheter use at least 23% of prevalent HD patients used a catheter in the UK, Belgium, Sweden, Canada and the USA in 2005–2007. The proportion of prevalent HD patients using a catheter has increased in many European countries, Canada and the USA (Figure 1(a) and (b)). Catheter use increased 2- to 3-fold in Italy, Germany, France and Spain between DOPPS I and DOPPS III. Increased dependence upon catheters is not limited to elderly patients with extensive comorbidities. In non-diabetic HD patients 18–70 years old, catheter use increased 2-fold in the USA and >3-fold in France, Germany, Italy and Spain, from DOPPS I–III (data not shown).
For patients on dialysis >180 days at study entry (Table 1) 1% of patients had never received a permanent access, 56% had received one permanent access, 25% had received two, 10% had received three and 8% had received four or more permanent accesses. Patients were significantly (P < 0.05) more likely to have ≥3 prior permanent VAs if they were female or black; had congestive heart failure, hypertension, a psychiatric disorder, or recurrent cellulitis/gangrene; had longer time with ESRD; or were treated in facilities taking at least 2 months to typically first cannulate an AVF (AOR = 1.39, P = 0.03 versus waiting ≤1 month) or in facilities not promptly performing VA procedures (AOR = 1.45, P = 0.003) (data not shown). The odds of having ≥3 prior permanent accesses were lower in Japan than the USA (AOR = 0.67, P = 0.03). The likelihood of patients using a catheter versus a permanent VA at study entry increased markedly as the number of prior permanent VAs increased (Table 1). For patients using a catheter, 9%, 56% and 35% had 0, 1–2 or >2 prior permanent VAs, respectively.
Table 1.
Number of permanent vascular accesses placed in patient prior to study entry and odds of using a catheter versus permanent access at study entry
| Number of permanent VA placed in patient prior to study entry | % of patients | AOR catheter versus permanent VA at study entry | P-value |
|---|---|---|---|
| 0 | 1.2 | – | – |
| 1 | 55.7 | 1.00 | Reference |
| 2 | 25.1 | 1.57 | <0.0001 |
| 3 | 9.6 | 2.91 | <0.0001 |
| ≥4 | 8.5 | 4.92 | <0.0001 |
DOPPS I and II (1996–2004): patients on dialysis >180 days, n = 24 795. Adjusted for age, race, gender, years with ESRD, 14 summary comorbid conditions and country. Accounted for facility clustering effects.
Likelihood of AVF use.
The relationship of patient chasracteristics with the likelihood of using an AVF versus any other VA type was examined for HD patients on dialysis >180 days in each DOPPS phase. Table 2 results indicate that patients were consistently less likely to use an AVF if they were older, had higher body mass index, diabetes mellitus, peripheral vascular disease or recurrent cellulitis (including gangrene). For these factors, the magnitude of the relationship was similar across all the three study phases. The odds of using an AVF versus other VA types increased from DOPPS I to III for males and patients with hypertension.
Vascular access use in incident patients
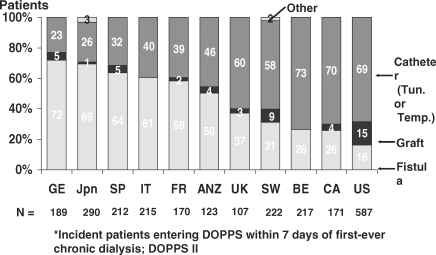
In nearly half of the countries during DOPPS II, 50% of patients initiated dialysis with a catheter, ranging from 23% to 73% (Figure 2). This high catheter use was observed despite 60–79% of patients seeing a nephrologist >4 months before starting dialysis and 69–88% seeing a nephrologist >1 month before starting dialysis. For patients seen by a nephrologist <1 month before dialysis start, the catheter use varied from 50% to >90% in all countries. For patients having seen a nephrologist >4 months prior to ESRD onset, catheter use varied from 10% in Japan to >50% in Canada, the USA and the UK (Table 3).
Fig. 2.
Vascular access use by country among incident (incident patients entering DOPPS within 7 days of first-ever chronic dialysis; DOPPS II) haemodialysis patients.
Table 3.
Percent catheter use by country in patients first seeing a nephrologist ≥4 months versus <1 month prior to initiating dialysis
| Percent catheter use in patients first seeing a | ||
|---|---|---|
| nephrologist ≥4 months versus <1 month | ||
| prior to initiating dialysis | ||
| <1 month | ≥4 months | |
| Country | (n = 221) | (n = 910) |
| Australia/New Zealand | 93.3 | 30.7 |
| Belgium | 88.9 | 47.5 |
| Canada | 92.9 | 57.1 |
| France | 73.3 | 25.0 |
| Germany | 64.3 | 15.1 |
| Italy | 60.0 | 31.5 |
| Japan | 50.0 | n/a |
| Spain | 81.0 | 25.6 |
| Sweden | 61.5 | 39.7 |
| UK | 81.0 | 64.4 |
| US | 88.6 | 60.6 |
| All countries | 77.4 | 35.7 |
DOPPS II (2002–2004); incident HD patients on dialysis <7 days when entering DOPPS and who completed a patient questionnaire indicating when first seeing a nephrologist prior to starting dialysis. Catheter use is based upon the vascular access in use at study entry.
Factors linked to the proportion of catheter use at dialysis start.
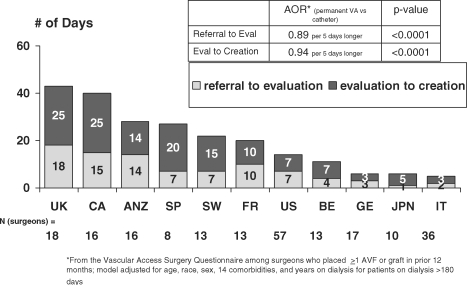
Timely accessibility to a VA surgeon was a factor influencing the proportion of patients starting dialysis with a permanent VA. The median time from referral to VA creation varied widely between countries from 5 to 6 days in Italy, Japan and Germany and from 40 to 43 days in Canada and the UK. In most countries the delay between referral and evaluation by the surgeon (1–18 days) was shorter than the delay between evaluation and permanent VA creation (7–25 days) (Figure 3). The likelihood of starting dialysis with a permanent VA versus a catheter was lower with longer time between referral and evaluation by the surgeon (AOR 0.89 per 5 days longer, P < 0.0001) and with longer time between evaluation and access creation (AOR 0.94 per 5 days longer, P < 0.0001). There was a wide variation between countries both in the median number of VA surgeons per 100 patients (0.5–5.9 per 100 patients) and the number of surgeons per facility (1.0–4.0 per facility) (Table 4). In most countries, vascular surgeons represent the larger group of VA operators. A significant proportion of VA operators were nephrologists in Italy (85%) and in Japan (25%), which explains the low proportion of surgeons per 100 patients in these two countries.
Fig. 3.
The median time from referral to vascular access surgery by country based on the vascular access surgery questionnaire distributed among surgeons who placed more than one arteriovenous fistula or graft in prior 12 months. Model was adjusted for age, race, sex, 14 comorbidities and years on dialysis for patients on dialysis >180 days.
Table 4.
The median number of surgeons per facility and per 100 patients and the median number of patients per facility, by country, DOPPS II
| Country | Number of surgeons per facility | Number of surgeons per 100 patients | Number of patients per facility |
|---|---|---|---|
| Australia/New Zealand | 2.5 | 4.8 | 53 |
| Belgium | 2.0 | 3.9 | 52 |
| Canada | 2.5 | 2.3 | 131 |
| France | 2.0 | 2.8 | 78 |
| Germany | 2.0 | 2.9 | 80 |
| Italy | 1.0 | 1.6 | 55 |
| Japan | 1.0 | 0.5 | 72 |
| Spain | 2.0 | 5.1 | 47 |
| Sweden | 3.0 | 5.8 | 50 |
| UK | 3.0 | 3.6 | 76 |
| USA | 4.0 | 5.9 | 76 |
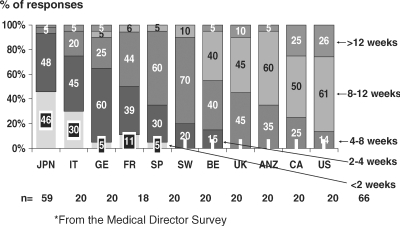
Time between AVF creation and cannulation varied. In a few countries, a high proportion of units (>60%) had their AVF first cannulated within 4 weeks after AVF creation, while in most countries units waited >4 weeks before first AVF cannulation (Figure 4).
Fig. 4.
Average time between arteriovenous creation and cannulation by country based on answers from the Medical Director Survey, DOPPS II.
Vascular access use and outcomes
A significantly higher proportion of patients with a catheter (27.5%) had an spKt/V <1.2 when compared with patients using a graft (9.9%) or fistula (19.2%) (P < 0.01 for catheter versus graft and fistula). When compared with patients with an AVF or a graft, patients with a catheter generally had shorter dialysis duration (except in France).
Discussion
The prevailing guideline goals during this study period [7] were to increase AVF use and limit catheter use. According to the NKF-KDOQI guidelines in 1997 [23], primary AVF should be constructed in at least 50% of all new patients. Ultimately, 40% of prevalent patients should be using an AVF and <10% maintained on a catheter as their permanent chronic dialysis access. Furthermore, the European Best Practice Guidelines (EBPG) for VA have strongly recommended an AVF as the VA of first choice for HD [10,24]. DOPPS II and III indicate large differences in VA use across countries. For prevalent patients, all countries except the USA met the standard of having >40% of patients dialysing via an AVF in DOPPS I and II, while some countries had more than twice this target level. Therefore, this prevailing standard was achievable and has led to a higher target level in revised guidelines [9]. Although the USA had a high proportion of graft use, it decreased by 29% between DOPPS I and III while AVF use increased to 47%, now meeting the 1997 KDOQI guidelines. The US increase in AVF use coincides with a substantial change in VA preference within US dialysis units as indicated by US dialysis unit medical director responses. In the USA, AVF is now the preferred access by 92% of medical director respondents in DOPPS II, compared to 79% in DOPPS I [13]. DOPPS I data have increased motivation towards AVF creation and implementation of initiatives, such as the Fistula First programme [25]. Despite attitude changes towards VA, this high proportion might be linked to the preference or experience of the VA surgeon in the USA [6]. In the other DOPPS I countries, the high proportion of AVF use slightly decreased between DOPPS I and III, suggesting that there might be an increased proportion of patients not suitable for the creation of an AVF. The increased prevalence of different comorbidities during the same period and their interaction could affect the quality of the vascular beds and impede successful AVF creation. The 2006 revised KDOQI clinical practice guidelines for VA have set a goal ≥65% functional AVF in HD patients. This target increase from 40% to 65% reflects ongoing changes in the US practice with an incentive to further increase AVF creation.
The <10% goal of prevalent patients with a catheter was met by five out of seven countries of DOPPS I. In DOPPS II, 6 out of 11 study regions met or nearly met the <10% goal. Japan was the only country in DOPPS III to meet the goal. The increase in diabetes from 18% to 33%, and vascular disease from 22% to 34% in HD patients between DOPPS I and III led to higher proportions of patients at risk for the failure of AVF creation. The increase in catheter use is impressive in the non-diabetic, non-elderly dialysis population and affects more females. The risk of having a catheter increases with the number of previous permanent VA attempts and may reflect practices of resorting to a catheter when repeated permanent access use has been unsuccessful. Some facility practices and patient characteristics are linked to high numbers of prior permanent VAs, which might suggest some changes in VA surgery practice patterns or expertise. These facility practices are modifiable, namely the time for first cannulation and the waiting time for permanent VA procedure. Surgical expertise most likely has an impact on the number and longevity of previous VAs. Our results consistently show from DOPPS I to III that patients were less likely to be using an AVF if they were older, obese, diabetic, had peripheral vascular disease or recurrent cellulitis. Different teams have addressed these problems and suggested solutions to increase AVF [26–29]. Despite such efforts, some patients might not be suitable for the creation of an AVF but could possibly benefit from a graft. Some countries with a significant proportion of patients with catheters have a proportionally low use of grafts. Given the higher mortality rate of catheter patients, a graft might be a better option for patients not suitable for AVF creation. The new 2006 KDOQI guidelines have kept the goal for catheter use to <10%. Meeting this goal will be a great challenge and will need great commitment, teamwork and the development of specific expertise.
Despite the different published guidelines, there is still a very high proportion of patients starting dialysis with a catheter. Late referral to a nephrologic team, which has been reported in many countries, could be one possible explanation for the high proportion of catheter use [6,30]. Predialysis care increases the likelihood of a permanent VA at dialysis start [6]. Our study supports this observation; we found that the proportion of new patients starting dialysis with a permanent access was higher when patients were seen for a longer predialysis period by a nephrologist. The mean proportion of catheter use in new patients was 77% and 36% for patients seen by a nephrologist <1 month and >4 months, respectively, before starting dialysis. The chance for a patient starting dialysis with a permanent VA also decreased with longer delays before being seen by a surgeon and with delays between evaluation and surgery. Countries with a long median time for AVF creation were also the countries most reluctant to cannulate AVF early. In Canada, the UK and the USA >90% of the facilities typically first cannulate AVF after 4 weeks, thus making the total time between referral and first AVF cannulation 2–3 months. In these countries, a patient can only start dialysis with a permanent VA if seen by a nephrologist for >3–4 months prior to dialysis initiation. Mendelssohn et al. [31] have discussed this problem in Canadian care. Fistula creation at least 4 months before starting chronic HD is associated with a lower risk of sepsis and death, primarily by reducing the use of HD catheters [32].
Inadequate dialysis (spKt/V <1.2) was found to be substantially more common for patients with a catheter. Previous studies have shown that when compared to AVF it is possible to provide adequate dialysis with catheters despite flow performances and slightly reduced dialysis doses [33,34]. Lengthening dialysis time is a simple and efficient tool to compensate for the reduced flow performances with catheter use and might explain the better Kt/V of French dialysis patients with catheters (data not shown).
AVF has a lower frequency of complications and a lower long-term cost than catheters and grafts, and should therefore be favoured as the VA of choice [13]. Catheter use has been shown to increase mortality risk after adjustment for many comorbidities or by the use of propensity scores [2,35,36]. However, other investigators did not find an increase in mortality in patients with catheters [37,38]. Despite careful adjustment for various and numerous factors it remains difficult to account for any bias by indication.
In summary, wide variation in VA use remains across countries. In the prevalent dialysis population, although the proportion of AVF meets the 1997 KDOQI guideline goal, the proportion of catheter use remains a problem. For incident patients, the high level of catheter use is a matter of concern and should lead to action. Delays between access creation and cannulation should be improved, particularly in view of Rayner et al.'s [18] observation of no detrimental consequences upon fistula survival when first cannulated 15 days or longer following creation. More in-depth studies of factors leading to delays and effects of healthcare management issues, such as impact of a VA coordinator and Fistula First program, should be initiated. Creation and maintenance of a functional AVF will remain the greatest challenge in the dialysis field. Achievement of target goals can only be met by dedicated and sustained teamwork and the development of specific expertise.
Acknowledgments
The Dialysis Outcomes and Practice Patterns Study is supported by research grants from Amgen and Kirin Pharma without restrictions on publications. The authors report no financial conflicts. Institutional review boards have approved the DOPPS in each country or facility, as required. Informed patient consent was obtained in accordance with the requirements of each country, review board and dialysis centre. Data collection was performed in a fashion that maintained patient anonymity. We acknowledge the great efforts of facility nurses, physicians and patients for contributing to the DOPPS, the editorial assistance of Shauna Leighton and the analytical support provided by Lin Tong during the manuscript review.
Conflict of interest statement. The Dialysis Outcomes and Practice Patterns Study is supported by research grants from Amgen and Kirin Pharma without restrictions on publications. The corresponding author and all other coauthors have no potential conflicts of interest to declare.
References
- 1.Hoen B, Kessler M, Hestin D, et al. Risk factors for bacterial infections in chronic haemodialysis adult patients: a multicentre prospective survey. Nephrol Dial Transplant. 1995;10:377–381. [PubMed] [Google Scholar]
- 2.Combe C, Pisoni RL, Port FK, et al. Dialysis Outcomes and Practice Patterns Study: data on the use of central venous catheters in chronic hemodialysis. Néphrologie. 2001;22:379–384. [PubMed] [Google Scholar]
- 3.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 4.Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002;39:92–101. doi: 10.1053/ajkd.2002.29886. [DOI] [PubMed] [Google Scholar]
- 5.Huber TS, Carter JW, Carter RL, et al. Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg. 2003;38:1005–1011. doi: 10.1016/s0741-5214(03)00426-9. [DOI] [PubMed] [Google Scholar]
- 6.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–316. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 7.NKF-KDOQI Clinical practice guidelines for vascular access: update 2000. Am J Kidney Dis. 2001;37(Suppl 1):S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 8.Ethier JE, Lindsay RM, Barre PE, et al. Clinical practice guidelines for vascular access. Canadian Society of Nephrology. J Am Soc Nephrol. 1999;10(Suppl 13):S297–S305. [PubMed] [Google Scholar]
- 9.NKF-KDOQI Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S248–S272. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Tordoir J, Canaud B, Haage P, et al. European best practice guidelines (EBPG) on vascular access. Nephrol Dial Transplant. 2007;22(Suppl 2):ii88–ii117. doi: 10.1093/ndt/gfm021. [DOI] [PubMed] [Google Scholar]
- 11.Besarab A. Vascular access in Europe and the US: striking contrasts. Contemp Dial Nephrol. 1999;20:22–28. [Google Scholar]
- 12.Stehman-Breen CO, Sherrard DJ, Gillen D, et al. Determinants of type and timing of initial permanent hemodialysis vascular access. Kidney Int. 2000;57:639–645. doi: 10.1046/j.1523-1755.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 13.Young EW, Dykstra DM, Goodkin DA, et al. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2002;61:2266–2271. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 14.O’Hare AM, Dudley RA, Hynes DM, et al. Impact of surgeon and surgical center characteristics on choice of permanent vascular access. Kidney Int. 2003;64:681–689. doi: 10.1046/j.1523-1755.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 15.Sesso R, Yoshihiro MM. Time of diagnosis of chronic renal failure and assessment of quality of life in haemodialysis patients. Nephrol Dial Transplant. 1997;12:2111–2116. doi: 10.1093/ndt/12.10.2111. [DOI] [PubMed] [Google Scholar]
- 16.Arora P, Obrador GT, Ruthazer R, et al. Prevalence, predictors, and consequences of late nephrology referral at a tertiary care center. J Am Soc Nephrol. 1999;10:1281–1286. doi: 10.1681/ASN.V1061281. [DOI] [PubMed] [Google Scholar]
- 17.Roubicek C, Brunet P, Huiart L, et al. Timing of nephrology referral: influence on mortality and morbidity. Am J Kidney Dis. 2000;36:35–41. doi: 10.1053/ajkd.2000.8241. [DOI] [PubMed] [Google Scholar]
- 18.Rayner HC, Pisoni RL, Gillespie BW, et al. Creation, cannulation and survival of arteriovenous fistulae: data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63:323–330. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 19.Ravani P, Brunori G, Mandolfo S, et al. Cardiovascular comorbidity and late referral impact arteriovenous fistula survival: a prospective multicenter study. J Am Soc Nephrol. 2004;15:204–209. doi: 10.1097/01.asn.0000103870.31606.90. [DOI] [PubMed] [Google Scholar]
- 20.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes And Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(Suppl 74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 21.Pisoni RL. Vascular access use and outcomes: results from the DOPPS. Contrib Nephrol. 2002;137:13–19. doi: 10.1159/000060262. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services . 2006 Annual Report. Baltimore, Maryland: Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of Clinical Standards & Quality; End Stage Renal Disease Clinical Performance Measures Project. January 2007. [Google Scholar]
- 23.Schwab S, Besarab A, Beathard G, et al. NKF-KDOQI clinical practice guidelines for hemodialysis vascular access. Am J Kidney Dis. 1997;30(Suppl 3):S137–S181. [Google Scholar]
- 24.Tordoir JH, Mickley V. European guidelines for vascular access: clinical algorithms on vascular access for haemodialysis. EDTNA ERCA J. 2003;29:131–136. doi: 10.1111/j.1755-6686.2003.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 25.Fistula First Website Fistula First National Vascular Access Improvement Initiative: Change Concepts. Available at: http://www.fistulafirst.org. (Accessed 16 August 2007)
- 26.Asif A, Unger SW, Briones P, et al. Creation of secondary arteriovenous fistulas: maximizing fistulas in prevalent hemodialysis patients. Semin Dial. 2005;18:420–424. doi: 10.1111/j.1525-139X.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 27.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 28.Lok CE, Oliver MJ. Overcoming barriers to arteriovenous fistula creation and use. Semin Dial. 2003;16:189–196. doi: 10.1046/j.1525-139x.2003.16038.x. [DOI] [PubMed] [Google Scholar]
- 29.Konner K. The initial creation of native arteriovenous fistulas: surgical aspects and their impact on the practice of nephrology. Semin Dial. 2003;16:291–298. doi: 10.1046/j.1525-139x.2003.16064.x. [DOI] [PubMed] [Google Scholar]
- 30.Mendelssohn DC, Kua BT, Singer PA. Referral for dialysis in Ontario. Arch Intern Med. 1995;155:2473–2478. [PubMed] [Google Scholar]
- 31.Mendelssohn DC, Ethier J, Elder SJ, et al. Haemodialysis vascular access problems in Canada: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II) Nephrol Dial Transplant. 2006;21:721–728. doi: 10.1093/ndt/gfi281. [DOI] [PubMed] [Google Scholar]
- 32.Oliver MJ, Rothwell DM, Fung K, et al. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol. 2004;15:1936–1942. doi: 10.1097/01.asn.0000131524.52012.f8. [DOI] [PubMed] [Google Scholar]
- 33.Canaud B, Leray-Moragues H, Kerkeni N, et al. Effective flow performances and dialysis doses delivered with permanent catheters: a 24-month comparative study of permanent catheters versus arterio-venous vascular accesses. Nephrol Dial Transplant. 2002;17:1286–1292. doi: 10.1093/ndt/17.7.1286. [DOI] [PubMed] [Google Scholar]
- 34.Tonelli M, Muirhead N. Access type as a predictor of dialysis adequacy in chronic hemodialysis patients. ASAIO J. 2000;46:279–282. doi: 10.1097/00002480-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Polkinghorne KR, McDonald SP, Atkins RC, et al. Vascular access and all-cause mortality: a propensity score analysis. J Am Soc Nephrol. 2004;15:477–486. doi: 10.1097/01.asn.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 36.Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62:620–626. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Iorio BR, Bellizzi V, Cillo N, et al. Vascular access for hemodialysis: the impact on morbidity and mortality. J Nephrol. 2004;17:19–25. [PubMed] [Google Scholar]
- 38.Duncan ND, Singh S, Cairns TD, et al. Tesio-Caths provide effective and safe long-term vascular access. Nephrol Dial Transplant. 2004;19:2816–2822. doi: 10.1093/ndt/gfh467. [DOI] [PubMed] [Google Scholar]