Abstract
We used the neonatal mouse model of rotavirus infection and virus strains SA11-clone 4 (SA11-Cl4) and Rhesus rotavirus (RRV) to examine the mechanism of the extraintestinal spread of viruses following oral inoculation. The spread-competent viruses, RRV and reassortant R7, demonstrated a temporal progression from the intestine, to the terminal ileum, to the mesenteric lymph nodes (MLN), and to the peripheral tissues. SA11-Cl4 was not found outside the intestine. Reassortant virus S7, which was unable to reach the liver in previous studies (E. C. Mossel and R. F. Ramig, J. Virol. 76:6502-6509, 2002), was recovered from 60% of the MLN, suggesting that there are multiple determinants for the spread of virus from the intestine to the MLN. Phenotypic segregation analysis identified RRV genome segment 6 (VP6) as a secondary determinant of the spread of virus to the MLN (P = 0.02) in reassortant viruses containing segment 7 from the spread-incompetent parent. These data suggest that in the orally infected neonatal mouse, the extraintestinal spread of rotavirus occurs via a lymphatic pathway, and the spread phenotype is primarily determined by NSP3 and can be modified by VP6.
Rotaviruses (Reoviridae family) are the major cause of viral gastroenteritis among children and animals, accounting annually for 450,000 to 650,000 deaths in children worldwide (U. D. Parashar, E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass, Abstr. Vaccines Enteric Dis., p. 76, 2001). The rotavirus particle consists of three concentric protein capsid layers surrounding the genome of 11 double-stranded RNA segments that encode six structural proteins (VP1 to VP4, VP6, and VP7) and six nonstructural proteins (NSP1 to NSP6) (5).
Rotavirus infection results in cytolytic lesions on the intestinal villi and a characteristic histopathology: villous blunting and atrophy, crypt hyperplasia, and sloughing of the enterocytes at the tip of the villus. Increasing clinical evidence suggests that rotavirus infection is not solely confined to the intestine, although occurrence outside of the intestine is rare, given the ubiquitousness of rotavirus infection. Numerous investigators report rotavirus antigen and/or viral RNA detected by enzyme immunoassay, immune electron microscopy, electron microscopy, or reverse transcription-PCR in the central nervous system (CNS) of children with rotavirus diarrhea and with benign to severe convulsions or encephalitis (3, 7-9, 12, 13, 17, 20, 21, 30-32). Additional reports discuss CNS complications with concomitant rotavirus infection but do not demonstrate CNS invasion (11, 26, 28). Rotavirus antigen and/or RNA was also observed in the microvasculature of the heart (not the myocytes or fibroblasts) of two children who presented with gastroenteritis but suffered cardio-respiratory failure and died (14) and in liver biopsy tissue from infants with cholestatic disease (22, 24). Finally, rotavirus antigen was detected in the liver and kidneys of four immunocompromised children with rotavirus-induced diarrhea at the time of death (6). Animal studies support these observations. Rotavirus and/or rotavirus antigen was recovered from the blood, liver, spleen, kidneys, lungs, and mesenteric lymph nodes (MLN) of orally inoculated mice (1, 2, 4, 10, 16, 27), and mouse models for rotavirus-induced hepatitis (29) and biliary atresia (25) were previously described.
A neonatal mouse model of heterologous rotavirus infection has been previously described and characterized (18, 23) and extensively used in rotavirus immunology and pathogenesis studies. While the extraintestinal spread of rotavirus in the neonatal mouse model is well documented, the determinants and mechanisms of the spread of the virus are not as clear. It is known that extraintestinal spread occurs in a rotavirus strain-dependent manner (16, 29). We previously demonstrated that among SA11-clone 4 (SA11-Cl4) and Rhesus rotavirus (RRV) reassortants, spread of the virus to the liver was significantly associated with RRV-derived genome segment 7, which encodes NSP3 (16). Murine rotavirus antigen was detected in the macrophages of Peyer's patches and mesenteric and inguinal lymph nodes of 14-day-old mice at 2 to 7 days postinoculation (2). In a subsequent study, rotavirus was found in Peyer's patches and mesenteric lymph nodes 1 to 10 days after the inoculation of adult mice with the RRV-based Rotashield vaccine preparation (15). Finally, rotavirus strains incompetent for extraintestinal spread following oral inoculation were observed to be unable to spread from a subcutaneous inoculation site, whereas viruses competent for extraintestinal spread were able to spread from the subcutaneous inoculation site (16). Taken together, these data suggest that the spread of rotavirus from the intestine may occur via the lymphoid system and may also involve gut-associated lymphoid tissue and/or mesenteric lymph nodes.
Kinetics of rotavirus extraintestinal spread.
To determine the kinetics of virus spread following oral inoculation, 5-day-old CD-1 mice were orally inoculated with 2 × 106 PFU of SA11-Cl4, RRV, or the single-segment reassortant viruses, S7 or R7 (Table 1), as previously described (16). Six, 12, 24, 48, and 72 h postinfection (hpi), whole blood, spleen, lungs, liver, mesenteric lymph nodes, and intestine were harvested. The intestine was excised last to prevent the contamination of peripheral tissues with gut contents. The intestine was divided into two sections. Because Peyer's patches could not be cleanly dissected from the suckling mice, a 1.5- to 2-cm section of Peyer's patch-rich terminal ileum (PP) was excised from each mouse and analyzed separately from the rest of the intestine to determine if virus concentrated in this region. The virus titer in each tissue was determined by plaque assay (16).
TABLE 1.
Frequency of detection in the MLN of RRV and SA11-Cl4 reassortant viruses following the oral inoculation of neonatal mice
| Virus | na | MLN growthb | MLN titerc | Parental origin of genome segmentd (gene product)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (VP1) | 2 (VP2) | 3 (VP3) | 4 (VP4) | 5 (NSP1) | 6 (VP6) | 7 (NSP3) | 8 (NSP2) | 9 (VP7) | 10 (NSP4) | 11 (NSP5) | ||||
| RRV | 17 | 100% | 2.8 | R | R | R | R | R | R | R | R | R | R | R |
| SA11-Cl4 | 17 | 0% | 0.0 | S | S | S | S | S | S | S | S | S | S | S |
| R7 | 10 | 80% | 3.7 | S | S | S | S | S | S | R | S | S | S | S |
| S1, 2, 3, 5, 7 | 17 | 77% | 3.1 | S | S | S | R | S | R | S | R | R | R | R |
| S7 | 10 | 60% | 3.8 | R | R | R | R | R | R | S | R | R | R | R |
| S4, 5, 7, 9 | 18 | 55% | 3.3 | R | R | R | S | S | R | S | R | S | R | R |
| R4, 6, 9 | 17 | 29% | 1.2 | S | S | S | R | S | R | S | S | R | S | S |
| R9 | 16 | 14% | 0.7 | S | S | S | S | S | S | S | S | R | S | S |
| R4 | 18 | 12% | 0.9 | S | S | S | R | S | S | S | S | S | S | S |
| R8, 9 | 17 | 0% | 0.0 | S | S | S | S | S | S | S | R | R | S | S |
| R10 | 17 | 0% | 0.0 | S | S | S | S | S | S | S | S | S | R | S |
| R11 | 18 | 0% | 0.0 | S | S | S | S | S | S | S | S | S | S | R |
n, the number of individual pups inoculated with each virus.
MLN growth is measured as the percentage of animals in which virus was recovered from the MLN at 3 days postinoculation.
Titers are the mean titers of the virus in virus-positive MLN at 3 days postinoculation.
RRV-derived genome segment numbers have been converted to SA11 functional equivalents.
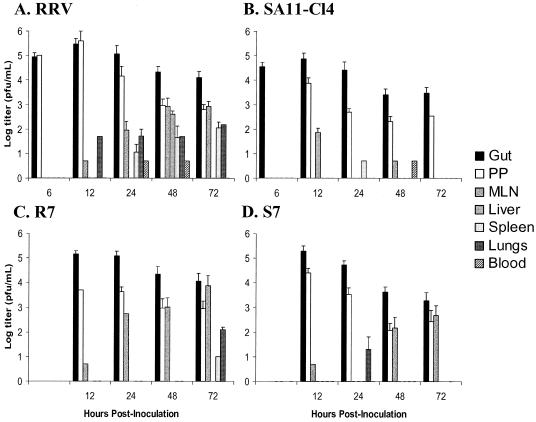
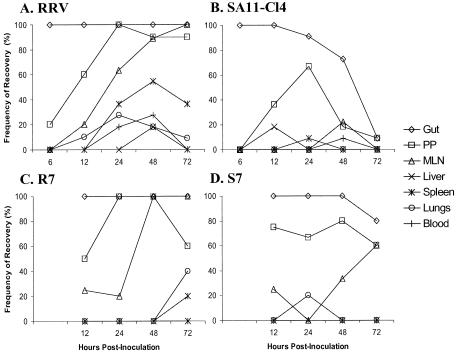
Virus was detected at high titers in the guts of all animals at early time points (Fig. 1A to D and 2A to D). The virus titers of SA11-Cl4 and S7 and their frequencies of detection in the gut declined at late time points. RRV and R7 titers also declined in the gut but less rapidly than the titers of SA11-Cl4 and S7. In the PP, virus titers were high and remained at relatively high levels at all time points for all viruses (Fig. 1). The number of inoculated animals with virus in the PP increased with time and reached 100% for both RRV and R7 (Fig. 2) Among SA11-Cl4 infected animals, the frequency of detection in the PP increased at early time points and then decreased. R7 differed from SA11-Cl4 in that the proportion of animals with R7 virus in the PP remained high throughout the experiments, but as with the detection of SA11-Cl4, the frequency of R7 detection did not reach 100%.
FIG. 1.
Virus titers in various tissues following oral inoculation of neonatal mice with (A) RRV, (B) SA11-Cl4, (C) R7, or (D) S7. Five-day-old mice were orally inoculated with 2 × 106 PFU of rotavirus. At the postinoculation times indicated, animals were sacrificed, tissues were harvested, and virus titers were determined by plaque assay. Values indicate mean titers of virus-positive samples. Error bars indicate standard deviations. Gut, large and small intestine less a 2-cm section of terminal ileum; PP, 2-cm section of Peyer's patch-rich terminal ileum. Results for RRV and SA11-Cl4 at 6 hpi and for R7 and S7 are from 5 to 6 animals. Results for RRV and SA11-Cl4 at 12 to 72 hpi are from 9 to 11 animals.
FIG. 2.
Frequency of virus recovery in various tissues following oral inoculation of neonatal mice with (A) RRV, (B) SA11-Cl4, (C) R7, or (D) S7. Five-day-old mice were orally inoculated with 2 × 106 PFU of rotavirus. At the postinoculation times indicated, animals were sacrificed, tissues were harvested, and virus presence was determined by plaque assay. Gut = large and small intestine less 2 cm section of terminal ileum; PP = 2 cm section of Peyer's patch-rich terminal ileum; MLN = mesenteric lymph nodes. Results for RRV and SA11-Cl4 at 6 hpi and for R7 and S7 are from 5 to 6 animals. Results for RRV and SA11-Cl4 at 12 to 72 hpi are from 9 to 11 animals.
Titers of RRV and R7 in the MLN increased over the course of the experiment (Fig. 1), as did the frequencies of virus isolation (Fig. 2). SA11-Cl4 was recovered from the MLN rarely and at extremely low titers. S7 showed increasing titers and frequencies of recovery from the MLN over time, but both measurements were lower than those observed for RRV and R7. For RRV, R7, and S7, the appearance of virus in the MLN lagged behind its appearance in the PP by about 24 h. The recovery of S7 in the MLN, when SA11-Cl4 was not found at that site, suggests that an RRV gene in S7 modifies the escape-incompetence phenotype previously associated with SA11-Cl4 segment 7 (16).
When the peripheral tissues (spleen, liver, and lung) were examined, the results were variable, as expected, since the spread-competent RRV escapes the gut to the liver in only about 30% of orally inoculated animals (16). RRV was found in the spleen and lungs only at low titers and only at late time points (Fig. 1). The appearance of RRV in these tissues lagged behind the appearance in the MLN, suggesting that the virus had passed through the MLN prior to reaching the target organ (Fig. 2). RRV was found in the liver of two animals at 48 hpi at titers similar to those reported previously. We did not detect RRV in the liver at 72 hpi, the time at which we had previously found the virus present in ∼30% of livers (16). This result is likely due to the small number of animals examined. As expected, SA11-Cl4 was not found in peripheral tissues, including MLN. R7 spread to spleen and lung only at the late 72-hpi time point and was found in these tissues at low titers. R7 was not found in the liver, again probably because of the small number of animals examined. The spread of S7 to the MLN was unexpected since it did not contain the RRV-derived segment 7, which was previously found to confer the spread phenotype (16). However, the unexpected spread of S7 to the MLN, coupled with its expected failure to spread to peripheral tissues, suggests that the modification of the spread phenotype of this virus affects spread only as far as the MLN and not beyond.
Virus was present at barely detectable titers in the blood of five RRV-inoculated animals at 24 and 48 h postinoculation and one SA11-Cl4-inoculated animal 48 h postinoculation. However, we cannot eliminate the possibility of an ongoing viremia below the limit of detection (50 PFU/ml) in our assays. Taken together, the kinetics of the spread of rotavirus from the intestine suggest a temporal progression of spread-competent virus through the gut, to the PP, to the MLN, and out to the peripheral tissues.
VP6 is a secondary determinant of extraintestinal spread.
To identify additional RRV-derived determinants that confer the phenotype for spread to the MLN on reassortant viruses containing a spread-incompetent segment 7, 5-day-old mice were inoculated with a panel of RRV and SA11-Cl4 reassortant viruses (Table 1). Since RRV-derived genome segment 7 is sufficient to confer the extraintestinal spread phenotype (16), all reassortant viruses examined contained SA11-Cl4-derived segment 7 and segregated the other 10 segments. Tissues were harvested 3 days pi (dpi), and MLN were examined by plaque assay for the presence of replication-competent virus.
RRV-derived segment 6 is present in each of the four reassortant viruses found most frequently and at the highest titers in the MLN after oral inoculation (Table 1). SA11-Cl4-derived segment 6 is present in each of the four reassortant viruses recovered least frequently from the MLN and at very low titers. Statistical analysis showed that RRV-derived segments 8, 10, and 11 are borderline significant by the t test. However, RRV-derived segment 6 is more than an order of magnitude more significant than any other segment (Table 2 according to the t test (P = 0.001), and it is the only significant segment according to the Wilcoxon ranked-sum test (P = 0.02) (see reference 16 for statistical methods).
TABLE 2.
Statistical analysis of the contribution of single genome segments to spread of reassortant viruses to the mesenteric lymph node
| Analysis |
P for genome segment
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Student’s t test | 0.10 | 0.10 | 0.10 | 0.13 | NAa | 0.001 | NAb | 0.05 | 0.36 | 0.05 | 0.05 |
| Wilcoxon ranked-sum test | 0.18 | 0.18 | 0.18 | 0.11 | 0.33 | 0.02 | NAb | 0.17 | 0.26 | 0.17 | 0.17 |
Statistical analysis by Student’s t test was not applicable (NA), since only one reassortant virus examined contained RRV-derived segment 5.
Statistical analysis was NA, since all reassortant viruses examined contained SA11-C14-derived segment 7.
In our studies we concentrated on the movement of infectious virus, since infectivity is required for the replication of rotavirus in peripheral tissues, as has been observed in humans (6) and in the mouse (25, 29). Our results indicate that rotaviruses with the ability to spread beyond the intestine do so via the lymphatic system. The studies presented here indicate that a bolus of virus, given in the stomach, moves sequentially to the gut, to the PP, to the MLN, and then to the peripheral tissues and organs (spleen, liver, and lungs). This pathway is similar to that elucidated for the extraintestinal spread of the closely related reovirus (19). We previously showed that genome segment 7, encoding protein NSP3, was the determinant of the spread of RRV beyond the gut (16). Here we found that, while RRV segment 7 was sufficient for a reassortant virus to spread beyond the gut, some reassortant viruses containing segment 7 from a spread-incompetent virus (SA11-Cl4) could also spread as far as the MLN but not beyond to the peripheral tissues. We found that this unexpected spread to the MLN of reassortant viruses with segment 7 from a spread-incompetent virus required the presence of segment 6, encoding VP6, from the spread-competent RRV parent. Thus, either RRV segment 6 or segment 7 conferred on a reassortant virus a phenotype for spread to the MLN, but as we described previously, RRV segment 7 is required for spread beyond the MLN to the peripheral tissues (16).
In summary, some rotavirus strains are capable of escape from the gut and movement to and infection of peripheral tissues. The pathway of escape is via cells of the lymphoid system, but the mechanisms of this escape from the gut remain to be defined. However, it is clear that NSP3 is the major determinant in this spread. Further characterization of the spread phenomenon is important for vaccine development efforts and for understanding how rotaviruses can be associated, though rarely, with severe disease at peripheral sites.
Acknowledgments
This work was supported by National Institutes of Health grants R01-AI 16687 (R.F.R.) and T32-AI 07471 (E.C.M.) and Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center.
REFERENCES
- 1.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. Rotavirus antigenemia/viremia: a common event? Lancet, in press. [DOI] [PubMed]
- 2.Brown, K. A., and P. A. Offit. 1998. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb. Pathog. 24:327-331. [DOI] [PubMed] [Google Scholar]
- 3.Contino, M. F., T. Lebby, and E. L. Arcinue. 1994. Rotaviral gastrointestinal infection causing afebrile seizures in infancy and childhood. Am. J. Emerg. Med. 12:94-95. [DOI] [PubMed] [Google Scholar]
- 4.Dharakul, T., M. Riepenhoff-Talty, B. Albini, and P. L. Ogra. 1988. Distribution of rotavirus antigen in intestinal lymphoid tissues: potential role in development of the mucosal immune response to rotavirus. Clin. Exp. Immunol. 74:14-19. [PMC free article] [PubMed] [Google Scholar]
- 5.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 6.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 7.Hongou, K., T. Konishi, S. Yagi, K. Araki, and T. Miyawaki. 1998. Rotavirus encephalitis mimicking afebrile benign convulsions in infants. Pediatr. Neurol. 18:354-357. [DOI] [PubMed] [Google Scholar]
- 8.Iturriza-Gomara, M., I. A. Auchterlonie, W. Zaw, P. Molyneaux, U. Desselberger, and J. Gray. 2002. Rotavirus gastroenteritis and central nervous system (CNS) infection: characterization of the VP7 and VP4 genes of rotavirus strains isolated from paired fecal and cerebrospinal fluid samples from a child with CNS disease. J. Clin. Microbiol. 40:4797-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keidan, I., I. Shif, G. Keren, and J. H. Passwell. 1992. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr. Infect. Dis. J. 11:773-775. [PubMed] [Google Scholar]
- 10.Kraft, L. M. 1962. Two viruses causing diarrhoea in infant mice, p. 115-130. In R. J. C. Harris (ed.), The problems of laboratory animal disease. Academic Press, Inc., New York, N.Y.
- 11.Lin, S. C., H. Y. Hsu, P. J. Wang, C. N. Lee, M. H. Chang, Y. Z. Shen, and S. M. Wang. 1996. Rotavirus gastroenteritis associated with afebrile seizure in childhood. Zhonghua Min Guo. Xiao. Er. Ke. Yi. Xue. Hui. Za Zhi. 37:204-207. [PubMed] [Google Scholar]
- 12.Lynch, M., B. Lee, P. Azimi, J. Gentsch, C. Glaser, S. Gilliam, H. G. Chang, R. Ward, and R. I. Glass. 2001. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin. Infect. Dis. 33:932-938. [DOI] [PubMed] [Google Scholar]
- 13.Makino, M., Y. Tanabe, K. Shinozaki, S. Matsuno, and T. Furuya. 1996. Haemorrhagic shock and encephalopathy associated with rotavirus infection. Acta Paediatr. 85:632-634. [DOI] [PubMed] [Google Scholar]
- 14.Morrison, C., T. Gilson, and G. J. Nuovo. 2001. Histologic distribution of fatal rotaviral infection: an immunohistochemical and reverse transcriptase in situ polymerase chain reaction analysis. Hum. Pathol. 32:216-221. [DOI] [PubMed] [Google Scholar]
- 15.Moser, C. A., D. V. Dolfi, M. L. Di Vietro, P. A. Heaton, P. A. Offit, and H. F. Clark. 2001. Hypertrophy, hyperplasia, and infectious virus in gut-associated lymphoid tissue of mice after oral inoculation with simian-human or bovine-human reassortant rotaviruses. J. Infect. Dis. 183:1108-1111. [DOI] [PubMed] [Google Scholar]
- 16.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura, S., H. Ushijima, H. Shiraishi, C. Kanazawa, T. Abe, K. Kaneko, and Y. Fukuyama. 1993. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 15:457-459. [DOI] [PubMed] [Google Scholar]
- 18.Offit, P. A., H. F. Clark, M. J. Kornstein, and S. A. Plotkin. 1984. A murine model for oral infection with a primate rotavirus (simian SA11). J. Virol. 51:233-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organ, E. L., and D. H. Rubin. 1998. Pathogenesis of reovirus gastrointestinal and hepatobiliary disease. Curr. Top. Microbiol. Immunol. 233:67-83. [DOI] [PubMed] [Google Scholar]
- 20.Pager, C., D. Steele, P. Gwamanda, and M. Driessen. 2000. A neonatal death associated with rotavirus infection—detection of rotavirus dsRNA in the cerebrospinal fluid. S. Afr. Med. J. 90:364-365. [PubMed] [Google Scholar]
- 21.Pang, X. L., J. Joensuu, and T. Vesikari. 1996. Detection of rotavirus RNA in cerebrospinal fluid in a case of rotavirus gastroenteritis with febrile seizures. Pediatr. Infect. Dis. J. 15:543-545. [DOI] [PubMed] [Google Scholar]
- 22.Qiao, H., G. Zhaori, Z. Jiang, Y. Chen, Y. Chen, and D. Hou. 1999. Detection of group C rotavirus antigen in bile duct and liver tissues of an infant with extrahepatic biliary atresia. Chin. Med. J. 112:93-95. [PubMed] [Google Scholar]
- 23.Ramig, R. F. 1988. The effects of host age, virus dose, and virus strain on heterologous rotavirus infection of suckling mice. Microb. Pathog. 4:189-202. [DOI] [PubMed] [Google Scholar]
- 24.Riepenhoff-Talty, M., V. Gouvea, M. J. Evans, L. Svensson, E. Hoffenberg, R. J. Sokol, I. Uhnoo, S. J. Greenberg, K. Schäkel, G. Zhaori, J. Fitzgerald, S. Chong, M. El-Yousef, A. Nemeth, M. Brown, D. Piccoli, J. Hyams, D. Ruffin, and T. Rossi. 1996. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J. Infect. Dis. 174:8-15. [DOI] [PubMed] [Google Scholar]
- 25.Riepenhoff-Talty, M., K. Schaekel, H. F. Clark, W. Mueller, I. Uhnoo, T. Rossi, J. Fisher, and P. L. Ogra. 1993. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatr. Res. 33:394-399. [DOI] [PubMed] [Google Scholar]
- 26.Salmi, T. T., P. Arstila, and A. Koivikko. 1978. Central nervous system involvement in patients with rotavirus gastroenteritis. Scand. J. Infect. Dis. 10:29-31. [DOI] [PubMed] [Google Scholar]
- 27.Shaw, D. P., L. G. Morehouse, and R. F. Solorzano. 1989. Experimental rotavirus infection in three-week-old pigs. Am. J. Vet. Res. 50:1961-1965. [PubMed] [Google Scholar]
- 28.Smeets, C. C., W. Brussel, Q. H. Leyten, and F. Brus. 2000. First report of Guillain-Barré syndrome after rotavirus-induced gastroenteritis in a very young infant. Eur. J. Pediatr. 159:224. [DOI] [PubMed] [Google Scholar]
- 29.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ushijima, H., K. Bosu, T. Abe, and T. Shinozaki. 1986. Suspected rotavirus encephalitis. Arch. Dis. Child. 61:692-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong, C. J., Z. Price, and D. A. Bruckner. 1984. Aseptic meningitis in an infant with rotavirus gastroenteritis. Pediatr. Infect. Dis. 3:244-246. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida, A., T. Kawamitu, R. Tanaka, M. Okumura, S. Yamakura, Y. Takasaki, H. Hiramatsu, T. Momoi, M. Iizuka, and O. Nakagomi. 1995. Rotavirus encephalitis: detection of the virus genomic RNA in the cerebrospinal fluid of a child. Pediatr. Infect. Dis. J. 14:914-916. [PubMed] [Google Scholar]