Abstract
Rationale: The predictive value of longitudinal change in BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index has received limited attention. We hypothesized that decrease in a modified BODE (mBODE) would predict survival in National Emphysema Treatment Trial (NETT) patients.
Objectives: To determine how the mBODE score changes in patients with lung volume reduction surgery versus medical therapy and correlations with survival.
Methods: Clinical data were recorded using standardized instruments. The mBODE was calculated and patient-specific mBODE trajectories during 6, 12, and 24 months of follow-up were estimated using separate regressions for each patient. Patients were classified as having decreasing, stable, increasing, or missing mBODE based on their absolute change from baseline. The predictive ability of mBODE change on survival was assessed using multivariate Cox regression models. The index of concordance was used to directly compare the predictive ability of mBODE and its separate components.
Measurements and Main Results: The entire cohort (610 treated medically and 608 treated surgically) was characterized by severe airflow obstruction, moderate breathlessness, and increased mBODE at baseline. A wide distribution of change in mBODE was seen at follow-up. An increase in mBODE of more than 1 point was associated with increased mortality in surgically and medically treated patients. Surgically treated patients were less likely to experience death or an increase greater than 1 in mBODE. Indices of concordance showed that mBODE change predicted survival better than its separate components.
Conclusions: The mBODE demonstrates short- and intermediate-term responsiveness to intervention in severe chronic obstructive pulmonary disease. Increase in mBODE of more than 1 point from baseline to 6, 12, and 24 months of follow-up was predictive of subsequent mortality. Change in mBODE may prove a good surrogate measure of survival in therapeutic trials in severe chronic obstructive pulmonary disease.
Clinical trial registered with www.clinicaltrials.gov (NCT 00000606).
Keywords: chronic obstructive pulmonary disease, survival, multidimensional index
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The BODE index has prognostic value with respect to chronic obstructive pulmonary disease mortality, although the importance of change in BODE has received limited attention.
What This Study Adds to the Field
In a cohort of patients with severe emphysema, an increase in modified BODE of more than 1 is associated with increased mortality. Change in modified BODE also predicted survival better than its separate components.
Modifying disease in chronic obstructive pulmonary disease (COPD) has become an increasingly feasible therapeutic option in patients with COPD. Decreasing mortality is particularly important in this disorder, which has exhibited a rising mortality over the past several decades (1). Clinical treatments that seek to improve mortality in COPD require large populations studied for long periods. A recently completed pharmacologic intervention resulted in a modest trend to improved survival despite more than 6,000 patients randomized to four treatment arms (2). It is very desirable to have a “marker” that can be used as a surrogate for survival to circumvent the need for large or prolonged trials. One such measure, the multidimensional BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index, has recently been shown to predict survival in cohort studies of COPD (3, 4). However, for a surrogate outcome to be valid, two other conditions should be satisfied. First, the measure should be responsive to treatments that improve survival. Second, the measure should show a dose–response relationship—that is, those patients who show the greatest improvement have the greatest increase in survival and those who show the least improvement (or greatest worsening) should have the greatest decrease in survival. The BODE index has partially satisfied the first of these conditions in an uncontrolled study of rehabilitation (5) and in three uncontrolled studies of lung volume reduction surgery (LVRS) (6–8). Moreover, those patients who demonstrated greater decreases in BODE index tended to have the best survival. Because these studies were uncontrolled, however, it is possible that these results could have reflected, in part, the natural variation in disease over time rather than the effect of therapeutic interventions alone.
The National Emphysema Treatment Trial (NETT) provides an ideal dataset to examine the relationship of changes in BODE index to survival insofar as it compares an active intervention (LVRS) with maximal medical therapy in a randomized trial of patients with severe COPD. The modified BODE (mBODE) has previously been shown to be a good predictor of survival in the NETT population (4). We hypothesized that the NETT population would allow a clear definition of the natural history of BODE, and that the wide distribution of longitudinal changes in BODE would allow a more clearly defined assessment of the magnitude of change that reliably predicts subsequent mortality. In the present analysis, the predefined specific goals were to answer the following questions:
Does the mBODE score improve with LVRS more than maximal medical therapy?
Does an improvement or worsening of mBODE correlate with improved or worsening survival within each treatment group?
Does the magnitude of change in BODE predict the magnitude of improvement in survival?
METHODS
Patient Selection
The study group consisted of all patients randomized to medical or surgical therapy at 17 NETT clinics (9). The design and methods of the trial have been previously detailed (9, 10). All patients provided written, informed consent, and the institutional review board at each clinic approved the study. Baseline measurements were completed after pulmonary rehabilitation and before randomization. All patients completed 16 sessions of pulmonary rehabilitation before randomization and 6 sessions of rehabilitation postrandomization. Some patients in both treatment arms of the study received further maintenance pulmonary rehabilitation either through outpatient or home-based pulmonary rehabilitation programs. Maximal medical therapy for patients also included medical therapies such as bronchodilators, inhaled corticosteroids, and close monitoring of abstinence from tobacco use.
Clinical Assessment
Demographic data and smoking and medical history were collected by patient interview using standardized instruments. Dyspnea was quantified using the University of California San Diego Shortness of Breath Questionnaire (UCSD SOBQ) (11).
Physiologic Testing
Patients underwent spirometry and plethysmographic lung volume measurement after the administration of albuterol; diffusing capacity, respiratory pressures, and arterial blood gases were also measured. The protocol used for six-minute-walk testing (6MWT) has been described in detail and provided the maximal distance walked (12).
Modified BODE
The BODE index is an 11-point composite score (0 through 10) in which higher scores indicate poorer outcomes (3). We modified the original BODE by using the UCSD SOBQ as the dyspnea measure because the Medical Research Council dyspnea scale was not used in NETT; this modified BODE demonstrated similar predictive ability in patients with severe emphysema (4).
Statistical Analysis
Means of mBODE over time were estimated and tested using the mixed models framework, which adjusts for bias due to patients being lost to attrition over time. Patient-specific mBODE trajectories were estimated using individual linear regressions applied to data available from randomization to 6, 12, or 24 months. For patients surviving beyond each of these times the estimated absolute change in mBODE from baseline was obtained. Vital status was assessed by review of records at each institution and supplemented by the use of the Social Security Death Master File ensuring complete capture of data.
On the basis of the absolute change in mBODE at 6, 12, and 24 months elapsed since randomization and available data, patients were classified into four distinct groups at each time point: (1) mBODE decreased by greater than 1 point, (2) mBODE remains stable, (3) mBODE increased greater than 1 point, (4) data missing for mBODE calculation. Survival was estimated both within each treatment arm and in the overall cohort, on the basis of multivariate Cox regression models, while adjusting for sex, ethnicity, baseline age, and baseline mBODE. To assess the Cox model fit and to directly compare mBODE mortality predictive power with that of each of its components, the index of concordance was computed. This measures model fit by calculating the percentage of times observed mortality pairs are correctly ordered by the model. Higher values are reflective of increasingly better model fit.
RESULTS
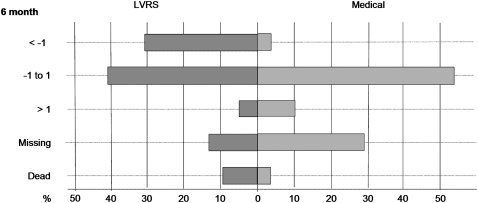
The entire cohort was characterized by elderly patients with severe airflow obstruction, moderate breathlessness, and increased mBODE (Table 1). At baseline, there were no differences in mBODE between the medical and surgical arms. Tables 2 and 3 give insight into the magnitude of mBODE differences between the two treatment arms over time and formally compare the two arms based on mixed model results that adjust for attrition of repeated measures. As shown in Table 3, surviving surgically treated (LVRS) patients presented negative slopes at 6, 12, and 24 months postrandomization on average, whereas medically treated patients had significantly higher (P value always less than 0.001) positive mBODE slopes at each time mark postrandomization. As such, LVRS patients showed a consistent pattern of mBODE decrease (improvement), whereas medically treated patients exhibited significant increases (deterioration) in mBODE. The distribution of change in mBODE among survivors at 6 (Figure 1), 12 (Figure E1A of the online supplement), and 24 months (Figure E1B) is illustrated by plot. It is evident that the entire cohort exhibits a wide range of scores at each landmark time, with negative changes in mBODE (improvements) more frequent in surgically treated patients. Significant missing data were seen, particularly in medically treated patients. Among those who survived to 6, 12, and 24 months after randomization (Table E1), subtle differences were observed in component variables of the mBODE at baseline in both the medical and surgical groups, making formal treatment comparisons among survivors over time inappropriate. When patients were grouped by survival duration, there was a clear gradient in baseline FEV1 and baseline dyspnea, with those who survived 24 months having higher FEV1 and lower dyspnea at baseline than those who did not survive as long.
TABLE 1.
PATIENT BASELINE CHARACTERISTICS
| Entire Cohort (n) | Medical (n) | LVRS (n) | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | 0.041* | |||||||||
| Male | 746 | 391 | 355 | |||||||
| Female | 472 | 219 | 253 | |||||||
| Total | 1,218 | 610 | 608 | |||||||
| Ethnicity | 0.751 | |||||||||
| White | 1,156 | 575 | 581 | |||||||
| African American | 42 | 23 | 19 | |||||||
| Hispanic | 5 | 3 | 2 | |||||||
| Other | 15 | 9 | 6 | |||||||
| Total | 1,218 | 610 | 608 | |||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |||||
| Age, yr | 1,218 | 67.14 (6.12) | 610 | 67.22 (5.90) | 608 | 67.05 (6.34) | 0.619 | |||
| FEV1% predicted | 1,218 | 26.75 (7.20) | 610 | 26.68 (7.03) | 608 | 26.83 (7.37) | 0.725 | |||
| 6MWT distance, ft | 1,218 | 1,217.70 (314.17) | 610 | 1218.9 (315.99) | 608 | 1216.5 (312.58) | 0.891 | |||
| UCSD SOBQ | 1,218 | 62.48 (18.33) | 610 | 63.39 (18.55) | 608 | 61.56 (18.07) | 0.082 | |||
| BMI | 1,217 | 24.6 (3.6) | 609 | 24.7 (3.5) | 608 | 24.5 (3.7) | 0.294 | |||
| mBODE | 1,217 | 5.02 (1.67) | 609 | 5.05 (1.68) | 608 | 5.00 (1.66) | 0.607 | |||
Definition of abbreviations: BMI = body mass index; LVRS = lung volume reduction surgery; mBODE = modified BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index; 6MWT = six-minute-walk test; UCSD SOBQ = University of California San Diego Shortness of Breath Questionnaire.
χ2 test comparing sex differences in medical and LVRS treatment arms: P value < 0.05.
TABLE 2.
MEAN (SD) mBODE INDEX IN MEDICALLY TREATED AND LUNG VOLUME REDUCTION SURGERY PATIENT GROUPS AT 6, 12, AND 24 MONTHS POSTRANDOMIZATION
| Time Since Randomization | Entire Cohort
|
Medical Arm
|
LVRS Arm
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | T Value | df | P Value | |
| 6 Months | 881 | 4.60 (1.44) | 412 | 5.07 (1.41) | 469 | 4.18 (1.33) | 9.58 | 850 | <0.001 |
| 12 Months | 711 | 4.43 (1.51) | 327 | 5.13 (1.44) | 384 | 3.84 (1.30) | 12.46 | 664 | <0.001 |
| 24 Months | 587 | 4.12 (1.65) | 260 | 5.23 (1.35) | 327 | 3.24 (1.29) | 18.15 | 543 | <0.001 |
Definition of abbreviations: df = degrees of freedom; LVRS = lung volume reduction surgery; mBODE = modified BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index.
Results are based on mixed model adjusting for patient sex, ethnicity, baseline age, and baseline mBODE.
TABLE 3.
MEAN mBODE SLOPES IN UNITS/TIME PERIOD (SD) IN MEDICALLY TREATED AND LUNG VOLUME REDUCTION SURGERY PATIENT GROUPS AT 6, 12, AND 24 MONTHS POSTRANDOMIZATION
| Time Since Randomization | Entire Cohort
|
Medical Arm
|
LVRS Arm
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | T Value | df | P Value | |
| 6 Months | 881 | −0.11 (0.35) | 412 | 0.14 (0.21) | 469 | −0.33 (0.32) | 26.07 | 813 | <0.001 |
| 12 Months | 711 | −0.30 (0.86) | 327 | 0.41 (0.44) | 384 | −0.90 (0.66) | 31.48 | 674 | <0.001 |
| 24 Months | 587 | −0.23 (0.62) | 260 | 0.38 (0.22) | 327 | −0.72 (0.33) | 47.80 | 568 | <0.001 |
For definition of abbreviations, see Table 2.
Results are based on mixed model adjusting for patient sex, ethnicity, baseline mBODE, and baseline age.
Figure 1.
The percentage of patients (x axis) experiencing changes in mBODE (modified Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) (y axis) 6 months after randomization to medical therapy or bilateral lung volume reduction surgery (LVRS). Patients who had died or were not able to provide data to calculate mBODE at each time point are also illustrated.
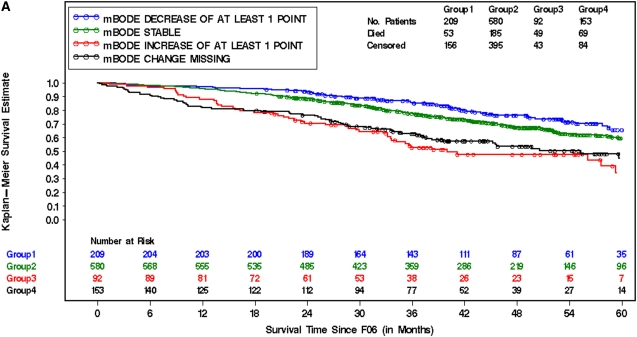
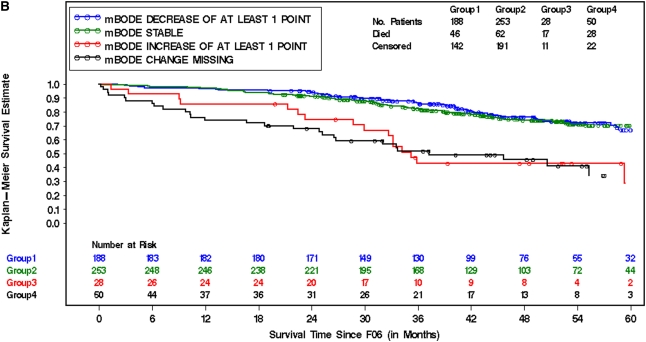
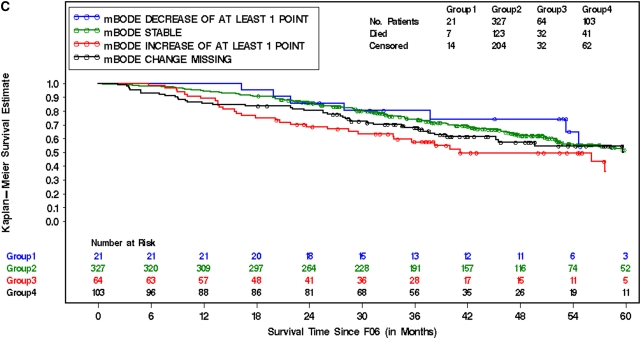
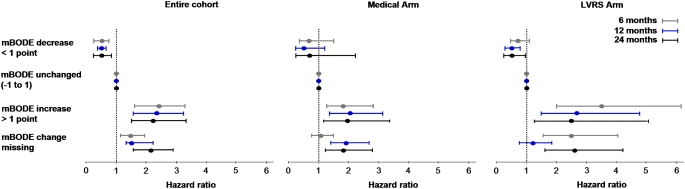
The influence of changes in mBODE on subsequent mortality was assessed by computing individual trajectories on the basis of each patient's available mBODE patient history available at 6 (Figure 2), 12 (Figure E2), and 24 months (Figure E2) postrandomization. The mortality characteristics of the entire cohort (Figure 2A), surgically treated patients (Figure 2B), and medically treated patients (Figure 2C) 6 months after randomization are illustrated in Figure 2 by prior change in mBODE. Similar curves for 12 and 24 months after randomization are illustrated in Figure E2. It is evident that there are distinct groups with significantly different survival experiences in the entire cohort, in surgically treated patients, and in medically treated patients. Figure 3 illustrates how a decrease in mBODE of greater than 1 point at 6 months postrandomization in the entire cohort was associated with a significant decrease in subsequent mortality (hazard ratio [HR], 0.57; 95% confidence interval [CI], 0.41–0.78; P < 0.001), whereas an increase in mBODE of more than 1 point was associated with a significantly increased mortality (HR, 2.35; 95% CI, 1.71–3.23; P < 0.001). Patients with missing data at 6 months experienced an intermediate increase in mortality (HR, 1.45; 95% CI, 1.10–1.92; P = 0.01). Similar results were noted in the entire cohort at 12 and 24 months postrandomization. In the surgically treated cohort, a decrease in mBODE of more than 1 point at 6 months was suggestive of improved subsequent survival (HR, 0.72; 95% CI, 0.48–1.08; P = 0.110), with a similar trend at 12 and 24 months postrandomization becoming statistically significant. Hazards associated with an increase in mBODE of greater than 1 point or missing data were somewhat higher in the surgical arm compared with the medical arm; otherwise, hazards in both treatment arms were ordered very similarly to the overall cohort and were highly statistically significant. An exploratory analysis confirmed that a change of 1 point in mBODE better distributed survival curves than other thresholds, including a 2-point change in mBODE (data not shown).
Figure 2.
Kaplan-Meier survival curves (starting at 6 months after randomization [F06]) for the entire cohort (A), surgically treated patients (lung volume reduction surgery [LVRS] arm) (B), and medically treated patients (C) in patients who survived and were able to provide data to calculate mBODE (modified Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) 6 (F06) months after randomization. Survival curves are segregated by groups who experienced a decrease in mBODE > 1 (blue), a rise in mBODE > 1 (red), no change in mBODE (−1 to 1 point change) (green), or missing data (black) from baseline to the starting time point. (A) Log rank = 46.9; degrees of freedom = 3; P value < 0.01. (B) Log rank = 53; degrees of freedom = 3; P value < 0.01. (C) Log rank = 7.58; degrees of freedom = 3; P value ≤ 0.06.
Figure 3.
Multivariate Cox survival models comparing the predictive value of change in mBODE (modified Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) at 6, 12, and 24 months postrandomization, adjusted for sex, ethnicity, baseline age, and baseline mBODE. LVRS = lung volume reduction surgery.
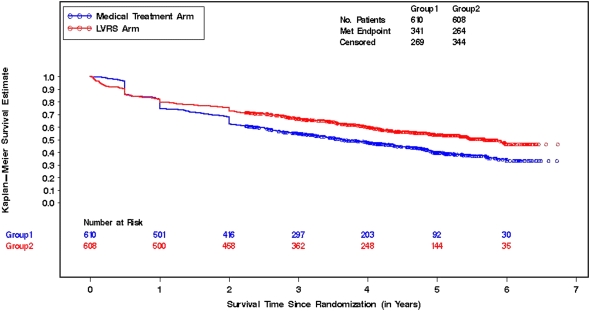
Figure 4 illustrates event-free rates associated with the composite outcome of death or an increase in mBODE of more than 1 point. The median time to this composite event was significantly shorter in medically treated patients (3.65 yr; 95% CI, 3.21–4.37 yr) compared with LVRS-treated patients (5.68 yr; 95% CI, 4.93 yr–not available). Table 4 demonstrates indices of concordance for mBODE, 6MWT, and UCSD SOBQ. The index of concordance allows us to quantify the predictive ability of a survival model. As can be seen from the table, mBODE exhibits indices of concordance consistently higher than those obtained for FEV1, 6MWT distance, and the UCSD SOBQ alone, indicating that the predictive ability of the mBODE for survival is better than any of the individual components. On the other hand, it appears that change in UCSD SOBQ likely accounted for much of the mBODE's predictive ability.
Figure 4.
Kaplan-Meier curves comparing a composite outcome of survival or increase in mBODE (modified Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) greater than 1 point between medical and surgical treatment groups. The red line reflects lung volume reduction surgery (LVRS)–treated patients, whereas the blue line reflects medically treated patients.
TABLE 4.
CONCORDANCE INDICES FOR (CHANGE IN) mBODE INDEX AND THREE OF ITS COMPONENTS (EXCEPT FOR BODY MASS INDEX) BY STUDY ARM AND FOR THE ENTIRE COHORT AT BASELINE AND 6, 12, AND 24 MONTHS POSTRANDOMIZATION
| Time Since Randomization
|
|||
|---|---|---|---|
| Variable | 6 Months | 12 Months | 24 Months |
| Entire cohort | |||
| n | 1,034 | 939 | 819 |
| mBODE | 0.66 | 0.68 | 0.69 |
| FEV1 | 0.58 | 0.59 | 0.61 |
| 6MWD | 0.61 | 0.63 | 0.64 |
| UCSD SOBQ | 0.64 | 0.65 | 0.65 |
| Medical arm | |||
| n | 515 | 476 | 393 |
| mBODE | 0.64 | 0.68 | 0.67 |
| FEV1 | 0.57 | 0.62 | 0.61 |
| 6MWD | 0.59 | 0.64 | 0.63 |
| UCSD SOBQ | 0.61 | 0.64 | 0.65 |
| LVRS arm | |||
| n | 519 | 463 | 426 |
| mBODE | 0.68 | 0.68 | 0.72 |
| FEV1 | 0.61 | 0.61 | 0.66 |
| 6MWD | 0.65 | 0.61 | 0.67 |
| UCSD SOBQ | 0.67 | 0.65 | 0.65 |
Definition of abbreviations: LVRS = lung volume reduction surgery; mBODE = modified BODE (Body mass index, airflow Obstruction, Dyspnea, and Exercise capacity) index; 6MWT = six-minute-walk test; UCSD SOBQ = University of California San Diego Shortness of Breath Questionnaire.
Indices are calculated based on Cox regression models adjusting for patient sex, ethnicity, and baseline age.
DISCUSSION
Reducing mortality has become increasingly feasible in COPD. Therapeutic trials designed to demonstrate improved survival require large study design with prolonged periods of follow-up. As such, identifying reliable surrogates of survival in COPD has become an important concept. Multidimensional indices, such as the BODE index, evaluated at baseline have been shown to exhibit greater predictive values than the individual components of the index. Recently, three uncontrolled studies have suggested that the BODE index changes after therapeutic intervention and that this change appears to relate to subsequent mortality (5, 6). These studies have been limited by lack of control groups. The current analysis describes longitudinal changes in a highly characterized, large cohort of patients with severe emphysema, half of whom underwent a therapeutic intervention that resulted in significant improvement. As such, we report the following: (1) the mBODE is responsive to longitudinal changes with greater improvement in surgically compared with medically treated patients; (2) a decrease in mBODE of more than 1 point was predictive of lesser mortality in the entire cohort and, particularly, the surgically treated cohort; (3) an increase of mBODE of more than 1 point was predictive of greater mortality, a finding more consistently seen in the medically treated patients; (4) analysis of concordance suggests that the mBODE is more predictive of survival than the changes in its individual components; and (5) short-term changes in a composite endpoint (mBODE increase of >1 point or death) may serve as a valuable surrogate of mortality in future COPD studies.
We confirm that the mBODE changes over 6 to 24 months in patients with severe COPD. Interestingly, the entire cohort illustrated similar numbers of patients who exhibited increases or decreases in mBODE. Importantly, medically treated patients appeared to experience a lesser change in mBODE, with most of the change seen in increasing values, suggesting a worsening score in this multidimensional index. A separate report noted similar, subtle change in BODE in a cohort of patients, some of whom were treated with pulmonary rehabilitation (5). Although the influence of additional pulmonary rehabilitation in the current cohort cannot be directly assessed, our work expands previous data by providing insight into the natural history of longitudinal change in mBODE in a large cohort of patients with severe emphysema treated with maximal medical therapy. Furthermore, these data allow the calculation of the expected natural history of the mBODE in such patients over a 2-year time frame. These data can be used to define expected changes in mBODE in future therapeutic trials that wish to use this composite index as a treatment outcome.
Our data provide a contrast in that surgically treated patients exhibit a wider distribution of changes in scores, with a larger number of patients experiencing decreasing, or improving, scores. These data expand on the original observations of the NETT that confirmed functional improvements in surgically compared with medically treated patients with severe emphysema (9). Two groups have recently presented results of short-term (3 mo) and intermediate-term (12–24 mo) changes in BODE in a small group of patients with emphysema treated with LVRS that document a similar distribution of change, with the majority of patients exhibiting decreases in scores (6–8). Our data extend these observations by illustrating the natural history of change in mBODE over a 2-year period, and are strengthened by having a similar medically treated patient group for comparison. Direct survival comparison of surgically versus medically treated patients by mBODE change is limited by differential mortality over the period of measurements. Nevertheless, the totality of these data provides insight into the potential change in mBODE in patients with severe COPD treated with a therapy that provides marked functional improvements.
Our data provide important insight into how short- and intermediate-term changes in a composite index can be used to predict mortality in a large group of medically and surgically treated patients with severe COPD. In both medically and surgically treated patients, an increase in mBODE was associated with an increased risk of mortality in subsequent follow-up. This was noted as early as 6 months and out to 2 years after randomization to intervention. Furthermore, a greater than 1 point increase in mBODE was associated with a 1.9- to 2.1-fold increase in mortality in medically treated patients and a 2.5- to 3.6-fold increase in surgically treated patients. We also noted that a decrease in mBODE of greater than 1 point after 12 months was associated with improved subsequent mortality only in surgically treated patients. These data expand on results from other groups that suggest that a decrease in BODE is associated with decreased subsequent mortality in surgically treated patients (6) by illustrating that changes in mBODE are predictive of subsequent mortality from as short as 6 months of follow-up to 24 months of follow-up. An important finding of our study is the ability to describe the time to death or to an increase in mBODE in medically and surgically treated patients. These data can be used to construct clinical trials of therapeutic interventions that may improve mortality with a smaller sample size over a period as short as 6 months.
We also provide insight into the operating characteristics of a composite index in contrast to some of the individual components. An analysis of concordance demonstrates that the composite index was better able to predict subsequent mortality in the entire cohort as well as in the medically and surgically treated patients. A higher index of concordance was evident for the composite mBODE index at 6, 12, and 24 months after intervention. Further examination suggests that the majority of change in mBODE was contributed by improved dyspnea. Our data extend the findings of others that have suggested that change in 6MWD over 1 year predicts survival (13) by contrasting change in exercise capacity with longitudinal changes in dyspnea, FEV1, and a composite index incorporating all of these factors.
Limitations of this study come from its highly selective sample population. All subjects were enrolled in NETT, a randomized trial of LVRS for patients with a predominantly emphysematous phenotype. As such, selection bias was imposed by requirements for a highly selected subset of patients with severe COPD. In addition, the mBODE was a modification of the original multidimensional index. On the other hand, the operating characteristic of the mBODE was remarkably similar with regard to prognostic ability (4) to the original index (3). In addition, the predictive ability of a greater than 1-unit change in mBODE was similar to that noted for changes in a broader spectrum of patients with COPD undergoing rehabilitation in a smaller, uncontrolled study (5). Finally, although no patients were lost to follow-up, we are missing data whenever a patient was unwilling or unable to complete any one component of the mBODE. This was particularly evident in medically treated patients. It is difficult to know if this led to any systematic bias in our reported results because the predictive ability of change in mBODE is conditional on the ability of patients to contribute data at the respective time points. Interestingly, we noted that those with missing data experienced a higher mortality than those with no change in mBODE, although those with rising mBODE generally appeared to exhibit even higher mortality.
In summary, we demonstrate that a composite index (mBODE) demonstrates short- and intermediate-term responsiveness to intervention in severe COPD. This was more evident in surgically treated than in medically treated patients. Furthermore, changes in mBODE at 6, 12, and 24 months of follow-up were predictive of subsequent mortality. An increase in mBODE of greater than 1 point proved predictive of mortality. Importantly, the changes in the individual parameters were not as predictive of subsequent survival as individual components. These changes provide support that a short- and intermediate-term change in a multidimensional composite index may be used as a surrogate for survival in clinical trials.
The National Emphysema Treatment Trial (NETT) was supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration), and the Agency for Healthcare Research and Quality. J.L.C. is supported by funding from a Research Enhancement Award Program (REAP) from the Biomedical Laboratory Research and Development Service, Department of Veterans Affairs.
Originally Published in Press as DOI: 10.1164/rccm.200709-1383OC on June 5, 2008
Conflict of Interest Statement: F.J.M. is a consultant for Altana Pharma and has received compensation greater than $10K. He has been a member of several advisory boards, CME committees, and the speaker's bureau for Boehringer Ingelheim (BI) and GlaxoSmithKline (GSK). His total compensation per company is greater than $10K. In addition, F.J.M. is on advisory boards for Dey and Novartis and the speaker's bureau for Sepracor, Schering Plough, Astra, and Pfizer, receiving less than $10K per company. F.J.M. has been an investigator for industry-sponsored studies for GSK, BI, and Actelion. M.K.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.-C.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.L.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.E.G. received speaker's fees of less than $50,000 from GSK, less than $15,000 from BI, less than $10,000 from Pfizer, and less than $5,000 from Schering for 2006. J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.L.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z.M. received $4,000 from AstraZeneca in 2007, $1,500 from BI in 2007, $2,000 from Pfizer in 2006. M.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.R.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Members of the NETT Research Group are as follows: Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, M.D. (Chair); Betsy Ann Bozzarello; Ameena Al-Amin. Clinical centers: Baylor College of Medicine, Houston, TX: Marcia Katz, M.D. (Principal Investigator); Carolyn Wheeler, R.N., B.S.N. (Principal Clinic Coordinator); Elaine Baker, R.R.T., R.P.F.T.; Peter Barnard, Ph.D., R.P.F.T.; Phil Cagle, M.D.; James Carter, M.D.; Sophia Chatziioannou, M.D.; Karla Conejo-Gonzales; Kimberly Dubose, R.R.T.; John Haddad, M.D.; David Hicks, R.R.T., R.P.F.T.; Neal Kleiman, M.D.; Mary Milburn-Barnes, C.R.T.T.; Chinh Nguyen, R.P.F.T.; Michael Reardon, M.D.; Joseph Reeves-Viets, M.D.; Steven Sax, M.D.; Amir Sharafkhaneh, M.D.; Owen Wilson, Ph.D.; Christine Young, P.T.; Rafael Espada, M.D. (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, M.D. (1999–2001); Katherine Hale, M.D. (1998–2000); Everett Hood, R.P.F.T. (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, M.D. (1998–2001); Karen King, R.P.F.T. (1998–1999); Charles Miller III, Ph.D. (1996–1999); Imran Nizami, M.D. (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, M.D. (Co-Principal Investigator 1996–2000); Robert Teague, M.D. (Co-Principal Investigator, 1999–2000); Kedren Williams (1998–1999). Brigham and Women's Hospital, Boston, MA: John Reilly, M.D. (Principal Investigator); David Sugarbaker, M.D. (Co-Principal Investigator); Carol Fanning, R.R.T. (Principal Clinic Coordinator); Simon Body, M.D.; Sabine Duffy, M.D.; Vladmir Formanek, M.D.; Anne Fuhlbrigge, M.D.; Philip Hartigan, M.D.; Sarah Hooper, E.P.; Andetta Hunsaker, M.D.; Francine Jacobson, M.D.; Marilyn Moy, M.D.; Susan Peterson, R.R.T.; Roger Russell, M.D.; Diane Saunders; Scott Swanson, M.D. (Co-Principal Investigator, 1996–2001). Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, M.D. (Principal Investigator); Zab Mohsenifar, M.D. (Co-Principal Investigator); Carol Geaga, R.N. (Principal Clinic Coordinator); Manmohan Biring, M.D.; Susan Clark, R.N., M.N.; Jennifer Cutler, M.D.; Robert Frantz, M.D.; Peter Julien, M.D.; Michael Lewis, M.D.; Jennifer Minkoff-Rau, M.S.W.; Valentina Yegyan, B.S., C.P.F.T.; Milton Joyner, B.A. (1996–2002). Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, M.D. (Principal Investigator); James Stoller, M.D. (Co-Principal Investigator); Yvonne Meli, R.N.C. (Principal Clinic Coordinator); John Apostolakis, M.D.; Darryl Atwell, M.D.; Jeffrey Chapman, M.D.; Pierre DeVilliers, M.D.; Raed Dweik, M.D.; Erik Kraenzler, M.D.; Rosemary Lann, L.I.S.W.; Nancy Kurokawa, R.R.T., C.P.F.T.; Scott Marlow, R.R.T.; Kevin McCarthy, R.C.P.T.; Pricilla McCreight, R.R.T., C.P.F.T.; Atul Mehta, M.D.; Moulay Meziane, M.D.; Omar Minai, M.D.; Mindi Steiger, R.R.T.; Kenneth White, R.P.F.T.; Janet Maurer, M.D. (Principal Investigator, 1996–2001); Terri Durr, R.N. (2000–2001); Charles Hearn, D.O. (1998–2001); Susan Lubell, P.A.-C. (1999–2000); Peter O'Donovan, M.D. (1998–2003); Robert Schilz, D.O. (1998–2002). Columbia University, New York, NY, in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, M.D. (Principal Investigator); Byron Thomashow, M.D. (Co-Principal Investigator); Patricia Jellen, M.S.N., R.N. (Principal Clinic Coordinator); John Austin, M.D.; Matthew Bartels, M.D.; Yahya Berkmen, M.D.; Patricia Berkoski, M.S., R.R.T. (Site Coordinator, Long Island Jewish); Frances Brogan, M.S.N., R.N.; Amy Chong, B.S., C.R.T.; Glenda DeMercado, B.S.N.; Angela DiMango, M.D.; Sandy Do, M.S., P.T.; Bessie Kachulis, M.D.; Arfa Khan, M.D.; Berend Mets, M.D.; Mitchell O'Shea, B.S., R.T., C.P.F.T.; Gregory Pearson, M.D.; Leonard Rossoff, M.D.; Steven Scharf, M.D., Ph.D. (Co-Principal Investigator, 1998–2002); Maria Shiau, M.D.; Paul Simonelli, M.D.; Kim Stavrolakes, M.S., P.T.; Donna Tsang, B.S.; Denise Vilotijevic, M.S., P.T.; Chun Yip, M.D.; Mike Mantinaos, M.D. (1998–2001); Kerri McKeon, B.S., R.R.T., R.N. (1998–1999); Jacqueline Pfeffer, M.P.H., P.T. (1997–2002). Duke University Medical Center, Durham, NC: Neil MacIntyre, M.D. (Principal Investigator); R. Duane Davis, M.D. (Co-Principal Investigator); John Howe, R.N. (Principal Clinic Coordinator); R. Edward Coleman, M.D.; Rebecca Crouch, R.P.T.; Dora Greene; Katherine Grichnik, M.D.; David Harpole, Jr., M.D.; Abby Krichman, R.R.T.; Brian Lawlor, R.R.T.; Holman McAdams, M.D.; John Plankeel, M.D.; Susan Rinaldo-Gallo, M.E.D.; Sheila Shearer, R.R.T.; Jeanne Smith, A.C.S.W.; Mark Stafford-Smith, M.D.; Victor Tapson, M.D.; Mark Steele, M.D. (1998–1999); Jennifer Norten, M.D. (1998–1999). Mayo Foundation, Rochester, MN: James Utz, M.D. (Principal Investigator); Claude Deschamps, M.D. (Co-Principal Investigator); Kathy Mieras, C.C.R.P. (Principal Clinic Coordinator); Martin Abel, M.D.; Mark Allen, M.D.; Deb Andrist, R.N.; Gregory Aughenbaugh, M.D.; Sharon Bendel, R.N.; Eric Edell, M.D.; Marlene Edgar; Bonnie Edwards; Beth Elliot, M.D.; James Garrett, R.R.T.; Delmar Gillespie, M.D.; Judd Gurney, M.D.; Boleyn Hammel; Karen Hanson, R.R.T.; Lori Hanson, R.R.T.; Gordon Harms, M.D.; June Hart; Thomas Hartman, M.D.; Robert Hyatt, M.D.; Eric Jensen, M.D.; Nicole Jenson, R.R.T.; Sanjay Kalra, M.D.; Philip Karsell, M.D.; Jennifer Lamb; David Midthun, M.D.; Carl Mottram, R.R.T.; Stephen Swensen, M.D.; Anne-Marie Sykes, M.D.; Karen Taylor; Norman Torres, M.D.; Rolf Hubmayr, M.D. (1998–2000); Daniel Miller, M.D. (1999–2002); Sara Bartling, R.N. (1998–2000); Kris Bradt (1998–2002). National Jewish Medical and Research Center, Denver, CO: Barry Make, M.D. (Principal Investigator); Marvin Pomerantz, M.D. (Co-Principal Investigator); Mary Gilmartin, R.N., R.R.T. (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, P.T.; Enrique Fernandez, M.D.; Lisa Geyman, M.S.P.T.; Connie Hudson; David Lynch, M.D.; John Newell, M.D.; Robert Quaife, M.D.; Jennifer Propst, R.N.; Cynthia Raymond, M.S.; Jane Whalen-Price, P.T.; Kathy Winner, O.T.R.; Martin Zamora, M.D.; Reuben Cherniack, M.D. (Principal Investigator, 1997–2000). Ohio State University, Columbus, OH: Philip Diaz, M.D. (Principal Investigator); Patrick Ross, M.D. (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, Ph.D.; Mark Gerhardt, M.D., Ph.D.; Mark King, M.D.; David Rittinger; Mahasti Rittinger. Saint Louis University, St. Louis, MO: Keith Naunheim, M.D. (Principal Investigator); Robert Gerber, M.D. (Co-Principal Investigator); Joan Osterloh, R.N., M.S.N. (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, D.O.; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, M.D.; Gregg Ruppel; Cary Stolar, M.D.; Janice Willey; Francisco Alvarez, M.D. (Co-Principal Investigator, 1999–2002); Cesar Keller, M.D. (Co-Principal Investigator, 1996–2000). Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Satoshi Furukawa, M.D. (Co-Principal Investigator); Anne Marie Kuzma, R.N., M.S.N. (Principal Clinic Coordinator); Roger Barnette, M.D.; Neil Brister, M.D.; Kevin Carney, R.N., C.C.T.C.; Wissam Chatila, M.D.; Francis Cordova, M.D.; Gilbert D'Alonzo, D.O.; Michael Keresztury, M.D.; Karen Kirsch; Chul Kwak, M.D.; Kathy Lautensack, R.N., B.S.N.; Madelina Lorenzon, C.P.F.T.; Ubaldo Martin, M.D.; Peter Rising, M.S.; Scott Schartel, M.D.; John Travaline, M.D.; Gwendolyn Vance, R.N., C.C.T.C.; Phillip Boiselle, M.D. (1997–2000); Gerald O'Brien, M.D. (1997–2000). University of California, San Diego, San Diego, CA: Andrew Ries, M.D., M.P.H. (Principal Investigator); Robert Kaplan, Ph.D. (Co-Principal Investigator); Catherine Ramirez, B.S., R.C.P. (Principal Clinic Coordinator); David Frankville, M.D.; Paul Friedman, M.D.; James Harrell, M.D.; Jeffery Johnson; David Kapelanski, M.D.; David Kupferberg, M.D., M.P.H.; Catherine Larsen, M.P.H.; Trina Limberg, R.R.T.; Michael Magliocca, R.N., C.N.P.; Frank J. Papatheofanis, M.D., Ph.D.; Dawn Sassi-Dambron, R.N.; Melissa Weeks. University of Maryland at Baltimore, Baltimore, MD, in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, M.D. (Principal Investigator); Henry Fessler, M.D. (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, M.D.; Jonathan Orens, M.D.; Steven Scharf, M.D., Ph.D.; David Shade; Stanley Siegelman, M.D.; Kenneth Silver, M.D.; Clarence Weir; Charles White, M.D. University of Michigan, Ann Arbor, MI: Fernando Martinez, M.D. (Principal Investigator); Mark Iannettoni, M.D. (Co-Principal Investigator); Catherine Meldrum, B.S.N., R.N., C.C.R.N. (Principal Clinic Coordinator); William Bria, M.D.; Kelly Campbell; Paul Christensen, M.D.; Kevin Flaherty, M.D.; Steven Gay, M.D.; Paramjit Gill, R.N.; Paul Kazanjian, M.D.; Ella Kazerooni, M.D.; Vivian Knieper; Tammy Ojo, M.D.; Lewis Poole; Leslie Quint, M.D.; Paul Rysso; Thomas Sisson, M.D.; Mercedes True; Brian Woodcock, M.D.; Lori Zaremba, R.N. University of Pennsylvania, Philadelphia, PA: Larry Kaiser, M.D. (Principal Investigator); John Hansen-Flaschen, M.D. (Co-Principal Investigator); Mary Louise Dempsey, B.S.N., R.N. (Principal Clinic Coordinator); Abass Alavi, M.D.; Theresa Alcorn, Selim Arcasoy, M.D.; Judith Aronchick, M.D.; Stanley Aukberg, M.D.; Bryan Benedict, R.R.T.; Susan Craemer, B.S., R.R.T., C.P.F.T.; Ron Daniele, M.D.; Jeffrey Edelman, M.D.; Warren Gefter, M.D.; Laura Kotler-Klein, M.S.S.; Robert Kotloff, M.D.; David Lipson, M.D.; Wallace Miller, Jr., M.D.; Richard O'Connell, R.P.F.T.; Staci Opelman, M.S.W.; Harold Palevsky, M.D.; William Russell, R.P.F.T.; Heather Sheaffer, M.S.W.; Rodney Simcox, B.S.R.T., R.R.T.; Susanne Snedeker, R.R.T., C.P.F.T.; Jennifer Stone-Wynne, M.S.W.; Gregory Tino, M.D.; Peter Wahl; James Walter, R.P.F.T.; Patricia Ward; David Zisman, M.D.; James Mendez, M.S.N., C.R.N.P. (1997–2001); Angela Wurster, M.S.N., C.R.N.P. (1997–1999). University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D. (Principal Investigator); James Luketich, M.D. (Co-Principal Investigator); Colleen Witt, M.S. (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, M.D.; Carl Fuhrman, M.D.; Robert Hoffman, M.D.; Joan Lacomis, M.D.; Joan Sexton; William Slivka; Diane Strollo, M.D.; Erin Sullivan, M.D.; Tomeka Simon; Catherine Wrona, R.N., B.S.N.; Gerene Bauldoff, R.N., M.S.N. (1997–2000); Manuel Brown, M.D. (1997–2002); Elisabeth George, R.N., M.S.N. (Principal Clinic Coordinator, 1997–2001); Robert Keenan, M.D. (Co-Principal Investigator, 1997–2000); Theodore Kopp, M.S. (1997–1999); Laurie Silfies (1997–2001). University of Washington, Seattle, WA: Joshua Benditt, M.D. (Principal Investigator), Douglas Wood, M.D. (Co-Principal Investigator); Margaret Snyder, M.N. (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, M.D.; Leighton Chan, M.D.; Cindy Chwalik; Bruce Culver, M.D.; Thurman Gillespy, M.D.; David Godwin, M.D.; Jeanne Hoffman; Andra Ibrahim, M.D.; Diane Lockhart; Stephen Marglin, M.D.; Kenneth Martay, M.D.; Patricia McDowell; Donald Oxorn, M.D.; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000). Other participants: Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, M.D., M.P.H.; Yen-Pin Chiang, Ph.D.; Carolyn Clancy, M.D.; Harry Handelsman, D.O. Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M. Berkowitz, Ph.D.; Tanisha Carino, Ph.D.; Joe Chin, M.D.; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora; Steven Sheingold, Ph.D. (1997–2004). Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, M.D., Ph.D. (Principal Investigator); James Tonascia, Ph.D. (Co-Principal Investigator); Patricia Belt; Amanda Blackford, Sc.M.; Karen Collins; Betty Collison; Ryan Colvin, M.P.H.; John Dodge; Michele Donithan, M.H.S.; Vera Edmonds; Gregory L. Foster, M.A.; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, Sc.M.; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, M.D.; Michael Smith; Brett Simon, M.D.; Paul Smith; Alice Sternberg, Sc.M.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Robert Wise, M.D. Cost-Effectiveness Subcommittee: Robert M. Kaplan, Ph.D. (Chair); J. Sanford Schwartz, M.D. (Co-Chair); Yen-Pin Chiang, Ph.D.; Marianne C. Fahs, Ph.D.; A. Mark Fendrick, M.D.; Alan J. Moskowitz, M.D.; Dev Pathak, Ph.D.; Scott Ramsey, M.D., Ph.D.; Steven Sheingold, Ph.D.; A. Laurie Shroyer, Ph.D.; Judith Wagner, Ph.D.; Roger Yusen, M.D. Cost-Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, M.D., Ph.D. (Principal Investigator); Ruth Etzioni, Ph.D.; Sean Sullivan, Ph.D.; Douglas Wood, M.D.; Thomas Schroeder, M.A.; Karma Kreizenbeck; Kristin Berry, M.S.; Nadia Howlader, M.S. CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, Ph.D. (Principal Investigator); Janice Cook-Granroth, B.S.; Angela Delsing, R.T.; Junfeng Guo, Ph.D.; Geoffrey McLennan, M.D.; Brian Mullan, M.D.; Chris Piker, B.S.; Joseph Reinhardt, Ph.D.; Blake Robinswood; Jered Sieren, R.T.R.; William Stanford, M.D. Data and Safety Monitoring Board: John A. Waldhausen, M.D. (Chair); Gordon Bernard, M.D.; David DeMets, Ph.D.; Mark Ferguson, M.D.; Eddie Hoover, M.D.; Robert Levine, M.D.; Donald Mahler, M.D.; A. John McSweeny, Ph.D.; Jeanine Wiener-Kronish, M.D.; O. Dale Williams, Ph.D.; Magdy Younes, M.D. Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Charles Soltoff, M.B.A. Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, M.D. (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, Ph.D.; James Kiley, Ph.D.; Margaret Wu, Ph.D. (1996–2001).
Other Acknowledgments: Arthur Gelb, M.D., Lakewood Regional Medical Center, Lakewood, CA.
References
- 1.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA 2005;294:1255–1259. [DOI] [PubMed] [Google Scholar]
- 2.Calverley P, Anderson J, Celli B, Ferguson G, Jenkins C, Jones P, Yates J, Vestbo J; for the TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–789. [DOI] [PubMed] [Google Scholar]
- 3.Celli B, Cote C, Marin J, Casanova C, Montes de Oca M, Mendez R, Pinto-Plata V, Cabral H. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]
- 4.Martinez F, Foster G, Curtis J, Criner G, Weinmann G, Fishman A, DeCamp M, Benditt J, Sciurba F, Make B, et al.; for the NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006;173:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cote C, Celli B. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J 2005;26:630–636. [DOI] [PubMed] [Google Scholar]
- 6.Imfeld S, Bloch KE, Weder W, Russi E. The BODE index after lung volume reduction surgery correlates with survival. Chest 2006;129:873–878. [DOI] [PubMed] [Google Scholar]
- 7.Lederer DJ, Thomashow BM, Ginsburg ME, Austin JH, Bartels MN, Yip CK, Jellen PA, Brogan FL, Kawut SM, Maxfield RA, et al. Lung-volume reduction surgery for pulmonary emphysema: improvement in body mass index, airflow obstruction, dyspnea, and exercise capacity index after 1 year. J Thorac Cardiovasc Surg 2007;133:1434–1438. [DOI] [PubMed] [Google Scholar]
- 8.Pompeo E, Mineo TC. Two-year improvement in multidimensional body mass index, airflow obstruction, dyspnea, and exercise capacity index after nonresectional lung volume reduction surgery in awake patients. Ann Thorac Surg 2007;84:1862–1869. [DOI] [PubMed] [Google Scholar]
- 9.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 10.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 11.Eakin E, Resnikoff P, Prewitt L, Ries A, Kaplan R. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619–624. [DOI] [PubMed] [Google Scholar]
- 12.Sciurba F, Criner G, Lee S, Mohsenifar Z, Shade D, Slivka W, Wise R; for the National Emphysema Treatment Trial Research Group. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. Am J Respir Crit Care Med 2003;167:1522–1527. [DOI] [PubMed] [Google Scholar]
- 13.Pinto-Plata V, Cote C, Cabral H, Taylor J, Celli B. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J 2004;23:28–33. [DOI] [PubMed] [Google Scholar]