Abstract
Rationale: Pseudomonas aeruginosa is one of the leading causes of gram-negative ventilator-associated pneumonia (VAP) associated with a mortality rate of 34 to 68%. Recent evidence suggests that P. aeruginosa in patients with VAP may persist in the alveolar space despite adequate antimicrobial therapy. We hypothesized that failure to eradicate P. aeruginosa from the lung is linked to type III secretory system (TTSS) isolates.
Objectives: To determine the mechanism by which infection with P. aeruginosa in patients with VAP may evade the host immune response.
Methods: Thirty-four patients with P. aeruginosa VAP underwent noninvasive bronchoalveolar lavage (BAL) at the onset of VAP and on Day 8 after initiation of antibiotic therapy. Isolated pathogens were analyzed for secretion of type III cytotoxins. Neutrophil apoptosis in BAL fluid was quantified by assessment of nuclear morphology on Giemsa-stained cytocentrifuge preparations. Neutrophil elastase was assessed by immunoenzymatic assay.
Measurements and Main Results: Twenty-five out of the 34 patients with VAP secreted at least one of type III proteins. There was a significant difference in apoptotic rate of neutrophils at VAP onset between those strains that secreted cytotoxins and those that did not. Neutrophil elastase levels were positively correlated with the rate of apoptosis (r = 0.43, P < 0.01). Despite adequate antimicrobial therapy, 13 out of 25 TTSS+ isolates were recovered at Day 8 post-VAP, whereas eradication was achieved in all patients who had undetectable levels of type III secretion proteins.
Conclusions: The increased apoptosis in neutrophils by the TTSS+ isolates may explain the delay in eradication of Pseudomonas strains in patients with VAP. Short-course antimicrobial therapy may not be adequate in clearing the infection with a TTSS secretory phenotype.
Keywords: ventilator-associated pneumonia, Pseudomonas aeruginosa, antimicrobial therapy, outcome
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Ventilator-associated pneumonia due to Pseudomonas aeruginosa has been associated with high rates of relapse despite adequate antimicrobial therapy.
What This Study Adds to the Field
Failure to eradicate P. aeruginosa in ventilator-associated pneumonia is linked to a type III secretory system, which is implicated in apoptosis of alveolar neutrophils.
Pseudomonas aeruginosa is a leading cause of nosocomial infections and is responsible for 10% of all hospital-acquired infections (1, 2). It is the most common antibiotic-resistant pathogen causing VAP (3), and the most common cause of fatal episodes of VAP (4). Unlike many other causes of VAP, Pseudomonas is consistently associated with a measurable attributable mortality (5, 6). Infections caused by P. aeruginosa are often severe and life threatening and are difficult to treat because of the limited susceptibility to antimicrobial agents and the high frequency of an emergence of antibiotic resistance during therapy (7), thus resulting in severe adverse outcomes.
Among its large arsenal of virulence factors, P. aeruginosa encodes a type III secretion system (TTSS) (8), which has attracted significant attention in recent years. Upon cell contact, the needlelike type III secretion machinery is deployed, allowing bacteria to inject toxins directly into the cytoplasm of the host cell (9). To date, four TTSS effector molecules have been described. Exoenzyme U (ExoU) is a necrotizing toxin with phospholipase activity (10) that leads to rapid lysis of mammalian cells (11–13). ExoY is an adenylate cyclase that increases intracellular levels of cAMP (14). The other two known effectors are ExoS and ExoT, highly homologous to each other, having a carboxy-terminal ADP-ribosyltransferase (ADPRT) domain and an amino-terminal GTPase-activating (GAP) domain (15, 16). The amino-terminal GAP activity acts on Rho family GTPases, whereas the carboxy-terminal ADPRT activity is directed toward Ras and other host cell proteins (17–19). As a result of these enzymatic activities, intoxication with ExoS is associated with several observable phenotypes, including cytotoxicity and inhibition of bacterial internalization by both phagocytic and nonphagocytic mammalian cells (12, 20).
Recently, we have demonstrated that P. aeruginosa could be isolated from the alveolar space 8 days from onset of VAP despite adequate antimicrobial therapy (21). Because the presence of a functional type III secretion system has been associated with a poor outcome in patients with VAP (22), we hypothesized that persistent alveolar infection with Pseudomonas VAP is correlated with the expression of TTSS phenotype. The objective of this investigation was to determine the mechanism by which infection with Pseudomonas infection in patients with VAP may evade the host immune response. Therefore, the aims of this study were to analyze the interaction between P. aeruginosa isolates and alveolar neutrophils in vivo and to evaluate the cytotoxic contribution of the type III secretion system on polymorphonuclear leukocyte (neutrophil) viability in vitro.
METHODS
Study Population
The study protocol was reviewed and approved by the institutional review board of the State University of New York at Buffalo. Written, informed consent was obtained from all subjects or their legal representatives. Only patients with first episode of P. aeruginosa VAP defined as bacterial growth of 104 colony forming units (cfu)/ml or more from bronchoalveolar lavage fluid (BALF) were enrolled. Exclusion criteria included polymicrobial infection and discordant antimicrobial therapy.
Data Collection
Clinical data recorded on study enrollment included age, sex, reasons for mechanical ventilation, duration of mechanical ventilation before study onset, prior antibiotic therapy, temperature, leukocyte count, ratio of PaO2/FiO2, time to first antibiotic dose from VAP onset, the Acute Physiology and Chronic Health Evaluation II score (23), and the Multiple Organ Dysfunction Score (MODS) (24).
Collection and Processing of Respiratory Specimens
BAL was obtained at time of suspected VAP onset before antimicrobial therapy and was repeated on Day 8 after completion of antibiotic therapy. One-half the collected samples were sent for microbiology processing, and the rest was filtered through two layers of sterile gauze and centrifuged at 500 × g for 10 minutes at 4°C to separate the supernatants from the cell pellet. BAL fluid total protein levels were measured by a modified Lowry assay (25).
To assess the clonal distribution of P. aeruginosa isolates, DNA typing was conducted on all samples obtained at VAP onset using repetitive-element–based polymerase chain reaction (26). Sample relationships were designated as follows: indistinguishable, no band differences; similar, one band difference; and different, two or more band differences.
Analysis of Type III Secretory Protein Phenotype
P. aeruginosa isolates were cultured under TTSS-inducing conditions in minimal media supplemented with nitrotriacetic acid and lacking calcium medium (27). Cultures were incubated with shaking overnight at 37°C before dilution to optical density, 600 (OD600) of 0.1 in fresh minimal media supplemented with nitrotriacetic acid and lacking calcium medium and cultured for a further 5 hours. Bacterial cells were harvested by centrifugation and the supernatant removed. Cell-free supernatant from each sample was concentrated using Centricon tubes (10 kD; MWCO, Millipore, MA). The concentration of protein in all preparations was determined by the Biorad Dc protein quantification kit (Bio-Rad Laboratories, Hercules, CA). Standardized protein concentrations (20 μg) were loaded onto 12.5% Tris polyacrylamide gels (Bio-Rad Laboratories) and run under denaturing conditions. Polyacrylamide gels were transferred to polyvinylidene fluoride membrane and immunoblotted with anti-PcrV, anti-ExoS or anti-ExoU as previously described (28).
Cytotoxicity Assay
PMNs were collected from whole blood obtained by venipuncture from healthy volunteers and purified by density gradient centrifugation. PMNs were washed twice and resuspended to 107/ml in modified HEPES (N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid)-buffered saline. The viability of PMNs, which was determined by trypan blue staining, was more than 95%.
Each of the clinical isolates was cultured overnight in Luria-Bertani medium. Bacterial cells were pelleted and washed three times in sterile phospate-buffered saline (PBS), diluted to an OD600 of 0.1, and regrown in Luria-Bertani medium for a further 1.5 hours. After this, cultures were harvested, washed with lactated Ringer's solution and finally resuspended in 100 μl Ringer's:PBS solution (2:1 ratio by volume). The viability of PMNs in the presence of bacterial isolates was assessed by coincubation of samples containing 5 × 106 cfu/ml of P. aeruginosa and 5 × 106 PMNs/ml. Cytotoxicity was assayed 2 hours after infection by lactate dehydrogenase release using the Cyto Tox96 kit (Promega, San Luis Obispo, CA) according to the manufacturer's instructions.
Neutrophil Apoptosis
Neutrophil apoptosis was assessed by light microscopy (×200) analysis of cytospin cells stained with Wright's Giemsa method and by identification of nuclear changes (condensation of chromatin and simplification of nuclear structure) characteristic of apoptosis (29, 30). Two blinded investigators assessed the percentage of neutrophil apoptosis on cytospin preparations by analyzing 500 cells per slide each. The analysis was performed on two different slides from the same patient. Data were reported as the percentage of apoptotic cells. To validate the light microscopic method of assessment of neutrophil apoptosis, we used a second independent method based on annexin V binding with quantification by flow cytometry (31). The extent of neutrophil apoptosis was compared with the percentage of neutrophil apoptosis determined by nuclear morphology and light microscopy (linear regression slope, 0.79; P = 0.03, n = 6).
Neutrophil elastase in BAL fluid was measured in duplicate by a commercial immunoenzymatic assay kit (PMN Elastase EIA; Alpco Diagnostics, Windham, NH).
Statistical Analysis
Continuous variables were compared using unpaired Student's t test or the Mann-Whitney U test if the variables were not normally distributed. Categorical variables were compared using χ2 test with Yates correction or Fisher's exact test when necessary. Correlations were analyzed with Spearman's rank correlation. Parametric data are presented as mean ± SD and nonparametric data as medians with 95% confidence intervals or ranges. A P value of less than 0.05 was determined as significant. Calculations were performed using SPPS 12.0 (SPSS, Inc., Chicago, IL).
RESULTS
Study Population
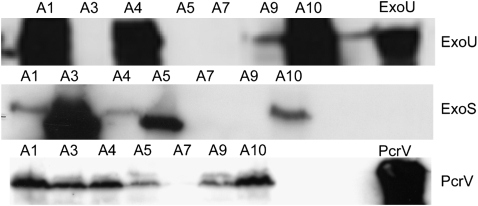
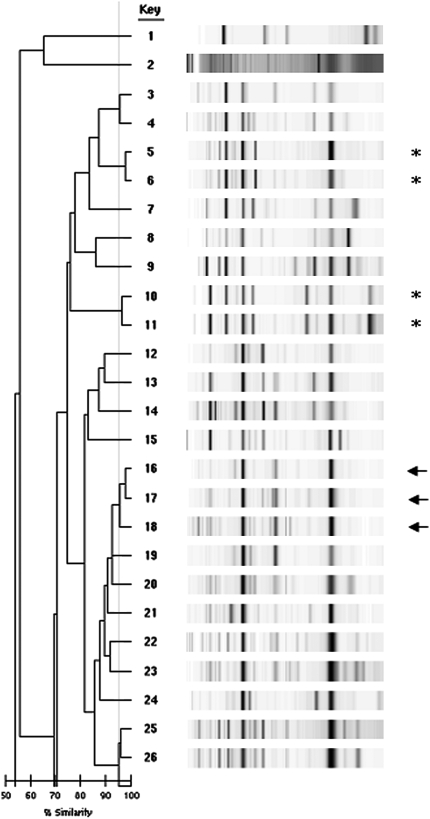
Thirty-four patients with P. aeruginosa VAP were enrolled between May 2003 and July 2006. Exacerbation of chronic obstructive pulmonary disease (21%) and severe community-acquired pneumonia (35%) accounted for the majority of the admission diagnoses followed by cerebrovascular accidents (24%) and abdominal pathology (9%). All participants received a total of 7 days of antibiotics therapy. The average time to first antibiotic dose from onset of VAP was 6.6 ± 3.2 hours (range, 3–16 h). The regimens consisted of a combination of vancomycin plus cefipime (n = 7), vancomycin plus piperacillin/tazobactam (n = 19), or vancomycin plus imipenem/cilastatin (n = 8). None of our patients has received an aminoglycoside as a single agent for the therapy of VAP. All isolates were analyzed in blinded fashion to the presence of a type III secretion system. Twenty-five patients had Pseudomonas strains that were capable of secreting detectable concentrations of at least one of the type III proteins assayed (PcrV, ExoU, and ExoS) (Figure 1). PcrV was secreted by the majority of strains (23/25) that exhibited either ExoU or ExoS cytotoxin secretion. ExoU secretion phenotype was detected in 12 isolates and ExoS in 11. Two isolates secreted PcrV alone without detectable ExoS or ExoU secretion. Of the 25 type III secretory Pseudomonas isolates, there were two distinct pairs considered as indistinguishable and three strains considered as similar. The 18 remainder isolates were distinct (Figure 2). Of interest, susceptibility profile showed fluoroquinolone resistance in 14 out of the 25 isolates, 11 of which (79%) were characterized as ExoU, PcrV secreters. Resistance to imipinem-cilastatin was observed in 6 out of the 25 isolates followed by cefipime (n = 3) and piperacillin/tazobactam (n = 2).
Figure 1.
Immunoblot analysis of exoenzyme U (ExoU), ExoS, and PcrV secretion from a subset of the Pseudomonas aeruginosa clinical isolates examined.
Figure 2.
DNA genotyping of Pseudomonas aeruginosa isolates by repetitive-element–based polymerase chain reaction assay. Row no. 26 is a replica of no. 25, which was used as a quality control. Sample relationships were designated as follows: *indistinguishable, no band differences; arrow, similar, one band difference, and different, two or more band differences.
Characteristics of Type III Secretory Phenotypes
Table 1 summarizes the clinical characteristics of patients with Pseudomonas-positive type III secretory phenotypes and those with Pseudomonas-negative type III secretory phenotypes. There were no significant differences between the two groups in terms of age, sex, burden of comorbidities, severity of hypoxemia, use of prior antibiotics, time to first dose of antibiotics, or severity of illness. Only the duration of mechanical ventilation before VAP onset was significantly longer in those patients who harbored isolates capable of type III cytotoxin secretion.
TABLE 1.
CLINICAL CHARACTERISTICS OF STUDY POPULATION AT THE TIME OF VENTILATOR-ASSOCIATED PNEUMONIA ONSET
| Type III Secretory Phenotype (+) (n = 25) | Type III Secretory Phenotype (−) (n = 9) | P Value | |
|---|---|---|---|
| Age, yr, mean ± SD | 64.2 ± 12.8 | 63.7 ± 11.5 | 0.90 |
| Sex, no. male/female | 18/7 | 5/4 | 0.42 |
| Charlson index, median (range) | 3 (1–6) | 3 (1–6) | 0.48 |
| Prior antibiotics | 14 (56%) | 3 (33%) | 0.44 |
| PaO2/FiO2, mean ± SD | 229.1 ± 98.6 | 234.1 ± 73.0 | 0.88 |
| Bilateral infiltrate | 16 (64%) | 4 (45%) | 0.43 |
| Days of MV before VAP, mean ± SD | 15.6 ± 4.7 | 12.2 ± 2.7 | 0.02 |
| Bacteremia | 3 (12%) | 0 | 0.55 |
| APACHE II score, mean ± SD | 25.7 ± 5.6 | 21.6 ± 4.9 | 0.07 |
| MODS, median (range) | 3 (2–8) | 3 (1–5) | 0.21 |
| Time to first dose of antibiotics from VAP onset, h, mean ± SD | 6.7 ± 3.2 | 6.3 ± 3.0 | 0.77 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; MODS = Multiple Organ Dysfunction Score; MV = mechanical ventilation; VAP = ventilator-associated pneumonia.
Upon examining the characteristics of the BAL fluid, the mean quantitative bacterial colony counts and the percentage of neutrophils were comparable between both groups (Table 2). However, the degree of neutrophilic apoptosis was increased significantly in patients with Pseudomonas-positive type III secretory phenotypes compared with Pseudomonas-negative type III secretory phenotypes. There was also a significant difference in the levels of neutrophils elastase levels between the two groups. Moreover, the degree of neutrophilic apoptosis was positively correlated with BAL elastase levels (r = 0.49, P = 0.003; Figure 3).
TABLE 2.
CHARACTERISTICS OF BRONCHOALVEOLAR LAVAGE OF STUDY POPULATION
| Type III Secretory Phenotype (+) (n = 25) | Type III Secretory Phenotype (−) (n = 9) | P Value | |
|---|---|---|---|
| BAL colony count, ×105 cfu/ml | 11.8 ± 4.9 | 12.6 ± 5.8 | 0.78 |
| BAL, % PMNs | 85.7 ± 7.4 | 83.9 ± 7.5 | 0.53 |
| Neutrophil elastase, ng/ml | 293.2 ± 161.8 | 78.8 ± 54.6 | <0.001 |
| Neutrophil apoptosis, % | 29.1 ± 12.4 | 16.0 ± 9.4 | 0.004 |
Definition of abbreviations: BAL = bronchoalveolar lavage; PMNs = neutrophils.
Figure 3.
Scatterplot between the rate of polymorphonuclear neutrophil (PMN) apoptosis and bronchoalveolar neutrophil elastase (r = 0.49, P = 0.003).
Cytotoxicity of Type III Secretory Phenotypes
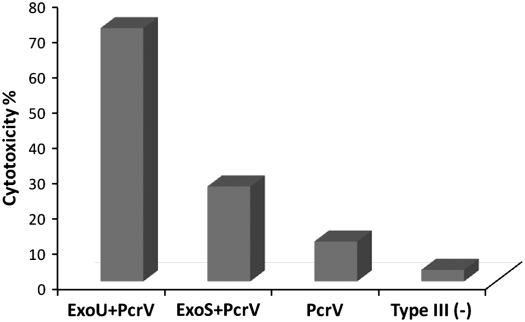
All type III secretory phenotype strains exhibited significant in vitro cytotoxicity against neutrophils (Figure 4). The highest toxicity was detected in isolates that cosecreted ExoU and PcrV, followed in decreasing order by strains that secreted both ExoS and PcrV, and PcrV alone. Of the phenotype-negative isolates, cytotoxicity was present albeit minimal compared with TTSS+ strains (P < 0.001).
Figure 4.
Cytotoxicity of Pseudomonas aeruginosa isolates toward human neutrophils. ExoS = exoenzyme S; ExoU = exoenzyme U.
Clinical Implications of Type III Secretory Phenotypes
A repeat BAL at Day 8 of VAP onset revealed the persistence of P. aeruginosa colonies in the alveolar space in 13 (52%) out of the 25 patients with type III secretory phenotypes. The bacterial burden ranged from 2 × 101 to 6 × 102 cfu/ml. In comparison, none of the patients with negative type III secretory phenotype had Pseudomonas isolates recovered from BAL culture. Analysis of the secretory pattern of these isolates revealed that nine had the ExoU/PcrV and four had the ExoS/PcrV phenotypes. Furthermore, BAL assays revealed higher levels of neutrophil elastase in the alveolar space, which corresponded to an advanced degree of in vitro neutrophil cytotoxicity in those patients with persistent Pseudomonas isolates compared with their counterparts who had cleared the bacterium (Figure 5).
Figure 5.
Comparison of in vitro polymorphonuclear neutrophil (PMN) cytotoxicity between patients with persistent alveolar Pseudomonas infection and those with bacterial clearance (left). Comparison of bronchoalveolar lavage neutrophil elastase between patients with persistent alveolar Pseudomonas infection and those with bacterial clearance (right).
Outcome
After VAP treatment, relapse occurred in 8 of the 13 patients who had persistent Pseudomonas in the alveolar space at end of therapy. The relapse was attributed to the same Pseudomonas strain in 7 of the 8 cases. Overall crude mortality was 59% (95% confidence interval [CI], 41 to 75%). Multiorgan failure was the immediate cause of death in 80% of cases. The survival rate for patients with nonsecretory type III phenotype was 66% (6 of 9) (95% CI, 30 to 93%) compared with 32% (8 of 25) for those with type III secretory phenotype isolates (95% CI, 15 to 54%). Patients exposed to prior antibiotics had no worse outcome that those who did not.
DISCUSSION
The results of the present study show that (1) VAP due to P. aeruginosa type III secretory phenotypes was associated with increased neutrophilic apoptosis in vivo and increased release of neutrophil elastase, (2) in vitro neutrophil cytotoxicity correlated significantly with the Pseudomonas ExoU/PcrV and ExoS/PcrV phenotypes, and (3) positive type III secretory phenotypes were associated with persistent alveolar infection and higher rate of relapse despite 7 days of adequate antimicrobial therapy.
Our analysis demonstrated that 71% of Pseudomonas isolates from patients with VAP were capable of secreting type III effector proteins. In contrast to patients with cystic fibrosis in whom the rate of type III secreting isolates ranged between 12 and 27.5% (32, 33), our observations agree with those of other investigators who noted that 77 to 91% of patients with Pseudomonas VAP harbor Pseudomonas strains that secrete TTSS proteins (22, 34). Although we have not examined the TTSS genetic constructs of these isolates, the phenotypes of the TTSS secretory isolates were predominantly either ExoU/PcrV or ExoS/PcrV. None of our isolates expressed both effector proteins. This mutually exclusive relationship between these two proteins has been documented previously (22, 34), the significance of which remains unclear.
Several in vitro and ex vivo investigations have studied the interaction between P. aeruginosa and eukaryotic cells using different infection models (35–37). These studies have made it possible to elucidate the mechanisms by which the type III secretion system enhances bacterial pathogenicity despite the host immune response. After a bacterial infection, successful eradication of bacterial infections depends on increased neutrophil survival (38). Once the invading pathogen has been neutralized, neutrophil apoptosis is initiated as an integral part of the normal resolution of the inflammatory response in the lung. Our study suggests that neutrophil apoptosis might be induced prematurely by P. aeruginosa isolates that secrete type III secretion proteins. In contrast, early neutrophil apoptosis did not occur in the presence of Pseudomonas strains that did not secrete type III proteins. Killing neutrophils may thus represent an important strategy for pathogen survival. Indeed, previous reports have shown cytotoxic TTSS+ Pseudomonas strains and isogenic noncytotoxic strains were equally well ingested by neutrophils and macrophages, but only the cytotoxic TTSS strains were able to escape the bactericidal activity by inducing oncosis of the host cells (39). In vitro experiments point to the fact that both ExoS and ExoU are primary virulence factors in acute P. aeruginosa infection (13, 14, 36, 37). The enzymatic activity of ExoS induces cytoskeletal alterations that have numerous deleterious effects on host cells, including altered DNA synthesis and decreased cellular adherence and viability (40, 41). ExoU, through its potent phospholipase A2 activity, induces cell death across several cell lines including neutrophils, epithelial cells, and macrophages (39, 42). ExoU also has recently been shown to inhibit caspase-1–driven proinflammatory cytokine production, thereby circumventing innate immune responses (43). We should indicate that there are likely other virulence products that help P. aeruginosa persist in the lungs of our patients. P. aeruginosa pigment, pyocyanin, has been implicated in apoptosis of human peripheral blood neutrophils via generation of reactive oxygen species and lowering of cAMP (44). Whether these two virulence systems, TTSS and pyocyanin, act in concert or operate independently of each other deserves further investigation.
Our results indicate that there were higher concentrations of neutrophil elastase in BAL fluid recovered from patients with TTSS+ P. aeruginosa isolates than in the BAL fluid from patients with TTSS− isolates. The correlation between the degree of apoptosis and BAL neutrophil elastase suggests an uncontrolled release of toxic mediators by the neutrophils in the alveolar milieu in an attempt to control the invading pathogens. Despite the increased concentrations of elastase, the TTSS+ strains were the strains that persisted on Day 8 post-therapy. These results suggest that the type III secretion proteins may successfully decrease host immune responses (43). Similar results were documented by the observation that isolates of P. aeruginosa were intact and viable even at extremely high neutrophil elastase concentrations (45).
Failure to sterilize the alveolar space despite adequate antimicrobial therapy has been reported previously (6, 21). In a retrospective study of VAP due to P. aeruginosa, Crouch Brewer and colleagues (6) reported persistent Pseudomonas infection after repeated BAL in 31% of all enrollees. After excluding patients who had received discordant antimicrobial therapy, half of those patients had BAL cultures still growing P. aeruginosa. Our results extend these findings by identifying a trait that would likely predict failure to cure Pseudomonas pneumonia in patients requiring mechanical ventilation. The latest American Thoracic Society guidelines for the management of VAP recommended shortening the duration of therapy for 7 days for patients with VAP provided that the etiologic agent was not P. aeruginosa (46). This recommendation of shorter duration of therapy might hold true for patients with TTSS− isolates of Pseudomonas but not for the TTSS+ strains. In the absence of an approved diagnostic test for type III secretory proteins, therapy for Pseudomonas VAP should be extended for more than 7 days, although the duration and the results of such therapy need to be examined further in light of our findings.
The mortality rate of 59% from P. aeruginosa is comparable to that reported 10 to 20 years ago (6, 47) despite improved intensive care unit care and the availability of more potent antimicrobial therapy. Even in the most recent studies of patients with P. aeruginosa pneumonia, TTSS+ strains were associated with poor clinical outcomes (22, 28). This unacceptably high mortality rate of Pseudomonas VAP warrants changes to the current approach to therapy. Innovative antibiotic regimens and longer duration should be considered, but adjuvant therapy might prove to be more efficacious in shifting the balance between the virulence of the infecting pathogens and the immune response of the host toward eradication of these microbes. Among these interventions, passive immunization against components of the type III secretory system could tilt this balance. In a mouse model of P. aeruginosa pneumonia, intravenous administration of polyclonal antibodies against PcrV (a protein involved in translocation of type III–secreted toxin) resulted in survival of all animals (48). Complementary studies in a rabbit model of P. aeruginosa–induced septic shock associated with lung injury showed that treatment with anti–PcrV IgG significantly reduced lung injury, bacteremia, and plasma tumor necrosis factor-α levels compared with animals treated with control IgG, as well as improved hemodynamic parameters (49). Other treatments would include directed vaccination against one or more of the TTSS components. Although this intervention would not eliminate P. aeruginosa VAP, it might decrease morbidity and/or mortality associated with this disease.
The current study is limited by the small number of patients recruited from a single institution, potentially limiting its generalizability. However, the poor prognosis of patients with Pseudomonas type III secretory phenotype observed in our study parallels that reported in other clinical investigations (5, 6). We have focused on two phenotypic expressions of the TTSS, ExoU/PcrV and ExoS/PcrV, which are considered the most cytotoxic of the effector proteins. The contribution of the other effector proteins (ExoT or ExoY), whether expressed alone or in conjunction with ExoU or ExoS, remains unknown. We should indicate that we have based our definition of relapse on phenotypic profile of Pseudomonas isolates. Although pulsed field gel electrophoresis remains the “gold standard” to assess Pseudomonas typing, Rello and colleagues (50) have shown previously using pulsed field gel electrophoresis that most recurrent episodes of P. aeruginosa pneumonia in ventilated patients occur due to persistence of strains present in a prior infection. Therefore, we consider our findings of recurrent pneumonia to be a relapse rather than reinfection.
In conclusion, Pseudomonas strains may differ markedly in their ability to cause severe infections. The secretion of type III secretion proteins appears to be an effective apoptosis-inducing agent for P. aeruginosa, allowing the bacteria to persist in the lungs of susceptible hosts. The current practice of identifying bacterial genus or species might be insufficient to characterize the disease potential, the duration of treatment, and the prognostic implications of this bacterium.
Supplementary Material
Acknowledgments
The authors thank Dr. Daniel Amsterdam for providing assistance in the repetitive-element–based polymerase chain reaction analysis.
Supported in part by the Research for Health in Erie County (A.A.E.S.) and by the Specialized Centers of Clinically-Oriented Research grant HL74005 and HL69809 from the National Institutes of Health, Bethesda, Maryland (J.P.W.-K., S.V.L.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200802-239OC on May 8, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Morrison AJ, Wenzel RP. Epidemiology of infection due to Pseudomonas aeruginosa. Rev Infect Dis 1984;6:627–642. [DOI] [PubMed] [Google Scholar]
- 2.National Nosocomial Infection Surveillance System. National Nosocomial Infection Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470–485. [DOI] [PubMed] [Google Scholar]
- 3.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903. [DOI] [PubMed] [Google Scholar]
- 4.Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 1993;104:1230–1235. [DOI] [PubMed] [Google Scholar]
- 5.Rello J, Jubert P, Valles J, Artigas A, Rue M, Niederman MS. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis 1996;23:973–978. [DOI] [PubMed] [Google Scholar]
- 6.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV Jr. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996;109:1019–1029. [DOI] [PubMed] [Google Scholar]
- 7.Carmeli Y, Troillet N, Eliopoulos G, Samore M. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob Agents Chemother 1999;43:1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yahr TL, Goranson J, Frank DW. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol Microbiol 1996;22:991–1003. [DOI] [PubMed] [Google Scholar]
- 9.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 1998;62:379–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauser A, Kang P, Engel J. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol 1998;27:807–818. [DOI] [PubMed] [Google Scholar]
- 11.Finck-Barbancon V, Frank DW. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J Bacteriol 2001;183:4330–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa–mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun 1997;65:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallis AJ, Finck-Barbancon V, Yahr TL, Frank DW. Biological effects of Pseudomonas aeruginosa type III–secreted proteins on CHO cells. Infect Immun 1999;67:2040–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 1998;95:13899–13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krall R, Sun J, Pederson K, Barbieri J. In vivo rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect Immun 2002;70:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krall R, Schmidt G, Aktories K, Barbieri J. Pseudomonas aeruginosa ExoT is a rho GTPase-activating protein. Infect Immun 2000;68:6066–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goehring U, Schmidt G, Pederson K, Aktories K, Barbieri J. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem 1999;274:36369–36372. [DOI] [PubMed] [Google Scholar]
- 18.Henriksson M, Sundin C, Jansson A, Forsberg A, Palmer R, Hallberg B. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities toward small GTPases in vivo. Biochem J 2002;367:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocha C, Coburn L, Rucks E, Olson J. Characterization of Pseudomonas aeruginosa exoenzyme S as a bifunctional enzyme in J774A.1 macrophages. Infect Immun 2003;71:5296–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman M, Jia J, Zeng L, Ha U, Chow M, Jin S. Pseudomonas aeruginosa–mediated apoptosis requires the ADP-ribosylating activity of ExoS. Microbiology 2000;146:2531–2541. [DOI] [PubMed] [Google Scholar]
- 21.El Solh A, Chio G, Schultz M, Pineda L, Mankowski C. Clinical and hemostatic responses to treatment in ventilator-associated pneumonia: role of bacterial pathogens. Crit Care Med 2007;35:490–496. [DOI] [PubMed] [Google Scholar]
- 22.Hauser A, Cobb E, Bodi M, Mariscal D, Valles J, Engel J, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 2002;30:521–528. [DOI] [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman J. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 24.Marschall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple Organ Dysfunction Score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995;23:1638–1652. [DOI] [PubMed] [Google Scholar]
- 25.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem 1985;150:76–85. [DOI] [PubMed] [Google Scholar]
- 26.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 1991;19:6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicas TI, Iglewski BH. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun 1984;45:470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 2001;183:1767–1774. [DOI] [PubMed] [Google Scholar]
- 29.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. J Clin Invest 1989;83:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med 1982;156:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown S, Bailey K, Savill J. Actin is cleaved during constitutive apoptosis. Biochem J 1997;323:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dacheux D, Toussaint B, Richard M, Brochier G, Croize J, Attree I. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect Immun 2000;68:2916–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain M, Ramirez D, Seshadri R, Cullina J, Powers C, Schulert G, Bar-Meir M, Sullivan C, McColley S, Hauser A. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol 2004;42:5229–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Lynch S, Flanagan J, Zhuo H, Tom W, Dotson RH, Baek MS, Rubio-Mills A, Singh G, Kipnis E, et al. Increased plasminogen activator inhibitor-1 concentrations in bronchoalveolar lavage fluids are associated with increased mortality in a cohort of patients with Pseudomonas aeruginosa. Anesthesiology 2007;106:252–261. [DOI] [PubMed] [Google Scholar]
- 35.Sawa T, Ohara M, Kurahashi K, Twining S, Frank D, Doroques D, Long T, Gropper M, Wiener-Kronish J. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun 1998;66:3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet J, Frank D. Exoproduct secretions of P. aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol 1994;267:L551–L556. [DOI] [PubMed] [Google Scholar]
- 37.Allewelt M, Coleman FT, Grout M, Priebe G, Pier G. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect Immun 2000;68:3998–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Droemann D, Aries S, Hansen F, Moellers M, Braun J, Katus H, Dalhoff K. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest 2000;117:1679–1684. [DOI] [PubMed] [Google Scholar]
- 39.Dacheux D, Attree I, Schneider C, Toussaint B. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect Immun 1999;67:6164–6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pederson K, Barbieri J. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas aeruginosa exoenzyme S is cytotoxic to eukaryotic cells. Mol Microbiol 1998;30:751–759. [DOI] [PubMed] [Google Scholar]
- 41.Pederson K, Krall R, Riese M, Barbieri J. Intracellular localization modulates targeting of ExoS, a type III cytotoxin, to eukaryotic signalling proteins. Mol Microbiol 2002;46:1381–1390. [DOI] [PubMed] [Google Scholar]
- 42.Finck-Barbancon V, Goranson V, Zhu L, Sawa T, Wiener-Kronish J, Fleiszig S, Wu C, Mende-Muller L, Frank D. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 1997;25:547–557. [DOI] [PubMed] [Google Scholar]
- 43.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med 2007;204:3235–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Usher LR, Lawson RA, Geary I, Taylor CJ, Bingle CD, Taylor GW, Whyte MK. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: a potential mechanism of persistent infection. J Immunol 2002;168:1861–1868. [DOI] [PubMed] [Google Scholar]
- 45.Sonawane A, Jyot J, During R, Ramphal R. Neutrophil elastase, an innate immunity effector molecule, represses flagellin transcription in Pseudomonas aeruginosa. Infect Immun 2006;74:6682–6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and health care–associated pneumonia: official statement of ATS and IDSA. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 47.Fagon J, Chastre J, Hance A, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 1993;94:281–299. [DOI] [PubMed] [Google Scholar]
- 48.Shime N, Sawa T, Fujimoto J, Faure K, Allmond LR, Karaca T, Swanson BL, Spack EG, Weiner-Kronish JP. Therapeutic administration of anti-PcrV F(ab)2 in sepsis associated with Pseudomonas aeruginosa. J Immunol 2001;167:5880–5886. [DOI] [PubMed] [Google Scholar]
- 49.Sawa T, Yahr T, Ohara M, Kurahashi K, Gropper MA, Weiner-Kronish JP, Frank DW. Active and passive immunization with Pseudomonas V antigen protects against type III intoxication and lung injury. Nature 1999;5:392–398. [DOI] [PubMed] [Google Scholar]
- 50.Rello J, Mariscal D, March F, Jubert P, Sanchez F, Valles J, Coll P. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: relapse or reinfection? Am J Respir Crit Care Med 1998;157:912–916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.