Abstract
Rationale: Microvascular injury, inflammation, and coagulation play critical roles in the pathogenesis of acute lung injury (ALI). Plasma protein C levels are decreased in patients with acute lung injury and are associated with higher mortality and fewer ventilator-free days.
Objectives: To test the efficacy of activated protein C (APC) as a therapy for patients with ALI.
Methods: Eligible subjects were critically ill patients who met the American/European consensus criteria for ALI. Patients with severe sepsis and an APACHE II score of 25 or more were excluded. Participants were randomized to receive APC (24 μg/kg/h for 96 h) or placebo in a double-blind fashion within 72 hours of the onset of ALI. The primary endpoint was ventilator-free days.
Measurements and Main Results: APC increased plasma protein C levels (P = 0.002) and decreased pulmonary dead space fraction (P = 0.02). However, there was no statistically significant difference between patients receiving placebo (n = 38) or APC (n = 37) in the number of ventilator-free days (median [25–75% interquartile range]: 19 [0–24] vs. 19 [14–22], respectively; P = 0.78) or in 60-day mortality (5/38 vs. 5/37 patients, respectively; P = 1.0). There were no differences in the number of bleeding events between the two groups.
Conclusions: APC did not improve outcomes from ALI. The results of this trial do not support a large clinical trial of APC for ALI in the absence of severe sepsis and high disease severity.
Clinical trial registered with www.clinicaltrials.gov (NCT 00112164).
Keywords: acute respiratory distress syndrome, acute lung injury, activated protein C, ventilator-free days, mortality
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
There are no pharmacologic therapies that have been proven to be effective for the treatment of acute lung injury (ALI) and acute respiratory distress syndrome. It is unknown whether activated protein C would benefit patients with ALI.
What This Study Adds to the Field
We tested activated protein C as a treatment for ALI. Although plasma protein C levels increased and pulmonary dead space fraction decreased, there was no benefit with regard to ventilator-free days (primary study endpoint), mortality, or lung injury score.
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) are a major cause of morbidity and mortality, with an incidence of approximately 200,000 patients per year in the United States (1, 2) and a mortality rate of 25 to 40%. Several pharmacologic treatments for clinical lung injury have been evaluated, but none have decreased mortality (2, 3). The use of a lung-protective ventilatory strategy produced the first major breakthrough in supportive care for patients with ALI, reducing mortality from 40 to 31% (4). However, there is still no effective pharmacologic therapy for the underlying lung injury (reviewed in References 3 and 5).
The pathogenesis of ALI involves both procoagulant and inflammatory mechanisms. Extravascular fibrin deposition in the lung (especially hyaline membranes in the alveoli) is a characteristic pathologic feature of ALI (6, 7), and fibrin deposition and small vessel thrombi develop within the lung circulation in patients with ALI (8, 9). In addition, we have reported that plasma protein C deficiency occurs in virtually all patients with ALI, and reduced plasma protein C levels are associated with a higher mortality and more nonpulmonary organ system dysfunction (10, 11). Normal fibrinolytic mechanisms are impaired in the alveolar compartment in patients with ALI. Elevated levels of plasminogen activator inhibitor (PAI)-1 in the plasma and pulmonary edema fluid are predictive of mortality in patients with ALI (11, 12). Therefore, the correlation of low protein C and elevated PAI-1 levels with poor clinical outcomes suggests that abnormalities of coagulation and fibrinolysis may play an important role in the pathogenesis of infectious and noninfectious ALI (10, 11). There is also persuasive evidence that activation of the coagulation cascade, specifically thrombin formation, can induce inflammatory events, including expression of IL-1, IL-6, and IL-8, and transmigration of inflammatory cells across the lung endothelium (13).
Activated protein C (APC) is a novel therapy with anticoagulant and antiinflammatory properties approved for the treatment of patients with severe sepsis and higher disease severity, based on the results of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) clinical trial (14). Given the evidence that procoagulant and inflammatory mechanisms play a critical role in the pathogenesis of ALI, we hypothesized that APC would be an effective pharmacologic therapy for the treatment of ALI. We therefore performed a randomized, double-blind, placebo-controlled phase II clinical trial to test this hypothesis.
Some of these results have been previously reported in the form of an abstract (15).
METHODS
Participants
Eligible subjects were critically ill patients at eight university medical centers who met the American/European consensus conference criteria for ALI (16). Reasons for exclusion are listed in the online supplement. Major reasons for exclusion included the following: the presence of ALI for more than 72 hours; the presence of sepsis with an Acute Physiology and Chronic Health Evaluation II (APACHE II) score greater than or equal to 25 (a group in whom the drug is currently approved); increased risk of bleeding due to trauma, liver dysfunction, or a known coagulation abnormality; inability to obtain consent; and irreversible medical conditions for which the estimated 6-month mortality exceeded 50%. Written, informed consent was obtained from the study subject or his or her surrogate. The institutional review board at each site approved the study, as did a National Heart, Lung, and Blood Institute data safety monitoring board (DSMB). The DSMB conducted prespecified interim analyses for safety, efficacy, and futility after the enrollment of 30 and 60 patients. The study was stopped by the DSMB after the interim analysis of the first 60 patients for futility; at that point, a total of 75 patients had been enrolled in the trial.
Study Design/Interventions
Subjects were randomly assigned to receive APC (at a dose of 24 μg/kg/h for 96 h) or placebo, with concealed allocation in permuted blocks of 2. Study participants and investigators were blinded throughout the treatment and follow-up period. All subjects were ventilated with the lung-protective, low tidal volume ventilation protocol and weaned from mechanical ventilation as described in Reference 4.
Outcomes
As described in detail in the online supplement, the original primary outcome of the study was pulmonary dead space fraction. However, during the Investigational New Drug Application process, the U.S. Food and Drug Administration strongly recommended that the primary outcome of the study should be a more clinically relevant endpoint. Thus, the primary outcome of the study was changed to ventilator-free days, defined as the number of days to Day 28 that the subject achieved unassisted breathing, assuming that a patient survived to Day 28 and remained free of assisted breathing. Subjects who did not survive to 28 days were assigned zero ventilator-free days. Secondary outcomes included Day 60 mortality, organ failure–free days as defined by the Brussels criteria (4, 17), and the change in the pulmonary dead space fraction.
Measurements
Biomarker measurements were made in stored plasma samples from the day of study enrollment and on Day 3 of the study as described in the online supplement. Dead space fraction was measured by volumetric capnography, adopting the procedure used during a recent validation study in patients with ALI/ARDS (18).
Statistical Methods
Continuous variables were expressed as mean ± SD or median with interquartile range, and were compared using Student's t test or the Wilcoxon rank sum test, where appropriate. Categorical variables were compared using χ2 tests or Fisher's exact tests, as appropriate. Ventilator-free days were compared using the Wilcoxon rank sum test, because these were not normally distributed. Multivariate linear regression was used to evaluate the association of APC with ventilator-free days, adjusting for baseline pulmonary dead space fraction and lung injury score (19). A generalized estimating equation approach was used to test the impact of APC treatment on PaO2:FiO2, lung injury score, and pulmonary dead space fraction, taking repeated measures into account and using an exchangeable correlation matrix. Analysis of covariance was used to analyze the impact of APC treatment on the biomarker levels at Day 3, controlling for baseline level. A two-sided P value of less than 0.05 was considered statistically significant. With the planned enrollment of 90 patients, the study had a statistical power of 80% to detect a difference of 6.5 ventilator-free days between the APC- and placebo-treated groups; additional detail on the power calculations is presented in the online supplement.
RESULTS
Study Protocol
Study participants were recruited at eight university medical centers in the United States from January 2005 until February of 2007. There were 38 patients assigned to receive placebo, and 37 patients assigned to receive APC. One participant received placebo instead of APC due to a pharmacy error; the analysis was conducted on an intention-to-treat basis as well as an as-treated basis (Figure 1). There were no significant differences in the trial outcomes between these two analyses; therefore, the intention-to-treat analysis results are reported here. The infusion was held before an invasive procedure (as described in the online supplement) and terminated before completion if there was any clinical concern for increased bleeding. A total of 31 of 38 patients in the placebo-treated group and 29 of 37 patients in the APC-treated group completed the 96-hour infusion (P = 0.73); there was no difference in the mean or median duration of infusion between the two groups.
Figure 1.
Enrollment and outcomes. Patients may have had more than one reason for exclusion. The full list of exclusion criteria can be found in the online supplement. APACHE = Acute Physiology and Chronic Health Evaluation; APC = activated protein C.
Baseline Data
There were no differences in the demographic characteristics or cause of ALI between the two groups. Specifically, the primary cause of lung injury, the APACHE II score, and the baseline physiologic variables were not different between the two groups. The only difference between the two groups at baseline was an increased pulmonary dead space fraction in the APC group (Table 1).
TABLE 1.
BASELINE CHARACTERISTICS
| Placebo (n = 38) | APC (n = 37) | P Value | |
|---|---|---|---|
| Age, yr | 51.6 ± 18.6 | 51.6 ± 15.5 | >0.99 |
| Male sex, n (%) | 26 (68) | 21 (57) | 0.30 |
| Race, n (%) | 0.53 | ||
| White | 23 (61) | 21 (57) | |
| African American | 7 (18) | 6 (16) | |
| Hispanic | 5 (13) | 9 (24) | |
| Other | 3 (8) | 1 (3) | |
| Primary etiology of lung injury, n (%) | 0.24 | ||
| Pneumonia | 16 (42) | 14 (38) | |
| Aspiration | 12 (32) | 14 (38) | |
| Sepsis | 7 (18) | 4 (11) | |
| Drug overdose | 2 (5) | 0 (0) | |
| Other | 1 (3) | 5 (13) | |
| Medical ICU admission, n (%) | 30 (79) | 33 (89) | 0.35 |
| APACHE II score | 20 ± 7 | 20 ± 8 | 0.72 |
| SAPS II score | 42 ± 14 | 42 ± 16 | 0.96 |
| Sepsis, n (%) | 17 (45) | 10 (27) | 0.11 |
| Mean arterial pressure, mm Hg | 82 ± 15 | 78 ± 14 | 0.19 |
| Vasopressor use, n (%) | 8 (21) | 10 (29) | 0.46 |
| Hematologic variables | |||
| Hemoglobin, g/dl | 10.8 ± 2.5 | 10.6 ± 3.6 | 0.81 |
| WBC, 106/ml | 14.3 ± 8.4 | 12.7 ± 6.8 | 0.37 |
| Platelets, 106/ml | 229 ± 122 | 221 ± 138 | 0.81 |
| Respiratory variables | |||
| Tidal volume, ml/kg PBW | 6.9 ± 1.5 | 6.7 ± 1.4 | 0.54 |
| Plateau pressure, cm H2O | 24 ± 5 | 25 ± 7 | 0.22 |
| PEEP, cm H2O | 8.5 ± 3.2 | 9.4 ± 4.6 | 0.34 |
| pH | 7.38 ± 0.05 | 7.38 ± 0.07 | 0.82 |
| PaO2/FiO2 | 174 ± 63 | 158 ± 67 | 0.30 |
| Lung injury score* | 2.5 ± 0.6 | 2.7 ± 0.6 | 0.10 |
| Dead space fraction | 0.55 ± 0.12 | 0.62 ± 0.12 | 0.03 |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; APC = activated protein C; ICU = intensive care unit; PaO2/FiO2 = ratio of the partial pressure of arterial oxygen and the fraction of the inspired oxygen; PBW = predicted body weight; PEEP = positive end-expiratory pressure; SAPS II = Simplified Acute Physiology Score; WBC = white blood cell.
Values are means ± SD unless otherwise noted.
Lung injury score is the 4-point score as described in Reference 19.
Study Outcomes
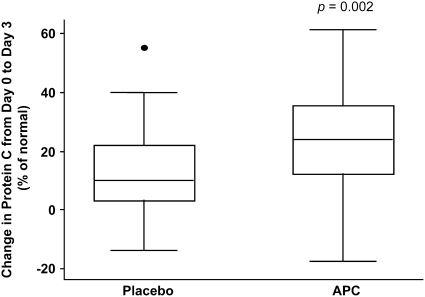
Compared with the placebo group, there was a significant increase in plasma protein C levels in the APC-treated group from baseline to Day 3 (P = 0.002; Figure 2). There was no statistically significant difference in ventilator-free days between the two groups (median: 19 d in the placebo group compared with 19 in the APC group, P = 0.78; 95% confidence interval [CI] for the difference, −3 to 4 d). There was also no statistically significant difference in 60-day mortality between the two groups (5/38 patients in the placebo group and 5/37 patients in the APC group, P = 1.0; 95% CI for the difference between the groups, −15 to 15.7%). Similarly, there was no statistically significant difference in ventilator-free days between the groups when only survivors were analyzed, nor was there a statistically significant difference in the number of organ failure–free days between the groups (Table 2). Also, after adjusting for the lung injury score and baseline dead space fraction, there was no statistically significant difference in the number of ventilator-free days between groups (95% CI, −3 to 9 d for treatment vs. placebo; P = 0.31).
Figure 2.
Change in protein C levels by treatment group. The horizontal line within the box represents the median, the boxes encompasses the 25th–75th percentile, and the whiskers encompass the 10th–90th percentile (solid dot is an outlier). Units for protein C levels are % of normal, so the y axis represents the change in protein C levels expressed as % of normal. There is a difference in the change between baseline and Day 3 levels in the activated protein C (APC)–treated group, compared with the control group, P = 0.002.
TABLE 2.
CLINICAL OUTCOMES BY GROUP
| Placebo (n = 38) | APC (n = 37) | P Value | |
|---|---|---|---|
| Ventilator-free days, median (IQR) | 19 (0–24) | 19 (14–22) | 0.78 |
| Death by Day 60, n (%) | 5 (13.5) | 5 (13.5) | 1.00 |
| Ventilator-free days among survivors, median (IQR) | 21 (5–25) | 20 (16–23) | 0.36 |
| Organ failure–free days, median (IQR) | 23 (14–27) | 23 (16–27) | 0.46 |
| Cardiovascular failure, median (IQR) | 25 (20–28) | 26 (23–28) | 0.30 |
| Coagulation failure, median (IQR) | 28 (28–28) | 28 (28–28) | 0.57 |
| Renal failure, median (IQR) | 28 (18.5–28) | 28 (28–28) | 0.41 |
| Hepatic failure, median (IQR) | 28 (27–28) | 28 (28–28) | 0.36 |
Definition of abbreviations: APC = activated protein C; IQR = interquartile range.
Physiologic and Biological Measurements
Given the anticoagulant and profibrinolytic properties of APC, we hypothesized that administration of APC would decrease the pulmonary dead space fraction. There was a significant difference in the dead space fraction between the two groups at baseline (0.55 ± 0.12 in placebo group vs. 0.62 ± 0.12 in the treatment group, P = 0.03). After adjusting for this baseline difference, there was a greater change in dead space fraction over the first 4 days of the study in the APC-treated group compared with the placebo group (P = 0.02; Table 3). Treatment with APC did not affect the change in PaO2:FiO2 or lung injury score over the first 4 days of the study (Table 3).
TABLE 3.
PULMONARY PHYSIOLOGY VARIABLES BY TREATMENT GROUP
| Baseline
|
Day 1
|
Day 4
|
||||
|---|---|---|---|---|---|---|
| Variable | Placebo | APC | Placebo | APC | Placebo | APC |
| PaO2:FiO2 | 174 ± 63 | 158 ± 67 | 178 ± 52 | 169 ± 63 | 197 ± 85 | 202 ± 74 |
| Tidal volume, ml/kg of PBW | 6.9 ± 1.5 | 6.7 ± 1.4 | 6.4 ± 1.0 | 6.4 ± 1.3 | 6.5 ± 1.1 | 6.3 ± 1.3 |
| Plateau pressure, cm H2O | 24 ± 5 | 25 ± 7 | 23 ± 4 | 24 ± 4 | 22 ± 5 | 23 ± 5 |
| Lung injury score | 2.5 ± 0.6 | 2.7 ± 0.6 | 2.4 ± 0.6 | 2.6 ± 0.6 | 2.3 ± 0.7 | 2.2 ± 0.7 |
| Dead space fraction | 0.55 ± 0.12 | 0.62 ± 0.12 | 0.55 ± 0.09 | 0.61 ± 0.13 | 0.57 ± 0.14 | 0.57 ± 0.14 |
| Change in dead space fraction | −0.01 ± 0.07 | −0.01 ± 0.09 | −0.001 ± 0.08 | −0.06 ± 0.09 | ||
Definition of abbreviation: APC = activated protein C, PBW = predicted body weight.
Values are means ± SD of the values recorded closest to 8 a.m. on Days 1 and 4 after enrollment. There was no difference in the change in PaO2:FiO2 (P = 0.38), plateau pressure (P = 0.21), or lung injury score (P = 0.22) between treatment groups. There was a significant change in the dead space fraction between the treatment groups, P = 0.02.
We also tested the impact of APC administration on coagulation and inflammatory markers. APC administration had no effect on PAI-1 or IL-6 levels (Table 4). The plasma protein C levels were decreased in both groups at baseline (Table 4). As would be expected, APC did increase the levels of protein C from baseline in the treatment group, but not in the control group (Table 4 and Figure 2).
TABLE 4.
BIOMARKER VALUES BY TREATMENT GROUP
| Placebo (n = 38) | APC (n = 37) | P Value | |
|---|---|---|---|
| IL-6, ng/ml | 0.67 | ||
| Baseline | 354 ± 644 | 374 ± 517 | |
| Day 3 | 147 ± 219 | 171 ± 235 | |
| PAI-1, ng/ml | 0.9 | ||
| Baseline | 56 ± 22 | 54 ± 22 | |
| Day 3 | 54 ± 24 | 53 ± 24 | |
| Protein C, % normal | 0.002 | ||
| Baseline | 68 ± 31 | 68 ± 28 | |
| Day 3 | 77 ± 32 | 90 ± 27 |
Definition of abbreviations: APC = activated protein C; PAI-1 = plasminogen activator inhibitor-1.
Levels are reported as mean ± SD. Analysis of covariance was used to test whether there was a difference in the change in biomarker levels between the APC- and placebo-treated groups.
Adverse Events
There were seven bleeding events (two severe adverse events) reported in the placebo arm compared with nine bleeding events (three severe adverse events) in the APC arm (P = 0.58) (see the online supplement for further details). There was no difference in the number of severe adverse events reported in the two treatment arms (14/38 in the placebo arm, 12/37 in the APC arm; P = 0.69).
DISCUSSION
On the basis of the established contribution of microvascular injury and inflammation to the pathogenesis of ALI, we designed this placebo-controlled, randomized clinical trial to test the efficacy of APC for the treatment of ALI in the absence of severe sepsis with a high risk of death. The National Heart, Lung, and Blood Institute DSMB stopped the trial for futility after 75 patients were enrolled when a planned interim analysis showed no difference in the primary outcome variable (ventilator-free days, a median of 19 in both groups; 95% CI, −3 to 4 d) and no difference in 60-day mortality.
Our study population differed significantly from that of most clinical trials of ALI because of the exclusion of patients with severe sepsis and an APACHE II score greater than or equal to 25. This exclusion was mandated by the DSMB. Interestingly, the mortality rate of subjects in our study was only 13%, compared with reported mortality rates of approximately 25% in large clinical trials of ALI that do not exclude septic patients with an APACHE II score greater than or equal to 25 (20–22). The mortality rate of subjects in our study is similar to that reported in the Administration of Drotrecogin Alfa in Early Stage Sepsis (ADDRESS) clinical trial in subjects with an APACHE II score of less than 20 (23), which focused on patients with sepsis and a low risk of death, primarily defined as those with either single-organ failure or an APACHE II score of less than 25. Our low mortality rate is likely due to lower overall severity of illness because of the exclusion of patients with severe sepsis at high risk of death or with increased risk of bleeding with APC therapy—for example, patients with evidence of coagulopathy or thrombocytopenia. Given the overall mortality rates of approximately 25% in recent clinical trials of ALI, a significant proportion of attributable mortality in those studies likely occurs in patients with ALI and a higher severity of sepsis. Indeed, in a reanalysis of data from a study of low-dose steroids for refractory septic shock, the mortality rate of patient with ALI and severe sepsis treated with placebo was 67% (24).
There are some limitations to our trial. First, there was a small but statistically significant difference in the baseline pulmonary dead space fraction between the APC-treated and placebo groups (0.62 vs. 0.55, P = 0.03). However, there was no difference between the two groups with regard to other baseline respiratory characteristics (PaO2:FiO2, pH, lung injury score). Furthermore, even after adjusting for the lung injury score and the baseline difference in dead space fraction, there was no statistically significant difference in the number of ventilator-free days between the two groups.
Second, the number of patients in our trial was modest (n = 75), and therefore we had limited statistical power to detect a difference in the primary endpoint, ventilator-free days. However, an important objective of phase II clinical trials is to identify physiologic and biological signals to suggest that a meaningful clinical difference would likely occur in a larger, phase III clinical trial. We did not observe a statistically significant difference in the primary endpoint of ventilator-free days, nor in the secondary endpoints of 60-day mortality or organ failure–free days, noting that we cannot completely exclude a difference between the two groups (either a benefit or harm) within the 95% CIs of the difference (−3 to 4 d in the case of ventilator-free days). However, there was no observed difference with APC treatment in any of the clinical endpoints, despite biological evidence that the administered treatment was active. Specifically, compared with placebo-treated control subjects, patients treated with APC had a significant increase in plasma protein C levels over the first 3 days of the study (Figure 2). In addition, given the anticoagulant and profibrinolytic mechanisms of action of APC, we hypothesized that administration of APC would improve the lung microcirculation, leading to better ventilation–perfusion matching, and therefore decrease the dead space in the lung. Indeed, dead space fraction decreased in patients treated with APC, compared with control subjects (P = 0.02). However, there was no change in other pulmonary physiologic parameters, including PaO2:FiO2 ratio or lung injury score with APC treatment. Given the lack of evidence from our data to support improved outcomes with APC in this patient population, and given the known bleeding risk associated with APC (14), the DSMB chose to stop the study at 75 patients, rather than allowing enrollment to the planned sample size of 90 patients. It is unlikely that inclusion of another 15 patients would have substantially changed the results of this trial because the difference in ventilator-free days was zero. Nonetheless, we recognize that our results do not exclude a small, beneficial effect of APC on the primary endpoint of ventilator-free days and that with a small phase II trial such as this, a type II error is always possible.
Furthermore, our results are consistent with the evidence that has emerged since the start of this clinical trial indicating that APC has limited benefit in patients who are less critically ill than the patients in the original PROWESS trial. In that trial, benefit was observed primarily in patients with higher disease severity as measured by an APACHE II score greater than or equal to 25 or multiorgan failure (14, 23). The ADDRESS clinical trial (n = 2,640) focused on patients with lower disease severity with severe sepsis and found no benefit (23). The RESOLVE (Researching Severe Sepsis and Organ Dysfunction in Children: a Global Perspective) clinical trial (n = 477) focused on children with sepsis, who are more likely to survive than adults, and also found no benefit (25). Thus, although our trial is relatively small, the results are consistent with other studies indicating that patients with lower disease severity may not benefit from APC. In particular, the results of our study suggest that patients with ALI and a lower risk of death do not benefit from APC. Also, patients treated with APC are at an increased risk of bleeding events; the increase in serious bleeding events in the treatment group was 1.5% in the PROWESS and 1.7% in the ADDRESS trials (14, 23). Given the known increased risk of bleeding with APC therapy, these results do not support a large, phase III clinical trial of APC as a therapy for ALI in the absence of sepsis and an associated high risk of death. In fact, given the extremely low mortality rate of this patient population, a trial adequately powered to test the hypothesis that APC reduces mortality associated with ALI would be prohibitively large. The impact of APC on outcomes in patients with persistent septic shock, a group with a high risk of death (26), is being examined in an ongoing phase III placebo-controlled trial, PROWESS-SHOCK (27).
Supplementary Material
Acknowledgments
The authors thank Drs. Carolyn Calfee, David Glidden, and Mark Looney for helpful comments, and Eli Lilly and Company for donating the study drug and placebo for this study. Lilly played no role in the study design, analysis, or presentation of the results.
Supported by NHLBI SCCOR HL74005; K.D.L. was supported by the National Institutes of Health Roadmap for Medical Research 8 K12 RR023262.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200803-419OC on June 19, 2008
Conflict of Interest Statement: K.D.L. owned shares in Eli Lilly until 2006. J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.H.K. received a speaker's fee of $1,000 from Respironics in 2006. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S. has received $160,000 in grant support from Eli Lilly and Co. (2005–2007) for participating in multicenter clinical trials; he has received $6,000 in 2005, $6,000 in 2006, and $10,500 in 2007 for speaking at conferences sponsored by Eli Lilly and Co., and provided consultant services to Eli Lilly in 2006. M.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.D.S. received $45,619 as a research grant for participation in a multicenter study sponsored by Eli Lilly investigating activated protein C with and without heparin for the treatment of severe sepsis. G.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.W.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.W. participated in a multicenter study looking at markers for acute graft dysfunction in lung transplant recipients funded by Eli Lilly. B.T.T. chaired the Data Safety Monitoring Board for the Lilly-sponsored XPRESS trial (sivelestat for ARDS) and received $12,475 for this activity between 2002 and 2005. B.T.T. is the co-principal investigator of the Lilly-sponsored PROWESS-SHOCK trial (rhAPC in septic shock) and has received $12,224 for work on this project to date. B.T.T. also chairs the Data Safety Monitoring Board for an AstraZeneca-sponsored phase II trial of anti-tumor necrosis factor antibodies in severe sepsis and has received $3,100 to date. B.T.T. received $2,500 from Pfizer to serve on an ARDS research advisory board. M.D.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The members of the National Heart, Lung, and Blood Data Safety Monitoring Board were as follows: Bennett deBoisblanc (Chair), Jonathan E. Gottlieb, R. Duncan Hite, Robert P. McMahon, Adrienne G. Randolph, Barbra B. Rothschild, Darrell J. Triulzi, Richard G. Wunderink, and Jacob Iasha Sznajder.
In addition to the study authors, the members of this National Heart, Lung, and Blood Acute Lung Injury Specialized Center of Clinically Oriented Research–sponsored clinical trial included the following: Baystate Medical Center: Lori-Ann Kozikowski, R.N., Krystal Kozikowski, Cynthia Kardo, R.N., Lesley De Souza; San Francisco General Hospital: John Luce, M.D., Rochelle Dicker, M.D.; Oregon Health and Science University: Jeffrey Gold, M.D., Kathy D. Bacon, Krista Thorne-Yocam; Stanford University: Geraldine O'Riordan, R.N., Marta Krupa-Plonowska, M.D., M.B.A.; University of California, San Francisco: Jeanine Wiener-Kronish, M.D., Magda Cepkova, M.D., Thomas Quinn, M.D., Brian Daniel, R.R.T., Sonia Sharma, R.R.T., Oscar Garcia, R.R.T., Ron Brown, Michelle Teng, Michelle Goldfinger; UCSF, Fresno: Jill Lanford, R.N., B.S.N., Wade Veneman; University of Southern California: Janice Liebler, M.D., Sujith Shetty, M.D., Travis Alexander; Yale University: Kathryn M. Engle, R.N., M.S., C.C.R.C.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 3.Cepkova M, Matthay MA. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med 2006;21:119–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 5.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest 2007;131:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis 1977;116:589–615. [DOI] [PubMed] [Google Scholar]
- 7.Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med 2000;21:435–466. [DOI] [PubMed] [Google Scholar]
- 8.Tomashefski J Jr, Davies P, Boggis C, Greene R, Zapol W, Reid L. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 9.Zapol W, Jones R. Vascular components of ARDS: clinical pulmonary hemodynamics and morthology. Am Rev Respir Dis 1987;136:471–474. [DOI] [PubMed] [Google Scholar]
- 10.Ware L, Fang X, Matthay M. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;285:L514–L521. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 2007;35:1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prabhakaran P, Ware L, White K, Cross M, Matthay M, Olman M. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003;285:L20–L28. [DOI] [PubMed] [Google Scholar]
- 13.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000;407:258–264. [DOI] [PubMed] [Google Scholar]
- 14.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001;344:699–709. [DOI] [PubMed] [Google Scholar]
- 15.Liu KD, Levitt J, Zhuo H, Brady S, Steingrub J, Siegel M, Soto G, Peterson M, Chesnutt M, Weinacker A, et al. Randomized, placebo-controlled trial of activated protein C for the treatment of acute lung injury [abstract]. Am J Respir Crit Care Med 2008;177:A766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 17.Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest 1997;112:164–172. [DOI] [PubMed] [Google Scholar]
- 18.Kallet RH, Daniel BM, Garcia O, Matthay MA. Accuracy of physiologic dead space measurements in patients with acute respiratory distress syndrome using volumetric capnography: comparison with the metabolic monitor method. Respir Care 2005;50:462–467. [PubMed] [Google Scholar]
- 19.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720–723. [DOI] [PubMed] [Google Scholar]
- 20.Brower R, Lanken P, MacIntyre N, Matthay M, Morris A, Ancukiewicz M, Schoenfeld D, Thompson B; National Heart, Lung, and Blood Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327–336. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213–2224. [DOI] [PubMed] [Google Scholar]
- 22.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E, Laterre PF, Garg R, Levy H, Talwar D, Trzaskoma BL, Francois B, Guy JS, Bruckmann M, Rea-Neto A, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med 2005;353:1332–1341. [DOI] [PubMed] [Google Scholar]
- 24.Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 2006;34:22–30. [DOI] [PubMed] [Google Scholar]
- 25.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 2007;369:836–843. [DOI] [PubMed] [Google Scholar]
- 26.Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, Holmes CL, Mehta S, Granton JT, Storms MM, Cook DJ, Presneill JJ, Ayers D; Vasopressin And Septic Shock Trial (VASST) Investigators. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008;358:877–887. [DOI] [PubMed] [Google Scholar]
- 27.Barie PS. “All in” for a huge pot: the PROWESS-SHOCK trial for refractory septic shock. Surg Infect (Larchmt) 2007;8:491–494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.