Abstract
Rationale: Bacterial pneumonia is a major cause of morbidity for HIV-infected persons and contributes to excess mortality in this population.
Objectives: To evaluate the frequency and risk factors for occurrence of bacterial pneumonia in the present era of potent antiretroviral therapy.
Methods: We evaluated data from a randomized trial of episodic antiretroviral therapy. The study, Strategies for Management of Antiretroviral Therapy, enrolled 5,472 participants at 318 sites in 33 countries. Study patients had more than 350 CD4 cells at baseline. Diagnosis of bacterial pneumonia was confirmed by a blinded clinical-events committee.
Measurements and Main Results: During a mean follow-up of 16 months, 116 participants (2.2%) developed at least one episode of bacterial pneumonia. Patients randomized to receive episodic antiretroviral therapy were significantly more likely to develop pneumonia than patients randomized to receive continuous antiretroviral therapy (hazard ratio, 1.55; 95% confidence interval, 1.07–2.25; P = 0.02). Cigarette smoking was a major risk factor: Current-smokers had more than an 80% higher risk of pneumonia compared with never-smokers (hazard ratio, 1.82; 95% confidence interval, 1.09–3.04; P = 0.02). Participants who were on continuous HIV treatment and were current smokers were three times more likely to develop bacterial pneumonia than nonsmokers. Current smoking status was significant, but a past history of smoking was not.
Conclusions: Bacterial pneumonia is a major source of morbidity, even for persons on potent antiretroviral therapy, including those with high CD4 cells. Efforts to reduce this illness should stress the importance of uninterrupted antiretroviral therapy and attainment and/or maintenance of nonsmoking status.
Clinical trial registered with www.clinicaltrials.gov (NCT 00027352).
Keywords: pneumonia, smoking, HIV, bacterial infections
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although bacterial pneumonia is known to be a major cause of morbidity and mortality in HIV-infected persons, no large studies have evaluated this problem in the current era of potent antiretroviral therapy.
What This Study Adds to the Field
This multinational study demonstrates that bacterial pneumonia remains a substantial problem, even in patients with high CD4+ cell counts on HIV therapy. Cigarette smoking was found to be the single greatest risk factor for the development of pneumonia.
Bacterial pneumonia is well known to occur at excess rates in persons with HIV infection (1–5). Since the early years of the HIV epidemic, high rates of bacterial infections were reported in patients with AIDS and HIV infection (1–3, 6, 7). These bacterial infections were typically bacteremia, skin and soft tissue infections, sinusitis, and, most commonly, bacterial pneumonia.
The increased morbidity and mortality associated with bacterial pneumonia in HIV-infected persons was recognized by the Centers for Disease Control in 1993, when it categorized two or more episodes of bacterial pneumonia within 1 year as an AIDS-defining event (8). Even a single episode of bacterial pneumonia is associated with substantial morbidity and mortality (2–6, 9).
The risk of development of bacterial pneumonia in HIV-infected persons has not been assessed in large, widely applicable groups since the early 1990s, which was before the availability of effective combination antiretroviral therapy. Multiple studies have demonstrated a clear correlation of an increased rate of pneumonia with decreasing CD4 cell counts (4, 5, 10). Cigarette smoking and the use of injection drugs have also have been reported for HIV-infected persons as factors associated with an increased risk of developing of bacterial pneumonia (4, 7, 10, 11).
Given the substantial impact of bacterial pneumonia on the health of HIV-infected persons, it is important to understand the epidemiology of this infection in the current era of potent antiretroviral therapy. Furthermore, knowledge of modifiable risk factors for bacterial pneumonia allows providers and patients to reduce morbidity from this illness.
We used data from a recently concluded study, Strategies for Management of Antiretroviral Therapy (SMART) (12), to evaluate the impact of continuous antiretroviral therapy on the incidence of bacterial pneumonia and to identify risk factors for the occurrence of this infection. Preliminary data from this study were previously reported as an abstract (13).
METHODS
A detailed description of the methods and the main results of the SMART study have been published elsewhere (12).
Study Population
Eligibility criteria included a CD4 count greater than 350 CD4 cells/mm3, age greater than 13 years, and a willingness to start or stop antiretroviral treatment according to the protocol. Participants could be on or off antiretroviral therapy at the time of study entry. Participants were randomized equally to one of two groups: the viral suppression (VS) group, who were assigned to stay on continuous potent antiretroviral therapy throughout the study, with a goal of full viral suppression, or the drug conservation (DC) group, who were to stop their antiretroviral therapy, if any, at study entry and remain off therapy until their CD4 cell count was less than 250 cells/mm3 and then to (re)start and continue potent antiretroviral therapy until their CD4 cells were confirmed to be greater than 350 cells/mm3. Participants in the DC group repeated this cycle of CD4 cell count–guided treatment and interruption throughout the trial.
This study was approved at all local institutional review boards and ethics committees, and all patients gave informed consent.
Data Collection and Follow-up
Before randomization, participants received a targeted physical examination and had their medical history taken. Baseline data included history of smoking (current, past, or never) and injection-drug use. Participants were asked at baseline about their history of prior AIDS-defining events, including history of prior/recurrent bacterial pneumonia. No information was collected about immunizations or about the routine use of antibiotics, including co-trimoxazole. Follow-up study visits were scheduled at 1 and 2 months after randomization, then every 2 months for the first year, and then every 4 months for the remainder of the study.
During the study, a standardized case report form was used to collect information on patients with suspected bacterial pneumonia. Patients were evaluated for pneumonia using local clinical standards and guidelines. A central clinical events committee, blinded to study arm, evaluated each report and classified participants into one of three categories: (1) “confirmed” (compatible clinical and radiographic evidence with histologic or microbiologic support); (2) “probable” (signs and symptoms of pneumonia with compatible radiographic abnormalities); or (3) “suspected” (signs or symptoms of pneumonia with no supporting radiographic evidence). Participants were categorized as having recurrent bacterial pneumonia (an AIDS-defining event) if, during a 12-month period, they had two or more episodes of probable or confirmed pneumonia with strong evidence that the first episode of pneumonia was resolved before the onset of the second episode. In this report, we include data on all patients with one or more episode of bacterial pneumonia that was classified by the clinical endpoint committee as “confirmed” or “probable.”
Statistical Methods
Analyses were by intention to treat. Kaplan-Meier survival curves and Cox proportional hazards models were used to compare the DC and VS treatment groups with respect to event rates for first bacterial pneumonia event. Follow-up data were censored on January 11, 2006, at the termination of the randomized portion of the trial, or when participants were lost to follow-up before that date. Baseline predictors for first occurrence of bacterial pneumonia were identified and tested using Cox proportional hazard models after adjustment for treatment group. Cox proportional hazard models were used to assess the effects of CD4+ counts and HIV RNA levels as time-dependent covariates during follow-up on the hazard ratio (HR) for first occurrence of bacterial pneumonia.
Person-years at specific CD4+ counts and HIV-RNA levels were calculated according to the latest measured value. Poisson regression was used to estimate relative risks for the rates of the outcomes in the DC versus VS treatment groups in strata according to latest CD4+ counts and HIV-RNA levels.
All reported P values are two-sided. Statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
Between January 8, 2002, and January 11, 2006, a total of 5,472 participants were randomized in SMART from a total of 318 sites in 33 countries. Selected baseline characteristics of this patient population are shown in Table 1. Participants in SMART had high baseline CD4 cell counts, with a median of 597 cells/mm3 at baseline. Cigarette smoking was common, with 41% of persons identified as smokers at baseline and an additional 25% of participants reporting a history of prior smoking. Injection drug use was reported by 10% of participants as their likely mode of HIV acquisition. Prior AIDS-defining recurrent bacterial pneumonia was reported for 1.8% of participants.
TABLE 1.
BASELINE CHARACTERISTICS OF SMART STUDY POPULATION
| Characteristic | Drug Conservation Group (n = 2,720) | Viral Suppression Group (n = 2,752) | All (n = 5,472) |
|---|---|---|---|
| Age, yr (median) | 43 | 44 | 43 |
| Sex, % female | 26.3 | 28.0 | 27.2 |
| Race, % | |||
| Black | 28.5 | 29.8 | 29.1 |
| White | 56.4 | 54.8 | 55.6 |
| Other | 15.1 | 15.4 | 15.3 |
| Mode of infection with HIV, % | |||
| Sexual contact | |||
| With person of same sex | 51.4 | 48.4 | 49.9 |
| With person of opposite sex | 44.4 | 45.6 | 45.0 |
| Injection drug use | 9.9 | 9.6 | 9.7 |
| Other/unknown | 7.5 | 8.7 | 8.1 |
| CD4 cells/mm3, median (IQR) | 597 (467–791) | 598 (465–489) | 597 (466–790) |
| CD4 nadir, cells/mm3, median (IQR) | 250 (152–358) | 250 (157–358) | 250 (154–358) |
| HIV-RNA ≤400 copies/ml, % | 71.9 | 71.5 | 71.7 |
| Smoking, % | |||
| Current | 41.3 | 39.7 | 40.5 |
| Past | 24.6 | 25.0 | 24.8 |
| Never | 34.1 | 35.3 | 34.7 |
| History of recurrent bacterial pneumonia, %* | 1.8 | 1.7 | 1.8 |
Definition of abbreviation: IQR = interquartile range; SMART = Strategies for Management of Antiretroviral Therapy.
Within 12 months, at least two episodes of pneumonia.
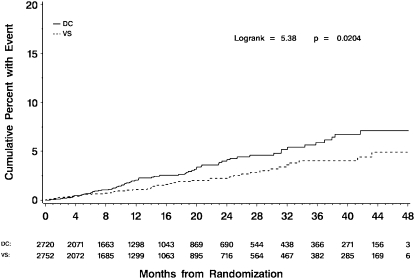
During a mean follow-up period of 16 months, 116 participants developed at least one episode of bacterial pneumonia (Table 2 and Figure 1). Overall, 98 (84%) of the bacterial pneumonia events were classified as “probable,” and 18 (16%) were classified as “confirmed.” An additional 29 persons had a “suspected” episode for which no radiologic confirmation was available. Participants randomized to receive CD4-guided treatment interruption (the DC group) were significantly more likely to develop bacterial pneumonia than patients on continuous antiretroviral therapy (the VS group), with an HR of 1.55 (95% confidence interval [CI], 1.07–2.25; P = 0.02). Only eight participants in the DC group and three participants in the VS group had recurrent bacterial pneumonia, defined as a second episode within 1 year. The most recent CD4 cell count just before the pneumonia event was lower in the DC group (median, 411; interquartile range [IQR], 299–572), compared with the VS group (median, 547; IQR, 398–733; P = 0.004). Most individuals with reported pneumonia had no organisms identified, but, of those with documented organisms, the most common were Streptococcus pneumoniae (10 cases) and Staphylococcus aureus (three cases, none of whom had a history of injection drug use). Two patients were infected with Haemophilus influenza and three patients were infected with other bacteria. No deaths were classified as being related to a pneumonia event.
TABLE 2.
BACTERIAL PNEUMONIA EVENTS
| DC Group, n (rate*) | VS Group n (rate*) | HR (95% CI) (DC/VS) | P Value | |
|---|---|---|---|---|
| Initial occurrence | 70 (2.0) | 46 (1.3) | 1.55 (1.07–2.25) | 0.02 |
| Categorization | ||||
| Confirmed | 9 | 9 | ||
| Probable | 61 | 37 | ||
| Recurrence | 8 (0.2) | 3 (0.1) | 2.67 (0.71–10.1) | 0.15 |
Definition of abbreviations: CI = confidence interval; DC = drug conservation; HR = hazard ratio; VS = viral suppression.
Per 100 person-years.
Figure 1.
Kaplan-Meier curves for time to first episode of bacterial pneumonia. The solid line represents the viral suppression (VS) group; the dashed line represents the drug conservation (DC) group. The overall hazard ratio of events (DC/VS) was 1.55 (95% confidence interval, 1.07–2.25; P = 0.02).
Table 3 shows the baseline predictors for the first occurrence of bacterial pneumonia for all participants in SMART. In the multivariable Cox regression analysis, factors that were significant predictors of bacterial pneumonia were having been randomized to the DC group; current cigarette smoking; and history of prior, recurrent bacterial pneumonia. In the univariate analysis, predictors were higher baseline HIV-RNA, past smoking, history of injection-drug use, and Black race. For participants in the VS group randomized to be on continuous therapy, significant baseline predictors for the first occurrence of bacterial pneumonia were current cigarette smoking, Black race, and baseline viral load (data not shown). Cigarette smoking was the strongest single predictor for the development of bacterial pneumonia in the VS group. In this arm, current smokers experienced a threefold increase in risk compared with persons who never smoked, with a HR of 3.01 (95% CI, 1.30–6.96; P = 0.01). The HR for past-smokers compared with never-smokers was 1.42 (95% CI, 0.52–3.88; P = 0.49).
TABLE 3.
BASELINE PREDICTORS FOR BACTERIAL PNEUMONIA BY STUDY ARMS—DRUG CONSERVATION AND VIRAL SUPPRESSION
| Univariate Cox Regression*
|
Multivariate Cox Regression†
|
||||||
|---|---|---|---|---|---|---|---|
| Predictors | n (%) | Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value |
| Treatment group | |||||||
| DC | 2720 (49.7) | 1.55 | 1.07–2.25 | 0.02 | 1.55 | 1.07–2.25 | 0.02 |
| VS | 2752 (50.3) | ||||||
| Baseline CD4 (per 100 cells/mm3) | 0.95 | 0.88–1.03 | 0.18 | 0.97 | 0.89–1.06 | 0.52 | |
| Nadir CD4 (per 100 cells/mm3) | 0.98 | 0.88–1.09 | 0.72 | 0.98 | 0.87–1.10 | 0.70 | |
| Baseline HIV-RNA, log10 copies/ml | 1.19 | 1.02–1.39 | 0.04 | 1.11 | 0.93–1.33 | 0.26 | |
| Cigarette smoking | |||||||
| Current | 2,215 (40.5) | 2.19 | 1.34–3.60 | 0.002 | 1.82 | 1.09–3.04 | 0.02 |
| Past | 1,358 (24.8) | 1.80 | 1.04–3.11 | 0.03 | 1.64 | 0.94–2.86 | 0.08 |
| Never (ref) | 1,899 (34.7) | 1.00 | 1.00 | ||||
| Injection drug use | |||||||
| Yes | 532 (9.7) | 2.04 | 1.32–3.16 | 0.001 | 1.50 | 0.95–2.38 | 0.09 |
| No (ref) | 4,940 (90.3) | ||||||
| Race | |||||||
| White (ref) | 3,041 (55.6) | 1.00 | 1.00 | ||||
| Black | 1,595 (29.1) | 1.66 | 1.13–2.45 | 0.01 | 1.45 | 0.96–2.20 | 0.08 |
| Other | 836 (15.3) | 0.80 | 0.43–1.52 | 0.50 | 0.79 | 0.41–1.50 | 0.47 |
| Sex | |||||||
| Male | 3,986 (72.8) | 0.99 | 0.65–1.51 | 0.97 | 1.04 | 0.67–1.61 | 0.86 |
| Female (ref) | 1,486 (27.2) | ||||||
| Prior recurrent bacterial pneumonia‡ | |||||||
| Yes | 97 (1.8) | 3.61 | 1.88–6.91 | 0.0001 | 2.60 | 1.33–5.09 | 0.005 |
| No (ref) | 5,375 (98.2) | ||||||
Definition of abbreviations: CI = confidence interval; DC = drug conservation; VS = viral suppression.
Adjusted for treatment group.
Adjusted for treatment group and all other variables.
Within 12 months, fewer than episodes of pneumonia.
The relationship between most recent CD4 cell count and the risk of developing bacterial pneumonia is shown in Table 4. Overall, the rate was highest in participants with fewer than 250 CD4 cell counts (3.3 events per 100 person-years of follow-up) and lowest in participants with 500 or more CD4 cell counts (1.4 events per 100 person-years of follow-up). The overall HR for bacterial pneumonia was 0.92 per 100 cells/mm3 increase in proximal CD4 cell count (P = 0.04). In comparing participants in the DC group with those in the VS group, there was a significant difference in the risk of developing bacterial pneumonia for those participants with 500 or more CD4 cells. In this group of patients with high CD4 cells, the development of bacterial pneumonia was over twice as great for persons on CD4-guided intermittent treatment as it was for persons assigned to continuous treatment (P = 0.01).
TABLE 4.
FIRST OCCURRENCE OF BACTERIAL PNEUMONIA BY PROXIMAL CD4 CELL COUNT CATEGORY
| DC Group
|
VS Group
|
Overall
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 Category | PY* | Events† | Rate‡ | PY* | Events† | Rate‡ | PY* | Events† | Rate‡ | RR (DC/VS) | P Value |
| <250 cells/mm3 | 317 | 11 | 3.5 | 72 | 2 | 2.8 | 388 | 13 | 3.3 | 1.25 | 0.775 |
| 250–349 cells/mm3 | 773 | 13 | 1.7 | 202 | 6 | 3.0 | 975 | 19 | 1.9 | 0.56 | 0.247 |
| 350–499 cells/mm3 | 1,193 | 19 | 1.6 | 820 | 12 | 1.5 | 2,013 | 31 | 1.5 | 1.09 | 0.818 |
| 500+ cells/mm3 | 1,295 | 27 | 2.1 | 2,541 | 26 | 1.0 | 3,836 | 53 | 1.4 | 2.04 | 0.010 |
| Total | 3,577 | 70 | 2.0 | 3,634 | 46 | 1.3 | 7,211 | 116 | 1.6 | 1.55 | 0.022 |
Definition of abbreviations: DC = drug conservation; PY = person-years; RR = relative risk; VS = viral suppression.
Time spent in a given CD4 category, censored at event.
Bacterial pneumonia with proximal CD4 cell count in the given category.
Per 100 person-years.
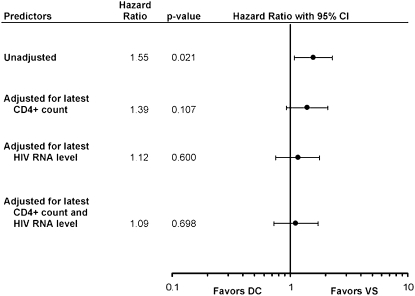
Figure 2 displays the unadjusted and adjusted HR for the development of bacterial pneumonia in the DC group compared with the VS group. Adjusting for latest CD4 cell count and for latest HIV-RNA level, each lowered the risk of developing bacterial pneumonia in the DC group. Adjusting for CD4 cell count and HIV-RNA level reduced the HR to 1.09 (P = 0.70). The control of viral load had a significant impact on the development of bacterial pneumonia (Table 5). For persons in the VS group with most recent viral loads of less than 400 copies/mL, the risk of developing bacterial pneumonia was 0.9 per 100 person-years of follow-up, compared with a risk of 2.3 per 100 person-years for persons with a viral load of greater than 400 copies/mL for a HR of 2.65 (95% CI, 1.49–4.72; P = 0.001). Control of viral load did not affect the risk of developing bacterial pneumonia for persons in the DC group.
Figure 2.
Estimated unadjusted and adjusted hazard ratios—drug conservation (DC) versus viral suppression (VS)—for first occurrence of bacterial pneumonia. The vertical line represents a hazard ratio of 1. Each solid circle represents the actual hazard ratios for that predictor, and each horizontal line represents the 95% confidence interval for that predictor.
TABLE 5.
RATES OF BACTERIAL PNEUMONIA BY TREATMENT GROUP AND BY MOST RECENT CD4 AND VIRAL LOAD
| Proximal HIV RNA ≤400
|
Proximal HIV-RNA >400
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4 Cell Count | Person-Years | Events | Rate | Person-Years | Events | Rate | RR (>400/≤400) (95% CI) | P Value |
| Viral suppression group | ||||||||
| <500 cells/mm3 | 627 | 7 | 1.1 | 468 | 13 | 2.8 | 2.49 (0.99–6.25) | 0.051 |
| 500+ cells/mm3 | 2,016 | 16 | 0.8 | 524 | 10 | 1.9 | 2.40 (1.09–5.30) | 0.03 |
| Overall | 2,643 | 23 | 0.9 | 990 | 23 | 2.3 | 2.65 (1.49–4.72) | 0.001 |
| Drug conservation group | ||||||||
| <500 cells/mm3 | 569 | 8 | 1.4 | 1,713 | 35 | 2.0 | 1.45 (0.67–3.13) | 0.34 |
| 500+ cells/mm3 | 457 | 10 | 2.2 | 837 | 17 | 2.0 | 0.93 (0.43–2.03) | 0.85 |
| Overall | 1,026 | 18 | 1.8 | 2,551 | 52 | 2.0 | 1.16 (0.68–1.99) | 0.58 |
Definition of abbreviations: CI = confidence interval; RR = relative risk.
DISCUSSION
We have shown that, in the era of potent antiretroviral therapy, bacterial pneumonia continues to be a major cause of morbidity in HIV-infected patients. In the SMART study, bacterial pneumonia was the single most frequent clinical event reported—occurring in 116 persons, or 2.1% of the population—during the mean follow-up period of 16 months. Most participants had only a single episode of bacterial pneumonia, although 11 persons had at least two episodes within a year (i.e., an AIDS-defining event).
This analysis has identified two factors strongly associated with an increased risk of bacterial pneumonia in HIV-infected patients: (1) the episodic use of antiretroviral therapy and (2) cigarette smoking.
The increased risk of bacterial pneumonia with a decrease in CD4 cell count, recognized in earlier cohort studies (4, 5, 10, 14), was also evident in SMART. Participants in the DC group were significantly more likely to develop bacterial pneumonia due, in large part, to the lower average CD4 cell count in the DC group. The mean CD4 cell count before a bacterial pneumonia event was 460 cells/mm3 in the DC group (IQR, 299–572), compared with 611 cells/mm3 in the VS group (IQR, 398–733) (P = 0.004). There was a clear trend toward higher rates of bacterial pneumonia as the CD4 cell counts declined (Table 4). For example, for all people in the study, the risk of bacterial pneumonia was 3.3 per 100 person-years for those with fewer than 250 CD4 cells, compared with a risk of 1.4 per 100 person-years for those with 500 or more CD4 cells. We demonstrated that this association holds for participants receiving continuous potent antiretroviral therapy: For participants in the VS group, the risk of bacterial pneumonia was 2.8 per 100 person-years for those with fewer than 250 CD4 cells compared with a rate of 1.0 per 100 person-years for those with 500 or more CD4 cells.
An additional important finding is the demonstration that antiretroviral therapy reduces the risk of bacterial pneumonia, even for persons with CD4 cell counts that are usually considered high (≥500). In SMART, participants with recent CD4 cell counts of 500 or more in the DC group (predominantly off antiretroviral therapy) had over twice the risk of developing bacterial pneumonia—a rate of 2.1 per 100 person-years—compared with participants in the VS group (predominantly on therapy) with a risk of 1.0 per 100 person-years (P = 0.01). Thus, even for individuals with CD4 cell counts above the range where HIV treatment is usually initiated, the use of antiretroviral therapy lessened the chance of developing bacterial pneumonia.
The impact of antiretroviral therapy seemed to occur, in part, due to a reduction in viral load. For persons in the VS group with 500 or more CD4 cells, the risk of bacterial pneumonia was over twice as high for individuals with a viral RNA of greater than 400 copies/ml as it was for those with fewer than 400 copies/ml (Table 5). Adjusting for latest HIV-RNA level decreases the HR (DC/VS) more than adjusting by latest CD4 cell count (Figure 2). The mechanism by which reduction of viral load reduces the risk of bacterial pneumonia is unknown; however, recent data have shown that in the SMART study, patients with uncontrolled viral replication had higher levels of inflammatory markers, including IL-6 and d-dimer, and that this was associated with an increased risk of death (15). It is possible that increased inflammation in patients with HIV-viral replication may be associated with a higher rate of pneumonia, although there is no direct evidence to support this hypothesis.
In the general population, cigarette smoking has been related to increased rates of infection (16). Increased rates of bacterial infections (including pneumococcal pneumonia and Legionnaires' disease) and of viral infections (including influenza) have been reported in smokers compared with nonsmokers. In the HIV-infected population, cigarette smoking has been demonstrated by several studies to be an added risk factor for the development of bacterial pneumonia and for increased mortality (4, 10, 11, 17, 18).
Cigarette smoking was also found to be a major risk factor for the development of bacterial pneumonia among SMART participants. In the overall univariate analysis, current smokers were found to have more than double the risk of pneumonia compared with never-smokers, with an HR of 2.19 (95% CI, 1.34–3.60; P = 0.002); after adjusting for other baseline variables, the risk of bacterial pneumonia remained approximately 80% higher for current smokers, with an HR of 1.82 (95% CI, 1.09–3.04; P = 0.02). Current smoking was the strongest single predictor (other than a prior history of bacterial pneumonia) of the HIV-infected patient developing bacterial pneumonia.
Cigarette smoking remains a major risk factor even in people with relatively high CD4 cell counts. In the 1995 report on bacterial pneumonia from the Pulmonary Complications of HIV Infection Study Group, smoking was an independent risk factor for bacterial pneumonia only in persons with 200 or fewer CD4 cells but not in persons with higher numbers of CD4 cells (4). In the Terry Beirn Community Programs for Clinical Research on AIDS 1996 report on the impact of cigarette smoking, bacterial pneumonia was 57% more likely to occur in current smokers; the baseline CD4 cell count for that cohort, however, was less than 300 (11). In SMART, the baseline CD4 cell count was 597, and the majority of bacterial pneumonia events occurred in people with a proximal CD4 cell count of 350 or higher (83 of 116 events); thus, the finding of this study that cigarette smoking is a major risk factor for the development of bacterial pneumonia is of importance to the many HIV-infected persons who have CD4 cell counts of 350 or higher.
The design of the SMART study also makes possible an analysis of the impact of smoking on patients taking potent antiretroviral treatment. The data show that the relative negative impact of smoking was greatest in this group of patients who are receiving standard therapy. Current smokers in the VS group were three times more likely to develop bacterial pneumonia than nonsmokers. Cessation of smoking was shown to be of value; former smokers in the VS group had a risk of bacterial pneumonia that was not significantly different from never-smokers.
In this trial, most bacterial pneumonia events were classified as “probable,” based on radiologic changes with compatible signs and symptoms. Similar diagnostic criteria have been used in other reports of bacterial pneumonia in HIV-infected persons (4, 5, 10, 11, 19). Most reports of bacterial pneumonia in HIV-infected individuals have emphasized the importance of S. pneumoniae, S. aureus, and Haemophilus influenzae as etiologic agents (2, 4, 5, 7, 9, 17, 19, 20). In most case series of pneumonia, however, an etiologic agent could not be identified. For example, in the Pulmonary Complications of HIV Study, 148 of 237 episodes of bacterial pneumonia had no organism identified (4). Some recent reports have noted a decreased incidence of invasive disease due to S. pneumonia in HIV-infected persons (21–24), perhaps due to improved efficacy of pneumococcal vaccine in the general population and perhaps in HIV-infected persons (25–29). We did not have information on the use of pneumococcal vaccination in the participants in SMART, but S. pneumoniae remained the most commonly documented cause of bacterial pneumonia in this study, with 10 patients diagnosed with disease due to S. pneumoniae. Only two other pathogens were identified as the cause of pneumonia in more than one person: S. aureus in three patients and H. influenza in two patients. Thus, the use of pneumococcal vaccine should be emphasized as part of standard care for HIV-infected patients because S. pneumoniae remains the leading identified cause of bacterial pneumonia in this population.
The SMART study provides a unique dataset for evaluation of the incidence and risk factors for bacterial pneumonia in a large, randomized, clinical trial of HIV-infected persons. The demographic and geographic diversity of the population makes the findings reported here widely applicable, including in resource-limited settings, where bacterial pneumonia is common (28). The data show that bacterial pneumonia remains a major source of morbidity, even for persons on potent antiretroviral therapy, including those with high CD4 cells. On the basis of the findings in this report, providers and patients have two ways to reduce the occurrence of this illness. First, for people on antiretroviral therapy, it is important to remain on effective treatment on a continuous basis: SMART participants who cycled on and off antiretroviral therapy were 56% more likely to develop bacterial pneumonia. Remaining on continuous antiretroviral therapy is important even for patients with CD4 cell counts of 500 or higher because those persons who were cycling on and off treatment were twice as likely as persons on continuous antiretroviral therapy to develop bacterial pneumonia. Second, for HIV-infected individuals who smoke cigarettes, smoking cessation should be a major health goal: SMART participants on continuous antiretroviral therapy who continued to smoke were three times more likely to develop bacterial pneumonia than those who never smoked, and former smokers had no increased risk compared with never-smokers. Finally, these data suggest that, in terms of avoiding the serious morbidity of bacterial pneumonia, there may be benefit in initiating antiretroviral therapy earlier in HIV-infected persons because there was a gradient of increased risk for bacterial pneumonia even at high CD4+ cell counts.
Acknowledgments
The authors thank the dedicated participants in the SMART Study; the clinical investigators, study coordinators, and site personnel; the Statistical and Data Management and Operations centers; the Division of AIDS (NIAID); and Janet Royal and Laura Mol for technical assistance in preparation of the manuscript.
Supported by The National Institute of Allergy and Infectious Diseases, National Institutes of Health (U01AI042170 and U01AI46362).
Originally Published in Press as DOI: 10.1164/rccm.200804-617OC on July 10, 2008
Conflict of Interest Statement: F.M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.P.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.M.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.D.L. has received funds from Abbott, Bristol-Myers Squibb, Roche, Tibotec, Gilead, GlaxoSmithKline, Pfizer, Merck, and Boehringer Ingelheim for delivering lectures, for consulting, and for clinical trials. J.M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.N.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.National Heart, Lung, and Blood Institute Workshop. Special report: pulmonary complications of the acquired immunodeficiency syndrome. N Engl J Med 1984;310:1682–1688. [DOI] [PubMed] [Google Scholar]
- 2.Polsky B, Gold JW, Whimbey E, Dryjanski J, Brown AE, Schiffman G, Armstrong D. Bacterial pneumonia in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1986;104:38–41. [DOI] [PubMed] [Google Scholar]
- 3.Selwyn PA, Feingold AR, Hartel D, Schoenbaum EE, Alderman MH, Klein RS, Friedland GH. Increased risk of bacterial pneumonia in HIV-infected intravenous drug users without AIDS. AIDS 1988;2:267–272. [DOI] [PubMed] [Google Scholar]
- 4.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, Markowitz N, Rosen MJ, Mangura BT, Hopewell PC; The Pulmonary Complications of HIV Infection Study Group. Bacterial pneumonia in persons infected with the human immunodeficiency virus. N Engl J Med 1995;333:845–851. [DOI] [PubMed] [Google Scholar]
- 5.Boschini A, Smacchia C, Di Fine M, Schiesari A, Ballarini P, Arlotti M, Gabrielli C, Castellani G, Genova M, Pantani P, et al. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: incidence, etiologies, and clinical aspects. Clin Infect Dis 1996;23:107–113. [DOI] [PubMed] [Google Scholar]
- 6.Witt DJ, Craven DE, McCabe WR. Bacterial infections in adult patients with the acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Am J Med 1987;82:900–906. [DOI] [PubMed] [Google Scholar]
- 7.Nagappan V, Kazanjian P. Bacterial infections in adult HIV-infected patients. HIV Clin Trials 2005;6:213–228. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 1992;41. [PubMed]
- 9.Cordero E, Pachón J, Rivero A, Girón JA, Gómez-Mateos J, Merino MD, Torres-Tortosa M, Gonzalez-Serrano M, Aliaga L, Collado A, et al.; for the Grupo Andaluz Para el Estudio de las Enfermedades Infecciosas. Community-acquired bacterial pneumonia in human immunodeficiency virus-infected patients. Am J Respir Crit Care Med 2000;162:2063–2068. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan JH, Moore RD, Keruly JC, Chaisson RE. Effect of antiretroviral therapy on the incidence of bacterial pneumonia in patients with advanced HIV infection. Am J Respir Crit Care Med 2000;162:64–67. [DOI] [PubMed] [Google Scholar]
- 11.Burns DN, Hillman D, Neaton JD, Sherer R, Mitchell T, Capps L, Vallier WG, Thurnherr MD, Gordin FM; for the Terry Beirn Community Programs for Clinical Research on AIDS. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. J Acquir Immune Defic Syndr 1996;13:374–383. [DOI] [PubMed] [Google Scholar]
- 12.The Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 13.Gordin F, Roediger M, Clezy K, Easterbrook PJ, Girard P, Lundgren J, Miro JM, Palfreeman A, Rodriguez-Barradas M, Slater L, et al.; for the SMART Study Group and INSIGHT Network. An increased risk of bacterial pneumonia in patients interrupting antiretroviral treatment: results from the SMART study. Presented at the Fourth IAS Conference on HIV Pathogenesis, Treatment and Prevention, Sydney, Australia, July 2007.
- 14.Wallace JM, Hansen NI, Lavange L, Glassroth J, Browdy BL, Rosen MJ, Kvale PA, Mangura BT, Reichman LB, Hopewell PC; The Pulmonary Complications of HIV Infection Study Group. Respiratory disease trends in the pulmonary complications of HIV infection study cohort. Am J Respir Crit Care Med 1997;155:72–80. [DOI] [PubMed] [Google Scholar]
- 15.Kuller L; SMART Study Group. Elevated levels of interleukin-6 and d-dimer are associated with an increased risk of death in patients with HIV. Presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, February 2008.
- 16.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med 2004;164:2206–2216. [DOI] [PubMed] [Google Scholar]
- 17.Tumbarello M, Tacconelli E, de Gaetano K, Ardito F, Pirronti T, Cauda R, Ortona L. Bacterial pneumonia in HIV-infected patients: analysis of risk factors and prognostic indicators. J Acquir Immune Defic Syndr 1998;18:39–45. [DOI] [PubMed] [Google Scholar]
- 18.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, Gibert CL, Butt AA, Justice AC. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med 2005;20:1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimland D, Navin TR, Lennox JL, Jernigan JA, Kaplan J, Erdman D, Morrison CJ, Wahlquist SP; Pulmonary Opportunistic Infection Study Group. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS 2002;16:85–95. [DOI] [PubMed] [Google Scholar]
- 20.Benito N, Rañó A, Moreno A, González J, Luna M, Agustí C, Danés C, Pumarola T, Miró JM, Torres A, et al. Pulmonary infiltrates in HIV-infected patients in the highly active antiretroviral therapy era in Spain. J Acquir Immune Defic Syndr 2001;27:35–43. [DOI] [PubMed] [Google Scholar]
- 21.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection: epidemiologic, clinical, and immunologic perspectives. Ann Intern Med 1992;117:314–324. [DOI] [PubMed] [Google Scholar]
- 22.Barry PM, Zetola N, Keruly JC, Moore RD, Gebo KA, Lucas GM. Invasive pneumococcal disease in a cohort of HIV-infected adults: incidence and risk factors, 1990–2003. AIDS 2006;20:437–444. [DOI] [PubMed] [Google Scholar]
- 23.Jordano Q, Falcó V, Almirante B, Planes AM, del Valle O, Ribera E, Len O, Pigrau C, Pahissa A. Invasive Pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004;38:1623–1628. [DOI] [PubMed] [Google Scholar]
- 24.Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 1995;191:2038–2045. [DOI] [PubMed] [Google Scholar]
- 25.Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE; Adult and Adolescent Spectrum of HIV Disease Project. Pneumococcal disease among human immunodeficiency virus–infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis 2001;32:794–800. [DOI] [PubMed] [Google Scholar]
- 26.Breiman RF, Keller DW, Phelan MA, Sniadack DH, Stephens DS, Rimland D, Farley MM, Schuchat A, Reingold AL. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected persons. Arch Intern Med 2000;160:2633–2638. [DOI] [PubMed] [Google Scholar]
- 27.Watera C, Nakiyingi J, Miiro G, Miiro G, Muwonge R, Whitworth JAG. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 2004;18:1210–1213. [DOI] [PubMed] [Google Scholar]
- 28.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet 2004;4:445–455. [DOI] [PubMed] [Google Scholar]
- 29.Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, Stone VE, Kaplan JE. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2004;39:609–628. [DOI] [PubMed] [Google Scholar]