Abstract
Rationale: Lung volume reduction surgery (LVRS) is inconsistently reported to improve arterial oxygenation in patients with chronic obstructive pulmonary disease.
Objectives: We studied the effects of surgery on oxygenation in a large cohort and identified predictors of postoperative oxygenation improvement.
Methods: We evaluated oxygenation in 1,078 subjects with chronic obstructive pulmonary disease enrolled in the National Emphysema Treatment Trial after LVRS compared with medical control subjects, including arterial blood gases, use of supplemental oxygen during treadmill walking, and self-reported use of oxygen during rest, exertion, and sleep.
Measurements and Main Results: PaO2 breathing room air was equal in medical and surgical subjects at baseline (64.8 vs. 65.0 mm Hg, P = not significant), but lower in medical subjects at 6 months (63.6 vs. 70.0 mm Hg, P < 0.001), 12 months (63.9 vs. 68.7 mm Hg, P < 0.001), and 24 months (62.4 vs. 68.0 mm Hg, P < 0.001). Fewer medical subjects required oxygen for treadmill walking at baseline compared with surgical subjects (46 vs. 53%, P = 0.02). However, more medical subjects required oxygen for this activity at 6 months (49 vs. 33%, P < 0.001), 12 months (50 vs. 36%, P < 0.001), and 24 months (52 vs. 42%, P = 0.02). Self-reported oxygen use was greater in medical than in surgical subjects at 6, 12, and 24 months. Multivariate modeling of preoperative characteristics showed baseline oxygenation status was the best predictor of postoperative oxygenation.
Conclusions: LVRS increases PaO2, and decreases treadmill and self-reported use of oxygen for up to 24 months post-procedure.
Clinical trial registered with www.clinicaltrials.gov (NCT 00000606).
Keywords: oxygen inhalation therapy; emphysema; lung diseases, obstructive
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Lung volume reduction surgery is reported to have benefits for patients with emphysema. It has been unclear whether this surgery has ameliorative effects on arterial oxygenation.
What This Study Adds to the Field
Lung volume reduction surgery improved arterial oxygenation and decreased supplemental oxygen use in a large cohort of subjects with emphysema compared with a medically treated control group.
Initial case series reports of modern lung volume reduction surgery (LVRS) suggested significant improvements in arterial blood oxygenation (1–3) and reduced need for supplemental oxygen after the procedure (1, 3, 4). However, improved oxygenation has not been consistently found in case series (5–7) or randomized trials of patients undergoing LVRS. Four trial reports described no significant differences in measures of arterial oxygenation after either surgical or medical treatment for emphysema (8–11), whereas two (12, 13) reported improvements in measures of oxygenation after LVRS.
Understanding the effects of LVRS on oxygenation and the corollary need for supplemental oxygen is important for several reasons. Oxygen therapy for people with chronic obstructive pulmonary disease (COPD) is expensive. One study estimated the cost at over $2 billion annually in the United States, representing one-third of all direct costs of medical care for COPD (14). Some patients find oxygen therapy burdensome. In a study by Eaton and colleagues, 14 of 34 (41%) subjects who responded to oxygen (defined as improvement in health-related quality of life or increased six-minute-walk distance or decreased dyspnea on exertion) declined ongoing ambulatory oxygen treatment (15). Data from the National Emphysema Treatment Trial (NETT) provide an important opportunity to study the effects of LVRS on oxygenation and oxygen use over time. The NETT is a large multicenter, randomized clinical trial of maximal medical therapy compared with LVRS for severe emphysema. During the trial, investigators collected arterial blood gas data, performed a standardized oxygen titration test, and queried patients about supplemental oxygen use at baseline and at scheduled intervals after randomization to LVRS or medical therapy. Our analysis was designed to determine the following: (1) does LVRS result in a change in PaO2, oxygen need during treadmill walking, and self-reported oxygen use during rest, exertion, and sleep; (2) does LVRS have an effect on qualification for supplemental oxygen using the most stringent commonly used indication (PaO2 ≤ 55 mm Hg); and (3) are there preoperative patient characteristics that allow us to predict changes in oxygen need or use after surgery?
METHODS
Additional details on the methods of this research are available in the online supplement.
NETT Design
The NETT is a multicenter randomized clinical trial comparing maximal medical therapy to maximal medical therapy plus LVRS for treatment of advanced emphysema. NETT study design and methods have been described previously (16, 17). The protocol was institutional review board approved at each of the centers.
Oxygenation assessments.
Arterial blood gas.
Arterial blood gas measurement was performed at every visit with patients resting and off supplemental oxygen 30 minutes before blood sampling.
Treadmill oxygen titration.
An oxygen titration protocol was used to identify the amount of supplemental oxygen needed at rest and while treadmill walking at low speed (≤1 mile per hour [mph]) and high speed (2 or 3 mph). The high-speed treadmill walking portion was optional. The oxygen titration protocol was performed at every visit up to and including at 24-month follow-up (see the online supplement for further details).
Self-reported oxygen use.
At each visit, participants were asked “yes/no” questions, as follows:
Do you use oxygen at rest (not sleeping)?
Do you use oxygen on exertion?
Do you use oxygen when sleeping?
Statistical Analysis
Medical and surgical group endpoints were compared between groups using the two-sample t test and the χ2 test. Endpoints included the following: PaO2; PaCO2; oxygen need at rest; oxygen need on low-speed and high-speed treadmill; self-reported oxygen use during rest, exertion, and sleep. Endpoints were compared separately between treatment groups at baseline and 6, 12, and 24 months after randomization. Data were analyzed only up to the 24-month follow-up time point because, by protocol, the oxygen titration was eliminated after that time point. We also compared the proportions of participants with PaO2 ≤ 55 mm Hg. The proportions were compared between the medical and surgical groups using the χ2 test.
Association of baseline characteristics with the above endpoints was also analyzed in the surgical subjects (for the follow-up at 24 mo) using logistic regression to determine if there were characteristics that might be predictive of oxygen need or use 24 months postoperatively. Baseline characteristics evaluated included upper lobe emphysema, exercise capacity, age, sex, baseline % predicted value of FEV1, FVC, RV, TLC, and diffusion capacity for carbon monoxide (DlCO). Multivariate logistic regression models for each of the six endpoints were constructed using the above characteristics. We used forward selection with P < 0.05 as a criterion for entry into the models. P values in the final models from stepwise regressions were not adjusted for multiple testing.
Data management was performed using Stata 8.2 software (Stata Corp., College Station, TX) and statistical analysis with multiple datasets and graphical displays were computed in R 2.0.1 (R Foundation for Statistical Computing, Vienna, Austria). All P values were based on two-sided tests, and P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population obtained after pulmonary rehabilitation, but before random treatment assignment, are shown in Table 1. We restricted data analysis to the 1,078 non–high-mortality-risk patients identified in a prior NETT publication (18).
TABLE 1.
POSTREHABILITATION BASELINE CHARACTERISTICS OF THE STUDY POPULATION
| Medical Therapy Group | Surgery Group | |
|---|---|---|
| Characteristics | (n = 540) | (n = 538) |
| Age at randomization, yr | 67.5 ± 5.8 | 67.5 ± 6.1 |
| Race or ethnic group, n (%) | ||
| Non-Hispanic white | 511 (95) | 513 (95) |
| Non-Hispanic black | 18 (3) | 17 (3) |
| Other | 11 (2) | 8 (1) |
| Sex, n (%) | ||
| Female | 200 (37) | 235 (44)* |
| Male | 340 (63) | 303 (56) |
| Distribution of emphysema on CT, n (%) | ||
| Upper lobe predominance | 364 (67) | 345 (64) |
| Non–upper lobe predominance | 176 (33) | 193 (36) |
| Heterogeneous | 314 (58) | 306 (57) |
| Homogeneous | 226 (42) | 232 (43) |
| Maximal workload, W | 41.3 ± 22.2 | 40.0 ± 21.1 |
| Distance walked in 6 min, ft | 1,247.4 ± 309.6 | 1,239.4 ± 307.5 |
| FEV1 after bronchodilator use, % of predicted value | 27.9 ± 6.5 | 28.1 ± 6.8 |
| TLC after bronchodilator use, % of predicted value | 127.6 ± 14.1 | 127.4 ± 15.0 |
| RV after bronchodilator use, % of predicted value | 216.7 ± 43.8 | 214.4 ± 45.6 |
| DlCO, % of predicted value | 29.5 ± 9.5 | 29.2 ± 9.2 |
| PaO2, mm Hg | 64.8 ± 10.1 | 65.0 ± 10.6 |
| PaCO2, mm Hg | 42.4 ± 5.5 | 42.8 ± 5.7 |
| PaO2 ≤ 55 mm Hg, n (%) | 107 (20) | 109 (20) |
| Oxygen need with oxygen titration, n (%) | ||
| At rest | 40 (7) | 37 (7) |
| Low-speed treadmill | 249 (46) | 287 (53)* |
| High-speed treadmill | 333 (68) | 333 (69) |
| Oxygen reported use, n (%) | ||
| Rest (not sleeping) | 280 (52) | 282 (52) |
| On exertion | 414 (77) | 406 (75) |
| Sleeping | 370 (69) | 351 (65) |
Baseline measurements were obtained after rehabilitation but before randomization except for carbon monoxide diffusing capacity which was obtained before rehabilitation. Plus-minus values are means ± SD.
P < 0.05 difference between medical and surgical groups.
Patients in both treatment groups had severe obstructive lung disease with reduced DlCO, hyperinflation, and reduced exercise capacity. The two groups were similar in all aspects except for sex (more surgical subjects were female) and need for oxygen with low-speed treadmill (more surgical subjects required oxygen with low-speed treadmill).
Arterial Blood Gases
We show the values for PaO2 and PaCO2 at baseline and 6, 12, and 24 months after randomization in the medical and surgical groups in Figures 1A and 1B, respectively. There was a significant increase in PaO2 and decrease in PaCO2 at all measured time points after LVRS.
Figure 1.
(A) PaO2 values and (B) PaCO2 values while breathing room air at baseline and 6, 12, and 24 months after randomization. Error bars are 95% confidence intervals. *P < 0.0001.
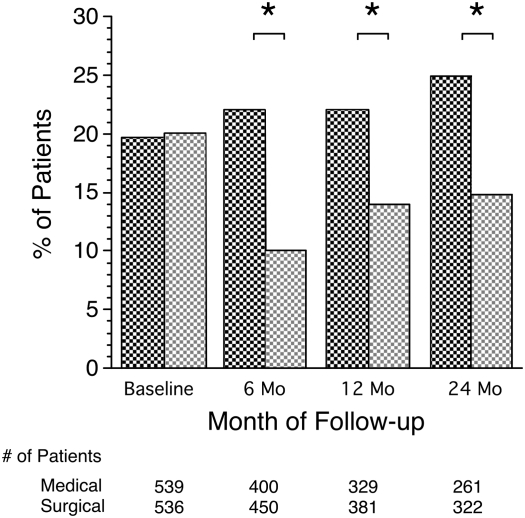
We also looked at the proportion of patients in each group who had a PaO2 ≤ 55 mm Hg, the most stringent cut point for oxygen prescription (Figure 2). Although the treatment groups were nearly identical at baseline, significantly larger percentages of medical patients had a PaO2 ≤ 55 mm Hg at each follow-up visit compared with surgically treated patients: 22 versus 10% (P < 0.001) at 6 months, 22 versus14% (P = 0.013) at 12 months, and 25 versus 15% (P = 0.002) at 24 months. Thirty-nine percent (103 of 261) of the medical group subjects had a PaO2 at 24 months that was improved or stable compared with baseline. Sixty-five percent (210 of 321) of surgically treated subjects were improved or stable at 24 months compared with baseline (P < 0.001, medical vs. surgical). To further analyze the number of individuals falling above or below the PaO2 55-mm Hg cut point, we constructed a 2 × 2 table of all individuals who had blood gas measurements at both baseline and 24 months postrandomization (see Table 2 for both medical subjects and for surgical subjects). Twenty-four months after randomization 8 of 48 (17%) of medical subjects with PaO2 55 mm Hg or less at baseline showed an increase to a PaO2 of greater than 55 mm Hg compared with 33 of 62 (53%) in the surgical group. Twelve percent (25/213) in the medical group and 7% (19/259) in the surgical group had a PaO2 that fell from more than 55 mm Hg at baseline to 55 mm Hg or less at 24 months.
Figure 2.
Percentage of patients with resting PaO2 ≤ 55 mm Hg at baseline and 6, 12, and 24 months after randomization. Dark cross-hatched bars, medical; light cross-hatched bars, surgical. *P < 0.05.
TABLE 2.
PaO2 ABOVE AND BELOW 55 mm Hg AT BASELINE AND AT 24 MONTHS POSTRANDOMIZATION FOR MEDICAL AND SURGICAL SUBJECTS
| PaO2 at 24 mo
|
|||
|---|---|---|---|
| Baseline PaO2 | ≤55 mm Hg | >55 mm Hg | Total n |
| Medical subjects | |||
| ≤55 mm Hg | 40 | 8 | 48 |
| > 55 mm Hg | 25 | 188 | 213 |
| Surgical subjects | |||
| ≤55 mm Hg | 29 | 33 | 62 |
| >55 mm Hg | 19 | 240 | 259 |
The initial NETT publication (17) identified subgroups of subjects with differential outcome based on distribution of emphysema (upper lobe vs. non–upper lobe) and baseline exercise capacity (high vs. low). PaO2 at 24 months in the surgically treated subjects was most likely to be stable or improved in the upper lobe disease subgroups (Table E2 of the online supplement).
Treadmill Titration Study
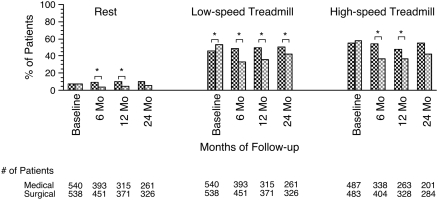
Medical subjects required oxygen more frequently at almost all time points at rest and during low-speed and high-speed treadmill measurements than surgical subjects after randomization (Figure 3).
Figure 3.
Percentage of patients requiring supplemental oxygen during treadmill titration at baseline and 6, 12, and 24 months after randomization. Dark shaded bars, medical; light shaded bars, surgical. *P < 0.05.
Self-Reported Oxygen Use
The percentages of subjects self-reporting use of supplemental oxygen at rest (not sleeping), on exertion, and while sleeping are displayed in Figure 4. At baseline, 50% or more of all subjects reported using oxygen with all three levels of activity. After randomization, larger percentages of medically treated subjects self-reported supplemental oxygen use compared with surgically treated subjects at each time point. A total of 282 surgical subjects reported using oxygen at rest at the baseline assessment. At 24 months, 114 reported using oxygen at rest, 77 reported not using oxygen at rest, and 91 had no 24-month data for this outcome.
Figure 4.
Percentage of patients self-reporting supplemental oxygen use during rest, activity, and while sleeping at baseline and 6, 12, and 24 months after randomization. Dark shaded bars, medical; light shaded bars, surgical. *P < 0.05.
Predictors
In the surgical group alone, we tested the association of other baseline characteristics with each of the outcome measures, including PaO2, no oxygen need during rest, or during low-speed and high-speed treadmill tests, and self-report of no oxygen use during rest, activity, or sleep. Our goal was to define patient characteristics at baseline in the surgical arm of the study that predicted no oxygen need or use at 24 months post-procedure. Upper lobe emphysema, low exercise capacity, baseline FEV1, baseline TLC, baseline DlCO, and baseline oxygen needs and use (defined as resting PaO2 > 55 mm Hg and no oxygen need or use with the specified levels of activity) were associated with some of the outcome measures (Table 3). The baseline oxygen need or use was most consistently associated with the various outcome measures; lack of need or use of oxygen at baseline predicted lack of use of oxygen post-LVRS.
TABLE 3.
UNIVARIATE MODELS (SURGICAL PATIENTS ONLY) FOR PaO2 GREATER THAN 55 mm Hg OR NO OXYGEN NEED WITH SPECIFIED ACTIVITY AT 24 MONTHS
| Predictors
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Upper Lobe Emphysema
|
Low Exercise Capacity*
|
Baseline FEV1 (% predicted)†
|
Baseline TLC (% predicted)†
|
Baseline DlCO (% predicted)†
|
Value of Outcome‡ at Baseline
|
||||||||||||
| Outcome Variable at 24 mo | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| PaO2 > 55 mm Hg | 2.34 | 1.26–4.36 | 0.007 | 0.82 | 0.44–1.53 | 0.5 | 1.60 | 1.00–2.58 | 0.052 | 0.74 | 0.60– 0.92 | 0.005 | 1.33 | 0.93–1.91 | 0.12 | 11.1 | 5.60–21.99 | <0.0001 |
| No rest O2 need (treadmill) | 1.28 | 0.51–3.19 | 0.6 | 1.69 | 0.64–4.47 | 0.3 | 0.71 | 0.38–1.32 | 0.3 | 0.82 | 0.61–1.09 | 0.20 | 1.21 | 0.73– 2.03 | 0.5 | 5.64 | 1.84–17.36 | 0.003 |
| No O2 need (low-speed treadmill) | 1.67 | 1.05–2.66 | 0.03 | 0.91 | 0.58–1.43 | 0.7 | 1.23 | 0.89–1.70 | 0.2 | 0.89 | 0.77– 1.03 | 0.13 | 1.51 | 1.16– 1.96 | 0.002 | 3.18 | 2.00–5.04 | <0.0001 |
| No O2 need (high-speed treadmill) | 1.44 | 0.85–2.45 | 0.2 | 1.38 | 0.85–2.25 | 0.2 | 1.20 | 0.85–1.71 | 0.3 | 0.90 | 0.76–1.07 | 0.2 | 1.76 | 1.32–2.36 | 0.0001 | 4.33 | 2.52–7.47 | <0.0001 |
| No resting O2 use (self-report) | 1.95 | 1.27–2.99 | 0.002 | 0.61 | 0.40–0.93 | 0.02 | 1.63 | 1.19– 2.22 | 0.002 | 0.95 | 0.83– 1.10 | 0.5 | 1.76 | 1.36– 2.27 | <0.0001 | 6.32 | 3.99–10.02 | <0.0001 |
| No O2 use on exertion (self-report) | 1.67 | 1.06–2.63 | 0.03 | 1.06 | 0.70– 1.62 | 0.8 | 1.30 | 0.96– 1.76 | 0.09 | 0.99 | 0.86– 1.14 | 0.9 | 1.43 | 1.12– 1.82 | 0.003 | 5.65 | 3.45–9.26 | <0.0001 |
| No sleep O2 use (self-report) | 1.69 | 1.09–2.62 | 0.02 | 0.70 | 0.46–1.06 | 0.09 | 1.30 | 0.97–1.75 | 0.08 | 1.04 | 0.90– 1.19 | 0.6 | 1.76 | 1.37– 2.25 | <0.0001 | 7.55 | 4.75–12.00 | <0.0001 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Values shown in bold are statistically significant.
Maximum work on the post-rehabilitation, pre-randomization bicycle ergometer test of ≤25 W for female subjects and ≤40 W for male subjects.
For values with % predicted, the OR is for a 10% increase in the value.
Baseline outcome indicates baseline PaO2 > 55 mm Hg or no O2 use with the specified activity.
Multivariate logistic regression was then used to model independent predictors and six multivariate models for each of the six endpoints were identified. These data are presented in Table 4. Upper lobe emphysema, higher DlCO, and the baseline status of the oxygen need or use measurement were the most frequent predictors of outcome at 24 months.
TABLE 4.
MULTIVARIATE LOGISTIC REGRESSION MODELS FOR PaO2 GREATER THAN 55 mm Hg OR NO OXYGEN USE WITH SPECIFIC ACTIVITY AT 24 MONTHS (SURGICAL PATIENTS ONLY)
| Model (for specified outcome at 24 mo) | Upper Lobe Emphysema | No Baseline Oxygen Use with Specific Activity | Baseline PaO2 > 55 mm Hg | Baseline DlCO (% predicted)* | Baseline TLC (% predicted)* | Low Exercise Capacity |
|---|---|---|---|---|---|---|
| Predict PaO2 > 55 mm Hg | — | — | 12.0 (5.94–24.4) | — | 0.74 (0.59–0.93) | — |
| Predict no O2 need (low-speed treadmill) | 2.01 (1.22–3.32) | 2.98 (1.83–4.83) | — | 1.35 (1.02–1.78) | — | — |
| Predict no O2 need (high-speed treadmill) | 1.82 (1.00–3.29) | 3.82 (2.16–6.76) | — | 1.82 (1.29–2.56) | — | 2.06 (1.15–3.70) |
| Predict no O2 at rest (self-report) | 2.42 (1.48–3.95) | 5.97 (3.68–9.68) | — | 1.49 (1.13–1.98) | — | — |
| Predict no O2 use on exertion (self-report) | 1.91 (1.16–3.14) | 6.03 (3.64–9.99) | — | — | — | — |
| Predict no O2 with sleep (self-report) | 2.30 (1.37–3.85) | 7.37 (4.50–12.07) | — | 1.44 (1.09–1.91) | — | — |
Models presented are those with more than one covariate. Candidate variates included: upper lobe emphysema, exercise capacity, age, sex, baseline % predicted value of FEV1, FVC, RV, TLC, and diffusion capacity for carbon monoxide (DlCO). Values presented are odds ratios and 95% confidence intervals in parentheses. Values shown in bold are statistically significant. Dashes indicate nonsignificant values.
The odds ratio is for a 10% increase in the value.
DISCUSSION
The major findings of this study are that arterial blood oxygenation as measured by three separate methods of assessment (two objective and one subjective) improved after LVRS when compared with medically treated subjects for up to 24 months after randomization to treatment.
Previously reported data concerning the effect of LVRS on arterial oxygenation and oxygen use have been conflicting. Nonrandomized case series have reported varying effects of LVRS (1–7, 19–23) on indices of oxygenation and it is clear from our analysis that the medical control group is important in analyzing temporal changes in oxygenation. As we show in Table 2, PaO2 can vary significantly over time in individuals with COPD. Thirteen percent of those in the medical control group had a change in PaO2 that moved them above or below the 55-mm Hg cut point at 24 months. Therefore, interpretation of findings from case series reports should be made cautiously. Previous randomized controlled trials of LVRS have shown varying results, with four studies reporting no change in oxygenation (8–11) whereas two reported some improvement in oxygenation (12, 13). It is possible that the conflicting results relate in part to the smaller sample size in most of the studies, which prevented the investigators from detecting changes. Different methods of assessing oxygenation and diverse periods of follow-up may also contribute to the varied results. In the initial publication from the Brompton trial, there were no differences in median PaO2 between medically and surgically treated subjects at 12 months of follow-up (9). However, longer follow-up (mean follow-up of 27.9 mo) assessing both immediate impact and change over time showed statistically significant improvements in oxygen saturation in the surgically treated subjects (13).
A major strength of NETT in addressing this issue is the large number of subjects enrolled in the trial and the fact that we had a number of measures of arterial oxygenation and oxygen use.
An important objective of this analysis was to attempt to identify preoperative characteristics that would allow us to predict which individuals requiring preoperative oxygen therapy might be able to discontinue oxygen therapy after surgery. Univariate models for each of the oxygenation endpoints were constructed and identified a number of predictors. Multiple logistic regression analysis yielded several models with more than one covariate. As would be expected, baseline PaO2 greater than 55 mm Hg or no oxygen use or need with each of the specified activities strongly predicted that same outcome 24 months postrandomization. Upper lobe emphysema was a positive predictor for no need for or use of oxygen for several of the outcome measures. A better outcome has been associated with upper lobe disease in other reports (1, 17, 24, 25), and it is perhaps not surprising that we found this association with oxygenation as well. We found that a higher DlCO was predictive of no need for oxygen with low- and high-speed treadmill activity and self-report of no use of oxygen at rest or while sleeping. A higher DlCO likely represents the presence of less severe tissue destruction of the lung in emphysema and it may be that improvement in those with less severe disease may result in a lesser need for supplemental oxygen. The percent predicted TLC was a negative predictor for a postsurgical PaO2 greater than 55 mm Hg. The reason for this is not intuitively clear; however, it is possible that a lower TLC is indicative of less severe disease and physiologic derangement and therefore is associated with a higher baseline PaO2.
We cannot comment on the mechanism of improvement of oxygenation post-LVRS because this study was not designed to determine the mechanism of improvement. PaCO2 did decrease as PaO2 increased, indicating that perhaps increased ventilation contributed in part to the improvement in PaO2. This is also supported by a slight increase in the alveolar to arterial oxygen gradient (Table E3). Previous studies have provided a possible alternative explanation for increased PaO2 with reductions in Vd/Vt during exercise (26). Albert and coworkers postulated that changes in ventilation–perfusion heterogeneity as opposed to changes in mean alveolar ventilation explained the effect of LVRS on arterial blood gas values (27).
There are limitations to our study. First and most notable is the fact that there was differential follow-up between the medical and surgical groups. More surgically treated subjects than medically treated subjects completed the various oxygen outcome measures in follow-up. Previously published studies have noted greater numbers of comorbid conditions (28, 29) and poorer pulmonary function (30) in people who do not complete study follow-up compared with those who do—leading to speculation that sicker people do not complete study follow-up. However, examining baseline characteristics of the NETT subjects who did not complete any part of the 24 months of follow-up compared with those who completed at least some part of the 24 months of follow-up, we noted that, overall, the groups had similar baseline characteristics. There were very slight differences noted in that medically treated subjects not completing any follow-up tended to have higher baseline PaO2 but achieved a smaller maximal workload on baseline bicycle ergometry, whereas surgically treated subjects not completing 24 months of follow-up had shorter mean six-minute-walk distance at baseline (Table E1). A second limitation is that the self-report of oxygen use is subject to substantial bias because this was an unblinded surgical trial. Those who underwent LVRS might expect in advance to discontinue supplemental oxygen post-procedure and might stop using this whether or not they objectively required oxygen. Bousamra and colleagues noted that some patients discontinued supplemental oxygen after LVRS despite demonstrated desaturation with activity (31). Because we include objective measures of oxygenation in addition to self-report, we believe that this helps to mitigate this concern. We noted that 77 surgical subjects who self-reported using oxygen at rest at the baseline assessment, and who also had 24-month data for this outcome reported no resting oxygen use at 24 months. Seventy (of the 77) also had 24-month PaO2 data and only one subject's PaO2 was less than 55 mm Hg. Finally, the NETT was performed at academic centers that had significant experience with LVRS before the study. Whether our results can be generalized to other centers remains to be determined.
In conclusion, we found that LVRS increases PaO2, and decreases treadmill and self-reported use of oxygen for up to 24 months post-procedure. The major predictor of not needing or using oxygen after LVRS was not needing or using oxygen preoperatively.
Supplementary Material
The National Emphysema Treatment Trial (NETT) was supported by contracts N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119 from the National Heart, Lung, and Blood Institute; the Centers for Medicare and Medicaid Services; and the Agency for Healthcare Research and Quality.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200712-1826OC on June 5, 2008
Conflict of Interest Statement: M.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.H.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.L.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Z.M. received lecture fees from AstraZeneca in 2006 and 2007 (less than $5,000 total), Pfizer in 2007 ($2,000), and Boehringer Ingelheim in 2007 ($3,000). R.A.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.O.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The members of the National Emphysema Treatment Trial Research Group were as follows: Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia: A. P. Fishman, B. A. Bozzarello, A. Al-Amin. Clinical Centers: Baylor College of Medicine, Houston: M. Katz, C. Wheeler, E. Baker, P. Barnard, J. Carter, S. Chatziioannou, K. Conejo-Gonzales, J. Haddad, D. Hicks, N. Kleiman, M. Milburn-Barnes, C. Nguyen, M. Reardon, J. Reeves-Viets, S. Sax, A. Sharafkhaneh, C. Young, R. Espada, R. Butanda, K. Dubose, M. Ellisor, P. Fox, K. Hale, E. Hood, A. Jahn, S. Jhingran, K. King, C. Miller, I. Nizami, T. Officer, J. Ricketts, J. Rodarte, R. Teague, K. Williams; Brigham and Women's Hospital, Boston: J. Reilly, D. Sugarbaker, C. Fanning, S. Body, S. Duffy, V. Formanek, A. Fuhlbrigge, P. Hartigan, S. Hooper, A. Hunsaker, F. Jacobson, M. Moy, S. Peterson, R. Russell, D. Saunders, S. Swanson; Cedars-Sinai Medical Center, Los Angeles: R. McKenna, Z. Mosenifar, C. Geaga, M. Biring, S. Clark, R. Frantz, P. Julien, M. Lewis, J. Minkoff-Rau, V. Yegyan, M. Joyner; Cleveland Clinic Foundation, Cleveland: M. DeCamp, J. Stoller, Y. Meli, J. Apostolakis, D. Atwell, J. Chapman, P. DeVilliers, R. Dweik, E. Kraenzler, R. Lann, N. Kurokawa, S. Marlow, K. McCarthy, P. McCreight, A. Mehta, M. Meziane, O. Minai, P. O'Donovan, M. Steiger, K. White, J. Maurer, C. Hearn, S. Lubell, R. Schilz, T. Durr; Columbia University, New York, and Long Island Jewish Medical Center, New Hyde Park: M. Ginsburg, B. Thomashow, P. Jellen, J. Austin, M. Bartels, Y. Berkman, P. Berkoski, F. Brogan, A. Chong, G. DeMercado, A. DiMango, B. Kachulis, A. Khan, B. Mets, M. O'Shea, G. Pearson, J. Pfeffer, L. Rossoff, S. Scharf, M. Shiau, P. Simonelli, K. Stavrolakes, D. Tsang, D. Vilotijevic, C. Yip, M. Mantinaos, M. McKeon; Duke University Medical Center, Durham: N. MacIntyre, R. D. Davis, J. Howe, R. E. Coleman, R. Crouch, D. Greene, K. Grichnik, D. Harpole, A. Krichman, B. Lawlor, H. McAdams, J. Plankeel, S. Rinaldo-Gallo, J. Smith, M. Stafford-Smith, V. Tapson, M. Steele, J. Norten; Mayo Foundation, Rochester: J. Utz, C. Deschamps, K. Mieras, M. Abel, M. Allen, D. Andrist, G. Aughenbaugh, S. Bendel, E. Edell, M. Edgar, B. Edwards, B. Elliot, J. Garrett, D. Gillespie, J. Gurney, B. Hammel, K. Hanson, L. Hanson, G. Harms, J. Hart, T. Hartman, R. Hyatt, E. Jensen, N. Jenson, S. Kalra, P. Karsell, D. Midthun, C. Mottram, S. Swensen, A.-M. Sykes, K. Taylor, N. Torres, R. Hubmayr, D. Miller, S. Bartling, K. Bradt; National Jewish Medical and Research Center, Denver: B. Make, M. Pomerantz, M. Gilmartin, J. Canterbury, M. Carlos, P. Dibbern, E. Fernandez, L. Geyman, C. Hudson, D. Lynch, J. Newell, R. Quaife, J. Propst, C. Raymond, J. Whalen-Price, K. Winner, M. Zamora, R. Cherniack; Ohio State University, Columbus: P. Diaz, P. Ross, T. Bees, H. Awad, J. Drake, C. Emery, M. Gerhardt, M. Kelsey, M. King, D. Rittinger, M. Rittinger; St. Louis University, St. Louis: K. Naunheim, F. Alvarez, J. Osterloh, S. Borosh, W. Chamberlain, S. Frese, A. Hibbit, M. E. Kleinhenz, G. Ruppel, C. Stolar, J. Willey, C. Keller; Temple University, Philadelphia: G. Criner, S. Furukawa, A. M. Kuzma, R. Barnette, N. Brister, K. Carney, W. Chatila, F. Cordova, G. D'Alonzo, M. Keresztury, K. Kirsch, C. Kwak, K. Lautensack, M. Lorenzon, U. Martin, P. Rising, S. Schartel, J. Travaline, G. Vance, P. Boiselle, G. O'Brien; University of California, San Diego, San Diego: A. Ries, R. Kaplan, C. Ramirez, D. Frankville, P. Friedman, J. Harrell, J. Johnson, D. Kapelanski, D. Kupferberg, C. Larsen, T. Limberg, M. Magliocca, F. J. Papatheofanis, D. Sassi-Dambron, M. Weeks; University of Maryland at Baltimore, Baltimore, and Johns Hopkins Hospital, Baltimore: M. Krasna, H. Fessler, I. Moskowitz, T. Gilbert, J. Orens, S. Scharf, D. Shade, S. Siegelman, K. Silver, C. Weir, C. White; University of Michigan, Ann Arbor: F. Martinez, M. Iannettoni, C. Meldrum, W. Bria, K. Campbell, P. Christensen, K. Flaherty, S. Gay, P. Gill, P. Kazanjian, E. Kazerooni, V. Knieper, T. Ojo, L. Poole, L. Quint, P. Rysso, T. Sisson, M. True, B. Woodcock, L. Zaremba; University of Pennsylvania, Philadelphia: L. Kaiser, J. Hansen-Flaschen, M. L. Geraghty, A. Alavi, T. Alcorn, J. Aronchick, S. Aukberg, B. Benedict, S. Craemer, R. Daniele, J. Edelman, W. Gefter, L. Kotler-Klein, R. Kotloff, D. Lipson, W. Miller, Jr., R. O'Connell, S. Opelman, W. Russell, H. Sheaffer, R. Simcox, S. Snedeker, J. Stone-Wynne, G. Tino, P. Wahl, J. Walter, P. Ward, D. Zisman, J. Mendez, A. Wurster; University of Pittsburgh, Pittsburgh: F. Sciurba, J. Luketich, C. Witt, G. Ayres, M. Donahoe, C. Fuhrman, R. Hoffman, J. Lacomis, J. Sexton, W. Slivka, D. Strollo, E. Sullivan, T. Simon, C. Wrona, G. Bauldoff, M. Brown, E. George, R. Keenan, T. Kopp, L. Silfies; University of Washington, Seattle: J. Benditt, D. Wood, M. Snyder, K. Anable, N. Battaglia, L. Boitano, A. Bowdle, L. Chan, C. Chwalik, B. Culver, T. Gillespy, D. Godwin, J. Hoffman, A. Ibrahim, D. Lockhart, S. Marglin, K. Martay, P. McDowell, D. Oxorn, L. Roessler, M. Toshima, S. Golden.
Other participants included the following: Agency for Healthcare Research and Quality, Rockville: L. Bosco, Y.-P. Chiang, C. Clancy, H. Handelsman; Centers for Medicare and Medicaid Services, Baltimore: S. Sheingold, T. Carino, J. Chin, J. Farrell, K. McVearry, A. Norris, S. Shirey, C. Sikora; Coordinating Center, Johns Hopkins University, Baltimore: S. Piantadosi, J. Tonascia, P. Belt, K. Collins, B. Collison, J. Dodge, M. Donithan, V. Edmonds, J. Fuller, J. Harle, R. Jackson, H. Koppelman, S. Lee, C. Levine, H. Livingston, J. Meinert, J. Meyers, D. Nowakowski, K. Owens, S. Qi, M. Smith, B. Simon, P. Smith, A. Sternberg, M. Van Natta, L. Wilson, R. Wise; Cost-effectiveness Subcommittee: R. M. Kaplan, J. S. Schwartz, Y.-P. Chiang, M. C. Fahs, A. M. Fendrick, A. J. Moskowitz, D. Pathak, S. Ramsey, S. Sheingold, A. L. Shroyer, J. Wagner, R. Yusen; Cost-effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle: S. Ramsey, R. Etzioni, S. Sullivan, D. Wood, T. Schroeder, R. Smith, K. Berry, N. Myers; CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City: E. Hoffman, J. Cook-Granroth, A. Delsing, J. Guo, G. McLennan, B. Mullan, C. Piker, J. Reinhardt, J. Sieren, W. Stanford; Data and Safety Monitoring Board: J. A. Waldhausen, G. Bernard, D. DeMets, M. Ferguson, E. Hoover, R. Levine, D. Mahler, A. J. McSweeny, J. Wiener-Kronish, O. D. Williams, M. Younes; Marketing Center, Temple University, Philadelphia: G. Criner, C. Soltoff; Project Office, National Heart, Lung, and Blood Institute, Bethesda: G. Weinmann, J. Deshler, D. Follmann, J. Kiley, M. Wu.
References
- 1.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112:1319–1329. [Discussion, pp. 1329–1330.] [DOI] [PubMed] [Google Scholar]
- 2.Norman M, Hillerdal G, Orre L, Jorfeldt L, Larsen F, Cederlund K, Zetterberg G, Unge G. Improved lung function and quality of life following increased elastic recoil after lung volume reduction surgery in emphysema. Respir Med 1998;92:653–658. [DOI] [PubMed] [Google Scholar]
- 3.Wisser W, Tschernko E, Senbaklavaci O, Kontrus M, Wanke T, Wolner E, Klepetko W. Functional improvement after volume reduction: sternotomy versus videoendoscopic approach. Ann Thorac Surg 1997;63:822–827. [Discussion, pp. 827–828.] [DOI] [PubMed] [Google Scholar]
- 4.McKenna RJ Jr, Brenner M, Gelb AF, Mullin M, Singh N, Peters H, Panzera J, Calmese J, Schein MJ. A randomized, prospective trial of stapled lung reduction versus laser bullectomy for diffuse emphysema. J Thorac Cardiovasc Surg 1996;111:317–321. [Discussion, p. 322.] [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto T, Teschler H, Hillejan L, Zaboura G, Stamatis G. Long-term results of lung volume reduction surgery. Eur J Cardiothorac Surg 2002;21:483–488. [DOI] [PubMed] [Google Scholar]
- 6.Malthaner RA, Miller JD. Lung volume reduction surgery: results of a Canadian pilot study. Canadian Lung Volume Reduction Surgery Study Group. Can J Surg 2000;43:377–383. [PMC free article] [PubMed] [Google Scholar]
- 7.Shade D Jr, Cordova F, Lando Y, Travaline JM, Furukawa S, Kuzma AM, Criner GJ. Relationship between resting hypercapnia and physiologic parameters before and after lung volume reduction surgery in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1405–1411. [DOI] [PubMed] [Google Scholar]
- 8.Criner GJ, Cordova FC, Furukawa S, Kuzma AM, Travaline JM, Leyenson V, O'Brien GM. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:2018–2027. [DOI] [PubMed] [Google Scholar]
- 9.Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, Agent P, Cullinan P, MacNeill SJ, Goldstraw P. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med 2000;343:239–245. [DOI] [PubMed] [Google Scholar]
- 10.Hillerdal G, Lofdahl CG, Strom K, Skoogh BE, Jorfeldt L, Nilsson F, Forslund-Stiby D, Ranstam J, Gyllstedt E. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest 2005;128:3489–3499. [DOI] [PubMed] [Google Scholar]
- 11.Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, Tan L, Road JD. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg 2006;81:314–320. [Discussion, pp. 320–321.] [DOI] [PubMed] [Google Scholar]
- 12.Pompeo E, Marino M, Nofroni I, Matteucci G, Mineo TC. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. Ann Thorac Surg 2000;70:948–953. [Discussion, p. 954.] [DOI] [PubMed] [Google Scholar]
- 13.Lim E, Ali A, Cartwright N, Sousa I, Chetwynd A, Polkey M, Geddes D, Pepper J, Diggle P, Goldstraw P. Effect and duration of lung volume reduction surgery: mid-term results of the Brompton trial. Thorac Cardiovasc Surg 2006;54:188–192. [DOI] [PubMed] [Google Scholar]
- 14.Ward MM, Javitz HS, Smith WM, Bakst A. Direct medical cost of chronic obstructive pulmonary disease in the U.S.A. Respir Med 2000;94:1123–1129. [DOI] [PubMed] [Google Scholar]
- 15.Eaton T, Garrett JE, Young P, Fergusson W, Kolbe J, Rudkin S, Whyte K. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J 2002;20:306–312. [DOI] [PubMed] [Google Scholar]
- 16.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. J Cardiopulm Rehabil 2000;20:24–36. [DOI] [PubMed] [Google Scholar]
- 17.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 18.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 19.Cassina PC, Teschler H, Konietzko N, Theegarten D, Stamatis G. Two-year results after lung volume reduction surgery in alpha1-antitrypsin deficiency versus smoker's emphysema. Eur Respir J 1998;12:1028–1032. [DOI] [PubMed] [Google Scholar]
- 20.Ciccone AM, Meyers BF, Guthrie TJ, Davis GE, Yusen RD, Lefrak SS, Patterson GA, Cooper JD. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513–525. [DOI] [PubMed] [Google Scholar]
- 21.Goto Y, Kurosawa H, Mori N, Kurokawa Y, Hida W, Kohzuki M. Improved activities of daily living, psychological state and health-related quality of life for 12 months following lung volume reduction surgery in patients with severe emphysema. Respirology 2004;9:337–344. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien GM, Furukawa S, Kuzma AM, Cordova F, Criner GJ. Improvements in lung function, exercise, and quality of life in hypercapnic COPD patients after lung volume reduction surgery. Chest 1999;115:75–84. [DOI] [PubMed] [Google Scholar]
- 23.Stammberger U, Thurnheer R, Bloch KE, Zollinger A, Schmid RA, Russi EW, Weder W. Thoracoscopic bilateral lung volume reduction for diffuse pulmonary emphysema. Eur J Cardiothorac Surg 1997;11:1005–1010. [DOI] [PubMed] [Google Scholar]
- 24.McKenna RJ Jr, Brenner M, Fischel RJ, Singh N, Yoong B, Gelb AF, Osann KE. Patient selection criteria for lung volume reduction surgery. J Thorac Cardiovasc Surg 1997;114:957–964. [Discussion, pp. 964–967.] [DOI] [PubMed] [Google Scholar]
- 25.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 26.Benditt JO, Lewis S, Wood DE, Klima L, Albert RK. Lung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometry. Am J Respir Crit Care Med 1997;156:561–566. [DOI] [PubMed] [Google Scholar]
- 27.Albert RK, Benditt JO, Hildebrandt J, Wood DE, Hlastala MP. Lung volume reduction surgery has variable effects on blood gases in patients with emphysema. Am J Respir Crit Care Med 1998;158:71–76. [DOI] [PubMed] [Google Scholar]
- 28.Butler CW, Snyder M, Wood DE, Curtis JR, Albert RK, Benditt JO. Underestimation of mortality following lung volume reduction surgery resulting from incomplete follow-up. Chest 2001;119:1056–1060. [DOI] [PubMed] [Google Scholar]
- 29.Gades NM, Jacobson DJ, McGree ME, Lieber MM, Roberts RO, Girman CJ, Jacobsen SJ. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC Med Res Methodol 2006;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yano E, Wang XR, Higashi H, Karita K, Nishi S. Selection effects on an estimation of long-term changes in pulmonary function. Environ Res 1999;80:165–171. [DOI] [PubMed] [Google Scholar]
- 31.Bousamra M II, Haasler GB, Lipchik RJ, Henry D, Chammas JH, Rokkas CK, Menard-Rothe K, Sobush DC, Olinger GN. Functional and oximetric assessment of patients after lung reduction surgery. J Thorac Cardiovasc Surg 1997;113:675–681. [Discussion, pp. 681–682.] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.