Abstract
Rationale: Puerto Ricans have the highest prevalence of and morbidity from asthma of all ethnic groups in the United States. One potential contributor to the high burden of asthma in Puerto Rican children is exposure to stress and violence.
Objectives: To examine whether exposure to stress and violence is associated with an increased risk of asthma among Puerto Rican children.
Methods: This study was a population-based probability sample of children in the San Juan and Caguas metropolitan areas in Puerto Rico. Information was collected in a household survey of 1,213 children and their primary caretakers.
Measurements and Main Results: The prevalence of lifetime physician-diagnosed asthma was 39.6%. In the year before the survey, 14% of children had witnessed an act of violence, 7% had been victims of violence, and 6% had been victims of physical or sexual abuse. Although stressful life events and exposure to neighborhood violence were not associated with asthma, a history of physical or sexual abuse was associated with approximately twice the odds of current asthma (odd ratio [OR], 2.52; 95% confidence interval [CI], 1.27–5.00), health care use for asthma (OR, 1.95; 95% CI, 0.96–3.96), and medication use for asthma (OR, 2.35; 95% CI, 1.05–5.26).
Conclusions: Physical or sexual abuse is associated with high asthma morbidity among Puerto Rican children. To our knowledge, this is the first report of an association between childhood abuse and asthma. Our findings highlight the importance of screening for asthma among victims of childhood abuse, and to be aware of the possibility of physical or sexual abuse among children with asthma.
Keywords: asthma, children, stress, violence, abuse
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Stress and violence have been associated with asthma in children. This association has never been examined specifically in Puerto Rican children, who bear a disproportionate burden of asthma morbidity compared with other groups.
What This Study Adds to the Field
Physical or sexual abuse was associated with high asthma morbidity among children living in urban areas of Puerto Rico. These findings highlight the importance of screening for asthma among victims of childhood abuse, and vice versa.
Asthma is the most common chronic disease of childhood in the United States, and it disproportionately affects ethnic minorities (1). Puerto Ricans have a higher prevalence of asthma and suffer more morbidity and mortality from asthma than do whites, blacks, and other Hispanics (2, 3). Among children living in the U.S. mainland in 2001, Puerto Ricans had a higher lifetime prevalence of asthma (25.8%) than whites (12.7%), blacks (15.8%), Mexicans (10.1%), Cubans (14.9%), and Dominicans (14.9%) (2). From 1990 to 1995, Puerto Ricans had the highest mortality rate due to asthma (40.9 per million) of all ethnic groups in the U.S. mainland (4). Recent evidence suggests that Puerto Rican children living in Puerto Rico (hereafter called island Puerto Rican children) have an even higher prevalence of asthma than Puerto Rican children in the U.S. mainland (5).
One potential contributing factor to the high burden of asthma in Puerto Rican children is exposure to stress and violence. This is an important avenue to explore because exposure and reaction to stress represent potentially modifiable risk factors for asthma in Puerto Ricans. There is evidence linking stressors such as negative life events and exposure to violence to childhood asthma (6–10). Studies from Puerto Rico (11) and from U.S. cities with large Puerto Rican populations suggest that Puerto Ricans are exposed to high levels of community and personal violence (12, 13). In addition, Puerto Ricans may be more vulnerable to suffering emotional distress in response to violence than members of other ethnic groups. Puerto Ricans were more likely to report post-traumatic stress disorder (PTSD) symptoms after the September 11th attacks than members of other ethnic groups. In addition, Puerto Ricans were more likely to have experienced a peri-event panic attack upon hearing of the events on that date (14). Among veterans from the Vietnam War, Puerto Ricans had increased risks of PTSD and severe PTSD symptoms (15). These data are particularly relevant because traumatic stress reactions to violence exposures—in addition to the exposures themselves—have been shown to predict health problems in childhood (16).
The purpose of this study was to examine whether exposure to stress and violence is associated with increased risk of asthma and allergies in a population of urban island Puerto Rican children. We hypothesized that more severe exposures (including personal victimization and abuse) would have the highest likelihood of being associated with asthma and allergy outcomes. To our knowledge, this is the first study of the relation among life stressors, violence, and asthma in Puerto Ricans.
METHODS
Detailed methods are provided in the online supplement.
Subject recruitment and study protocols have been described in detail elsewhere (17). In brief, this was a prospective cohort study of randomly sampled Puerto Rican children in the standard metropolitan areas of San Juan and Caguas (Puerto Rico). Participants were enrolled from July 2001 through August 2003 and interviewed annually in three waves. The current cross-sectional analysis is based on data from the 2-year follow-up interview, which had detailed questions about asthma. The study was approved by the Institutional Review Board of the University of Puerto Rico Medical School.
The study used a multistage probability sample method. Primary sampling units were randomly selected neighborhood clusters based on the 1990 U.S. Census and subsequently adjusted to the 2000 census. Secondary sampling units were randomly selected households within each individual primary sampling unit. A household was eligible if one or more resident was a child (age 5–13 yr) identified by his/her parents or primary caretakers as Puerto Rican and one or more of the child's parents or primary caretakers in the household self-identified as Puerto Rican. In households with more than one eligible child, up to three children were randomly selected to participate.
Procedures
Structured questionnaire interviews were conducted in the participants' homes in English and/or Spanish, based on the preferences of the participants. Whenever space permitted, children were interviewed separately from their parents. Interviewers were trained on the protocol regarding the reporting of traumatic experiences (abuse, neglect, excessive fear of adults “in charge”) to staff supervising the study. If children were judged to be in possible jeopardy from child abuse, a referral was made to local authorities.
Exposure to violence in the previous year was assessed using children's responses to a modified version of the Exposure to Community Violence Scale (18). The child's history of physical and sexual abuse in the previous year was elicited separately from parents and children using the modified Traumatic Experience Questionnaire (19). Children completed the Stressful Life Events Scale (20, 21), which asked about 20 major life stressors (e.g., death of a family member, parents' divorce) during the previous year (17).
Information about asthma and allergy outcomes was elicited from the child's parent or primary caregiver. “Current asthma” was defined as having ever received a physician's diagnosis of asthma and wheeze in the previous year, and “lifetime allergic rhinitis” as having ever received a physician's diagnosis of allergic rhinitis or allergic conjunctivitis. “Health care use for asthma” referred to a child's visit(s) to a physician's office for asthma in the previous year, and “medication use for asthma” to use of prescription medications for asthma in the previous year by children with physician-diagnosed asthma.
Covariates considered for inclusion in multivariable models included demographic data (child's age and sex, and indicators of socioeconomic status [SES], such as household income [primarily defined as <$18,000 vs. ≥$18,000/yr, the median income in Puerto Rico in 2005 (22)], and receipt of public assistance in the previous year), maternal and paternal history of asthma and allergic diseases, and caregiver perceived stress (assessed by the Perceived Stress Scale [23]).
Data Analysis
The samples were weighted to represent the age and sex distribution of children in the standard metropolitan areas in Puerto Rico on the basis of the 2000 U.S. Census. Weighted analyses were conducted with SUDAAN software (version 8; Research Triangle Institute, Research Triangle Park, NC) to adjust standard errors for intraclass correlations induced by multistage sampling, with children nested within households and households nested within primary sampling units. We used logistic regression to examine potential predictors of current asthma, allergies, health care use for asthma, and medication use for asthma. Multivariable models were constructed by entering all predictors with P ≤ 0.20 in bivariate analyses and then conducting a backward stepwise selection procedure. Our primary exposures of interest were exposure to stressful life events, exposure to neighborhood violence, and physical or sexual abuse. Other covariates remained in the final models if they were statistically significant (P < 0.05) or satisfied a change-in-estimate criterion of 10% or greater.
RESULTS
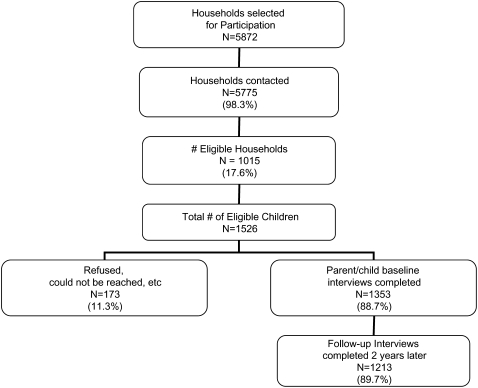
Figure 1 shows the schema for enrollment into the study. On the basis of the sampling design, 5,872 households were randomly selected for initial inclusion in the study during the first wave, and 5,775 (98.3%) were successfully contacted. Of these 5,775 households, 1,015 (17.6%) households with 1,526 children were eligible for the study. However, 173 (11.3%) of the 1,526 eligible children did not participate in the study because of parental refusal or repeated unavailability (three or more unsuccessful attempts). The original study population consisted of 1,353 child–parent dyads. Follow-up at 2 years was obtained on 89.7% of the original study population, such that 1,213 parent–child dyads from 826 households completed the interview for the current study. Although the proportion of families with an income of less than $18,000 per year was lower in the 1,213 participants who completed the follow-up interview at 2 years than in those who did not (65 vs. 53%, P < 0.01), baseline characteristics were otherwise similar between the two groups in terms of age, sex, maternal education, and receipt of public assistance (P > 0.20 in all cases).
Figure 1.
Enrollment of subjects.
Table 1 summarizes the characteristics of the study population. The mean age of the children at the time of the follow-up interview was 11.7 years (range, 6.8–16.6 yr). The percentage of children who reported at least two stressful life events in the past year was high (45%). In terms of exposure to stress and violence in the year before the survey, 14.4% of children reported having witnessed an act of violence in their neighborhood, 7% reported that they had been a victim of violence in their neighborhood, and 6.3% of children had a history of physical or sexual abuse. Estimates of the lifetime prevalence of physician-diagnosed asthma (39.6%) and physician-diagnosed allergic rhinitis (26.6%) were high. Of the 478 children ever diagnosed with asthma, 144 (30%) had asthma symptoms in the previous year, and 113 (24%) required medical attention for their asthma. Weighted percentages adjusted for the age and sex distribution of the general population were similar to the unweighted percentages and are presented in Table E1 in the online supplement.
TABLE 1.
CHARACTERISTICS OF STUDY POPULATION*
| Characteristic | No. | % |
|---|---|---|
| Females | 588/1,213 | 48.5 |
| Income <$18,000 in past year | 729/1,189 | 61.3 |
| Received public assistance in past year | 452/1,212 | 37.3 |
| Maternal history of asthma | 247/1,191 | 20.7 |
| Paternal history of asthma | 153/1,119 | 13.7 |
| Physician visit for routine care, past year | 840/1,212 | 69.3 |
| Two or more stressful life events in past year | 518/1,149 | 45.1 |
| Victim of neighborhood violence, past year | 84/1,201 | 7.0 |
| Witness of neighborhood violence, past year | 173/1,198 | 14.4 |
| Victim of physical or sexual zbuse, past year | 75/1,199 | 6.3 |
| Diagnosed with asthma by physician | 478/1,206 | 39.6 |
| Wheeze/whistling in chest during past 12 mo | 168/1,206 | 13.9 |
| Current asthma (diagnosed with asthma by physician and wheeze/whistling in chest during past 12 mo) | 144/1,205 | 12.0 |
| Diagnosed with allergic rhinitis/conjunctivitis by physician | 321/1,206 | 26.6 |
| Child brought to physician's office/clinic for asthma in past 12 mo | 113/1,207 | 9.4 |
| Taken prescription medication for asthma in past 12 mo (among patients with asthma only) | 225/478 | 47.1 |
Numbers and percentages vary because of missing information on some variables.
Table 2 summarizes the results of the bivariate (unadjusted) analyses of the association between the variables of interest and asthma and allergy outcomes. Maternal and paternal history of asthma and history of physical and/or sexual abuse were each associated with increased odds of current asthma, lifetime allergic rhinitis, and health care use for asthma. Increasing age was associated with decreased odds of current asthma, health care use for asthma, and medication use for asthma. Of note, although SES indicators (income, public assistance, and maternal education) were associated with decreased odds of lifetime allergic rhinitis, these indicators were not associated with any of the asthma outcomes. Finally, stressful life events (analyzed as both a dichotomous and a continuous variable), exposure to neighborhood violence, and caregiver perceived stress were not associated with asthma or allergies among study participants.
TABLE 2.
BIVARIATE PREDICTORS OF ASTHMA AND ALLERGY OUTCOMES
| Current Asthma* | Lifetime Allergic Rhinitis† | Health Care Use for Asthma‡ | Medication Use for Asthma§ (patients with asthma only) | |
|---|---|---|---|---|
| Age | 0.91 | 1.03 | 0.85 | 0.87 |
| (0.85–0.98) | (0.97–1.09) | (0.79–0.91) | (0.81–0.95) | |
| P = 0.0176 | P = 0.3156 | P < 0.0001 | P = 0.0012 | |
| Female sex | 0.97 | 0.88 | 1.01 | 1.22 |
| (0.68–1.38) | (0.62–1.25) | (0.67–1.52) | (0.80–1.87) | |
| P = 0.8695 | P = 0.4822 | P = 0.9763 | P = 0.3476 | |
| Income <$18,000/yr | 0.78 | 0.53 | 0.85 | 1.14 |
| (0.54–1.12) | (0.39–0.73) | (0.55–1.30) | (0.78–1.67) | |
| P = 0.1777 | P = 0.0001 | P = 0.4453 | P = 0.4950 | |
| Received public assistance in past year | 1.10 | 0.59 | 1.30 | 0.99 |
| (0.70–1.73) | (0.43–0.81) | (0.84–1.99) | (0.69–1.42) | |
| P = 0.6631 | P = 0.0012 | P = 0.2351 | P = 0.9638 | |
| Maternal history of asthma | 2.98 | 1.73 | 2.42 | 1.86 |
| (1.81–4.93) | (1.27–2.35) | (1.46–4.00) | (1.09–3.17) | |
| P < 0.0001 | P = 0.0006 | P = 0.0008 | P = 0.0235 | |
| Paternal history of asthma | 2.28 | 2.42 | 2.80 | 1.34 |
| (1.30–3.99) | (1.52–3.87) | (1.61–4.85) | (0.81–2.20) | |
| P = 0.0045 | P = 0.0003 | P = 0.0004 | P = 0.2528 | |
| Physician visit for routine care, past year | 0.77 | 1.29 | 1.03 | 1.46 |
| (0.49–1.21) | (0.85–1.95) | (0.65–1.64) | (0.92–2.33) | |
| P = 0.2498 | P = 0.2317 | P = 0.8989 | P = 0.1046 | |
| Two or more stressful life events in past year | 0.91 | 1.02 | 0.92 | 0.93 |
| (0.61–1.35) | (0.74–1.43) | (0.60–1.41) | (0.66–1.30) | |
| P = 0.6921 | P = 0.8879 | P = 0.7004 | P = 0.6526 | |
| Victim of neighborhood violence | 0.75 | 0.66 | 0.69 | 0.47 |
| (0.31–1.80) | (0.35–1.24) | (0.24–2.01) | (0.20–1.08) | |
| P = 0.5177 | P = 0.1928 | P = 0.4909 | P = 0.0758 | |
| Witnessed neighborhood violence | 1.16 | 1.07 | 0.93 | 1.08 |
| (0.66–2.06) | (0.67–1.70) | (0.51–1.69) | (0.65–1.81) | |
| P = 0.5988 | P = 0.7890 | P = 0.8170 | P = 0.7572 | |
| Physical or sexual abuse | 2.15 | 1.91 | 1.99 | 2.67 |
| (1.07–4.36) | (1.11–3.27) | (0.96–4.12) | (1.21–5.90) | |
| P = 0.0329 | P = 0.0200 | P = 0.0638 | P = 0.0157 | |
| Parent's score on perceived stress scale | 1.01 | 0.94 | 0.98 | 1.05 |
| (0.94–1.10) | (0.87–1.01) | (0.90–1.07) | (0.96–1.15) | |
| P = 0.7378 | P = 0.0980 | P = 0.6772 | P = 0.2563 |
Values shown are odds ratios (with confidence intervals in parentheses) and corresponding P values.
Defined as ever having a physician's diagnosis of asthma and wheeze or whistling in the chest in the previous 12 months.
Defined as ever having a physician's diagnosis of allergic rhinitis or conjunctivitis.
Defined as a doctor's office or clinic visit for asthma in the previous 12 months.
Defined as medication use for asthma in the previous 12 months.
Table 3 presents a comparison of asthma and allergy outcomes for children with and without a reported history of abuse in the previous year. Children with a history of abuse had higher frequencies of all outcomes of interest than those without a history of abuse. The results of multivariable analyses of the association between abuse and asthma and allergic rhinitis are shown in Table 4. After adjusting for relevant covariates, history of abuse was associated with an approximate doubling of the odds of current asthma, health care use for asthma, medication use for asthma, and allergic rhinitis. There was no modification of the effect of abuse on any of the outcomes by selected variables (sex, age, or parental history of asthma) (data not shown). However, we had limited statistical power to detect any effect modification.
TABLE 3.
ASTHMA/ALLERGY OUTCOMES IN CHILDREN WITH REPORTED HISTORY OF ABUSE COMPARED WITH CHILDREN WITHOUT HISTORY OF ABUSE
| Outcome Variable | Children with Abuse History in the Previous Year, n (%) | Children without Abuse History in the Previous Year, n (%) |
|---|---|---|
| Diagnosed with asthma by physician | 38/75 (50.7) | 436/1,118 (39) |
| Wheeze/whistling in chest during past 12 mo | 18/75 (24) | 149/1,118 (13.3) |
| Current asthma | 15/75 (20) | 128/1,117 (11.5) |
| Diagnosed with allergic rhinitis/conjunctivitis by physician | 27/75 (36) | 292/1,118 (26.1) |
| Child brought to physician's office/clinic for asthma in past 12 mo | 10/75 (13.3) | 102/1,119 (9.1) |
| Taken prescription medication for asthma in past 12 mo (among patients with asthma only) | 24/38 (63.1) | 200/436 (45.9) |
TABLE 4.
MULTIVARIABLE ANALYSIS OF THE RELATION BETWEEN REPORTED ABUSE AND ASTHMA/ALLERGY OUTCOMES
| Variable | Current Asthma | Lifetime Allergic Rhinitis | Health Care Use for Asthma | Medication Use for Asthma (patients with asthma only) |
|---|---|---|---|---|
| Abuse | 2.52 | 2.09 | 1.95 | 2.35 |
| (1.27–5.00) | (1.05–4.16) | (0.96–3.96) | (1.05–5.26) | |
| P = 0.0085 | P = 0.0354 | P = 0.0650 | P = 0.0377 | |
| Maternal history of asthma | 3.05 | 1.66 | 2.25 | 1.78 |
| (1.80–5.18) | (1.1–2.34) | (1.29–3.94) | (1.02–3.10) | |
| P = 0.0001 | P = 0.0038 | P = 0.0048 | P = 0.0415 | |
| Paternal history of asthma | 1.80 | 2.23 | 2.27 | |
| (0.97–3.34) | (1.27–3.91) | (1.30–3.98) | ||
| P = 0.0615 | P = 0.0055 | P = 0.0046 | ||
| Age | 0.92 | 0.87 | 0.88 | |
| (0.85–1.00) | (0.82–0.93) | (0.81–0.95) | ||
| P = 0.0638 | P = 0.0001 | P = 0.0016 | ||
| Family income <$18,000/yr | 0.64 | 0.67 | ||
| (0.42–0.96) | (0.45–1.95) | |||
| P = 0.0335 | P = 0.0258 | |||
| Family received public assistance in past year | 0.62 | |||
| (0.41–0.95) | ||||
| P = 0.0276 |
Values shown are odds ratios (with confidence intervals in parentheses) and corresponding P values.
DISCUSSION
In this population-based study of island Puerto Rican children, exposure to high levels of psychosocial stressors (stressful life events, community violence, and child abuse) and current asthma were common. Although stressful life events and community violence were not associated with current asthma or related outcomes, a history of child abuse was associated with an approximately twofold increase in the odds of current asthma and lifetime allergic rhinitis. To our knowledge, this is the first report of a direct association between child abuse and asthma and asthma-related outcomes.
In contrast to our findings, several studies have shown an association between neighborhood violence and asthma. Among Scottish children, a stressful life event increased the risk of an asthma attack (adjusted odds ratio [OR] at 4–6 wk, 2.2; 95% confidence interval [CI], 1.3–3.6). The risk of asthma was further increased when a stressful event was superimposed on chronic exposure to life stress (adjusted OR, 3.0; 95% CI, 1.2–7.4) (24). Exposure to violence may be a specific stressor that contributes to the pathogenesis of asthma among families living in poverty (8). Among 851 low-income children, increased exposure to community violence was associated with a higher number of daytime and nighttime asthma symptoms (7). In a birth cohort study, violence exposure was associated with an increased risk of wheezing at age 2–3 years (25).
One explanation for the discrepancy between our findings and those published in the literature may be the relative impact of the stressors we measured on the Puerto Rican children in this study. Current evidence suggests that it is not simply exposure to a particular stressor but the psychological response to that stressor that predicts physical health outcomes. Among 160 low-income children in Michigan, symptoms of PTSD were associated with a diagnosis of asthma (16). In a telephone survey of patients with asthma in lower Manhattan 5 to 9 weeks after the September 11th attacks, those who experienced a peri-event panic attack, had PTSD symptoms related to the attack, or had two or more life stressors during the year before the attack were more likely to report increased severity of asthma symptoms. In models adjusted for SES and difficulty breathing because of smoke and debris, having two or more life stressors was associated with increased asthma severity (9). In a separate survey 6 to 9 months after the attacks, PTSD was associated with increased symptom severity, unscheduled physician visits, and emergency department visits among adults with asthma (26). Our finding that child abuse was the only stress exposure with a significant association to asthma is consistent with the notion that emotional distress is a mediator on the causal pathway between a stressor and adverse health outcomes, because abuse victims are at higher risk for a traumatic stress response than children who witness isolated episodes of neighborhood violence or have exposure to other stressors that may not be as traumatic as physical or sexual abuse (27). Because our data only indicate an association, it is possible that childhood asthma preceded abuse, or vice versa. However, it is plausible that childhood abuse increases the severity of asthma, thus leading to increased medication use and an increased risk of asthma exacerbations.
Puerto Rican culture itself may have protective features that contribute to the lack of association between community violence and asthma in children seen in prior studies (7). Previous data suggest that island Puerto Rican children are more likely to come from families in which the parents are married, to have mothers who have completed at least a high school education, and to have better relationships with their parents than Puerto Rican children living in the U.S. mainland (5, 17). Certain goals within the Latino culture, including familismo (unity of the family) (28), respeto (harmonious interpersonal relationships and respect for the family) (29), and simpatía (the need for politeness and respect in relationships) (30), place an emphasis on social supports that may buffer the effects of poverty and community violence experienced by children in Puerto Rico. Because of these protective factors, it may be that a stressor has to be chronic or traumatic to have a significant impact on a Puerto Rican child's physical health.
One potential mechanism that may explain the association between abuse and asthma in this study population is alterations in the hypothalamic–pituitary–adrenal (HPA) axis. Studies have shown an association between decreased levels of endogenous cortisol and asthma (31, 32). Patients with asthma exhibit a reduced glucocorticoid response to stress, which may result in decreased suppression of inflammation in the airway, leading to increased airway responsiveness and airflow obstruction (33–35). Several studies have shown altered HPA axis responses among child victims and adult survivors of child abuse, especially among those with PTSD. Newport and colleagues administered dexamethasone suppression tests to women with a history of child abuse who did and did not have concomitant depression and/or PTSD and control subjects without a history of abuse. Survivors of abuse who had depression and/or PTSD exhibited greater cortisol suppression than did women with a history of abuse but no psychiatric illness, or women without an abuse history (36). Similar results were found in a study of hospitalized adolescents. Adolescents hospitalized with PTSD from sexual abuse had lower adrenocorticotropic hormone (ACTH) and cortisol levels after a dexamethasone suppression test compared with control adolescents who were hospitalized for other reasons (37).
In unadjusted and adjusted analyses, low SES (as measured by income and receipt of public assistance) was associated with decreased odds of allergic rhinitis. This finding is consistent with other studies of populations both within and outside the United States. Using the data from the International Study of Asthma and Allergies in Childhood (ISAAC), researchers in Cape Town, South Africa, found a positive association between higher SES and the prevalence of allergic rhinitis (38). An ISAAC study in Brazil also found a higher prevalence of physician-diagnosed rhinitis among those with higher versus lower SES (defined as “purchasing power”) (39). In a population-based study of 1.2 million Swedish males (ages 17 to 20 yr) conducted over three decades, low SES was associated with reduced odds of allergic rhinitis among those born in the earliest cohorts (OR, 0.84; 95% CI, 0.82–0.85). Of note, the effect of SES on risk of allergic rhinitis seemed to decrease among cohorts born in later years (40). In a study of United States adults from California, education level was positively associated with self-reported hay fever such that women with a postgraduate education had 30% higher odds of hay fever, and men with a postgraduate education had 60% higher odds of hay fever (41). Because we lack data on objective markers of atopy (e.g., allergy skin tests), we cannot establish whether the observed inverse association between low SES and allergic rhinitis in Puerto Rican children is due to underdiagnosis of allergic rhinitis (e.g., because of inadequate access to health care) or a truly reduced prevalence of this disease among study participants.
We recognize several limitations of our study. First, our study is cross-sectional. Thus, a plausible alternative explanation for our findings is that Puerto Rican children with asthma are more likely to be victims of abuse than those who are healthy. Regardless of the cause(s) of the observed association, our findings highlight the importance of both screening for illnesses such as asthma in children who are abused and of being aware of the possibility of abuse in children with asthma. Second, our outcomes and exposures are based on self-report. With regard to asthma outcomes, the question “Has a doctor ever diagnosed your child with asthma” has been used and validated in several studies (42), and the question “Has your child had wheezing/whistling in the chest in the past 12 months” is taken directly from the previously validated ISAAC questionnaire (43, 44), which has been used in 56 countries (45) and has been validated in Spanish (46). To facilitate a more rigorous estimate of asthma among our study population, we chose to examine “current asthma,” defined as having both a physician's diagnosis of asthma and asthma symptoms during the previous year. Although a history of child abuse was ascertained separately from children and their caregivers, it is likely that these results underestimate the prevalence of abuse among Puerto Rican children and adolescents because the majority of perpetrators of child abuse are the child's parents. Despite these conservative estimates of both outcomes and exposures, a significant positive association was still detected between child abuse and asthma. Third, our study population was limited to island Puerto Rican children, and thus our findings—especially with regard to the lack of association between life stressors, community violence, and asthma—may not be generalizable to children of different cultural backgrounds.
In summary, we have shown for the first time that physical or sexual abuse was associated with asthma and asthma morbidity among Puerto Rican children. Our findings suggest the need for further study of the mechanisms to explain this association. This study also highlights the importance of screening for otherwise unforeseen physical health problems—specifically asthma—among victims of childhood abuse, and to be aware of the possibility of physical or sexual abuse among children with asthma.
Supplementary Material
Supported by the National Institute of Mental Health through grant R01 MH56401 “Antisocial Behaviors in US and Island Puerto Rican Youth” (The Boricua Youth Study) (H.R.B., principal investigator), by the National Center for Minority Health and Health Disparities grant P60 MD002261-01 (to G.J.C.), and by the National Heart, Lung, and Blood Institute grants 5 T32 HL07424 (to R.T.C.), and HL04370 and HL073373 (to J.C.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200711-1629OC on June 12, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma: United States, 1980–1999. MMWR Surveill Summ 2002;51:1–13. [PubMed] [Google Scholar]
- 2.Lara M, Akinbami L, Flores G, Morgenstern H. Heterogeneity of childhood asthma among Hispanic children: Puerto Rican children bear a disproportionate burden. Pediatrics 2006;117:43–53. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Asthma prevalence and control characteristics by race/ethnicity: United States, 2002. MMWR Morb Mortal Wkly Rep 2004;53:145–148. [PubMed] [Google Scholar]
- 4.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med 2000;161:504–509. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RT, Canino GJ, Bird HR, Shen S, Rosner BA, Celedon JC. Area of residence, birthplace, and asthma in Puerto Rican children. Chest 2007;131:1331–1338. [DOI] [PubMed] [Google Scholar]
- 6.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol 2005;5:23–29. [DOI] [PubMed] [Google Scholar]
- 7.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, Gold DR. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health 2004;94:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright RJ, Steinbach SF. Violence: an unrecognized environmental exposure that may contribute to greater asthma morbidity in high risk inner-city populations. Environ Health Perspect 2001;109:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center–Manhattan, New York, 2001. MMWR Morb Mortal Wkly Rep 2002;51:781–784. [PubMed] [Google Scholar]
- 10.Rietveld S, Everaerd W, Creer TL. Stress-induced asthma: a review of research and potential mechanisms. Clin Exp Allergy 2000;30:1058–1066. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Taboas A, Canino G, Wang MQ, Garcia P, Bravo M. Prevalence and victimization correlates of pathological dissociation in a community sample of youths. J Trauma Stress 2006;19:439–448. [DOI] [PubMed] [Google Scholar]
- 12.Purugganan OH, Stein RE, Silver EJ, Benenson BS. Exposure to violence among urban school-aged children: is it only on television? Pediatrics 2000;106(4, Suppl):949–953. [PubMed] [Google Scholar]
- 13.Vermeiren R, Schwab-Stone M, Deboutte D, Leckman PE, Ruchkin V. Violence exposure and substance use in adolescents: findings from three countries. Pediatrics 2003;111:535–540. [DOI] [PubMed] [Google Scholar]
- 14.Galea S, Vlahov D, Tracy M, Hoover DR, Resnick H, Kilpatrick D. Hispanic ethnicity and post-traumatic stress disorder after a disaster: evidence from a general population survey after September 11, 2001. Ann Epidemiol 2004;14:520–531. [DOI] [PubMed] [Google Scholar]
- 15.Ortega AN, Rosenheck R. Posttraumatic stress disorder among Hispanic Vietnam veterans. Am J Psychiatry 2000;157:615–619. [DOI] [PubMed] [Google Scholar]
- 16.Graham-Bermann SA, Seng J. Violence exposure and traumatic stress symptoms as additional predictors of health problems in high-risk children. J Pediatr 2005;146:349–354. [DOI] [PubMed] [Google Scholar]
- 17.Bird HR, Canino GJ, Davies M, Duarte CS, Febo V, Ramirez R, Hoven C, Wicks J, Musa G, Loeber R. A study of disruptive behavior disorders in Puerto Rican youth: I. Background, design, and survey methods. J Am Acad Child Adolesc Psychiatry 2006;45:1032–1041. [DOI] [PubMed] [Google Scholar]
- 18.Richters JE, Martinez P. The NIMH community violence project: I. Children as victims of and witnesses to violence. Psychiatry 1993;56:7–21. [DOI] [PubMed] [Google Scholar]
- 19.Finkelhor D, Dziuba-Leatherman J. Children as victims of violence: a national survey. Pediatrics 1994;94:413–420. [PubMed] [Google Scholar]
- 20.Johnson JH, McCutcheon SM. Assessing life stress in older children and adolescents: preliminary findings with the life events checklist. In: Sarason IG, Speilberger CD, editors. Stress and anxiety. Washington, DC: Hemisphere; 1980. pp. 111–125.
- 21.Goodman LA, Corcoran C, Turner K, Yuan N, Green BL. Assessing traumatic event exposure: general issues and preliminary findings for the stressful life events screening questionnaire. J Trauma Stress 1998;11:521–542. [DOI] [PubMed] [Google Scholar]
- 22.American FactFinder; U.S. Census Bureau [Internet]. United States–data sets–American FactFinder. 2006. American Community Survey [includes Puerto Rico Community Survey]. Washington: The Bureau [accessed 2008 Jul 28]. Available from: http://factfinder.census.gov/servlet/DatasetMainPageServlet?_program=ACS&_submenuId=datasets_3&_lang=en&_ts=
- 23.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park, CA: Sage; 1988. pp. 31–67.
- 24.Sandberg S, Paton JY, Ahola S, McCann DC, McGuinness D, Hillary CR, Oja H. The role of acute and chronic stress in asthma attacks in children. Lancet 2000;356:982–987. [DOI] [PubMed] [Google Scholar]
- 25.Berz JB, Carter AS, Wagmiller RL, Horwitz SM, Murdock KK, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. J Pediatr Psychol 2006;32:154–166. [DOI] [PubMed] [Google Scholar]
- 26.Fagan J, Galea S, Ahern J, Bonner S, Vlahov D. Relationship of self-reported asthma severity and urgent health care utilization to psychological sequelae of the September 11, 2001. terrorist attacks on the World Trade Center among New York City area residents. Psychosom Med 2003;65:993–996. [DOI] [PubMed] [Google Scholar]
- 27.Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Arch Gen Psychiatry 2007;64:577–584. [DOI] [PubMed] [Google Scholar]
- 28.Zayas LH. Childrearing, social stress, and child abuse: clinical considerations with Hispanic families. J Soc Distress Homeless 1992;1:291–309. [Google Scholar]
- 29.Halgunseth LC, Ispa JM, Rudy D. Parental control in Latino families: an integrated review of the literature. Child Dev 2006;77:1282–1297. [DOI] [PubMed] [Google Scholar]
- 30.Marin G. AIDS prevention among Hispanics: needs, risk behaviors, and cultural values. Public Health Rep 1989;104:411–415. [PMC free article] [PubMed] [Google Scholar]
- 31.Kauffmann F, Guiochon-Mantel A, Neukirch F. Is low endogenous cortisol a risk factor for asthma? Am J Respir Crit Care Med 1999;160:1428. [DOI] [PubMed] [Google Scholar]
- 32.Landstra AM, Postma DS, Boezen HM, van Aalderen WM. Role of serum cortisol levels in children with asthma. Am J Respir Crit Care Med 2002;165:708–712. [DOI] [PubMed] [Google Scholar]
- 33.Buske-Kirschbaum A, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: a general feature of atopic disease? Psychosom Med 2003;65:806–810. [DOI] [PubMed] [Google Scholar]
- 34.Wamboldt MZ, Laudenslager M, Wamboldt FS, Kelsay K, Hewitt J. Adolescents with atopic disorders have an attenuated cortisol response to laboratory stress. J Allergy Clin Immunol 2003;111:509–514. [DOI] [PubMed] [Google Scholar]
- 35.Ball TM, Anderson D, Minto J, Halonen M. Cortisol circadian rhythms and stress responses in infants at risk of allergic disease. J Allergy Clin Immunol 2006;117:306–311. [DOI] [PubMed] [Google Scholar]
- 36.Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry 2004;55:10–20. [DOI] [PubMed] [Google Scholar]
- 37.Duval F, Crocq MA, Guillon MS, Mokrani MC, Monreal J, Bailey P, Macher JP. Increased adrenocorticotropin suppression following dexamethasone administration in sexually abused adolescents with posttraumatic stress disorder. Psychoneuroendocrinology 2004;29:1281–1289. [DOI] [PubMed] [Google Scholar]
- 38.Mercer MJ, Joubert G, Ehrlich RI, Nelson H, Poyser MA, Puterman A, Weinberg EG. Socioeconomic status and prevalence of allergic rhinitis and atopic eczema symptoms in young adolescents. Pediatr Allergy Immunol 2004;15:234–241. [DOI] [PubMed] [Google Scholar]
- 39.Borges WG, Burns DA, Felizola ML, Oliveira BA, Hamu CS, Freitas VC. Prevalence of allergic rhinitis among adolescents from Distrito Federal, Brazil: comparison between ISAAC phases I and III. J Pediatr (Rio J) 2006;82:137–143. [DOI] [PubMed] [Google Scholar]
- 40.Braback L, Hjern A, Rasmussen F. Social class in asthma and allergic rhinitis: a national cohort study over three decades. Eur Respir J 2005;26:1064–1068. [DOI] [PubMed] [Google Scholar]
- 41.Chen JT, Krieger N, Van Den Eeden SK, Quesenberry CP. Different slopes for different folks: socioeconomic and racial/ethnic disparities in asthma and hay fever among 173,859 US men and women. Environ Health Perspect 2002;110:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunekreef B, Groot B, Rijcken B, Hoek G, Steenbekkers A, de Boer A. Reproducibility of childhood respiratory symptom questions. Eur Respir J 1992;5:930–935. [PubMed] [Google Scholar]
- 43.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995;8:483–491. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins MA, Clarke JR, Carlin JB, Robertson CF, Hopper JL, Dalton MF, Holst DP, Choi K, Giles GG. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol 1996;25:609–616. [DOI] [PubMed] [Google Scholar]
- 45.Asher MI, Weiland SK. The International Study of Asthma and Allergies in Childhood (ISAAC). ISAAC Steering Committee. Clin Exp Allergy 1998;28(Suppl 5):52–66. [Discussion, pp. 90–51.] [DOI] [PubMed] [Google Scholar]
- 46.Mata Fernandez C, Fernandez-Benitez M, Perez Miranda M, Guillen Grima F. Validation of the Spanish version of the phase III ISAAC questionnaire on asthma. J Investig Allergol Clin Immunol 2005;15:201–210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.