Abstract
We examined the role of proline-rich tyrosine kinase (Pyk) 2 in the spreading and migration of human blood eosinophils after β2-integrin ligation. Western blot analysis showed that Pyk2 was activated by phosphorylation at Y402 after eosinophil adhesion to BSA-coated plates after activation with IL-5, platelet-activating factor (PAF), formyl-met-leu-phe (fMLP), or Mn2+. To determine the role of Pyk2 in regulating eosinophil migration, we used a transducable dominant-negative inhibitor of Pyk2, TAT-mediated protein transduction of dominant-negative C-terminal Pyk2 (TAT-Pyk2-CT), a fusion protein in which TAT peptide was fused to the C-terminal Pyk2. TAT-Pyk2-CT blocked tyrosine phosphorylation of Pyk2 caused by β2-integrin adhesion, but did not block adhesion of eosinophils to plated BSA. TAT-Pyk2-CT also blocked subsequent spreading and migration of eosinophils caused by IL-5, PAF, or fMLP. Spreading eosinophils stained with FITC-conjugated phalloidin showed elongation and formation of multiple fillopodia and lamellipodia, whereas nonspreading eosinophils were smaller and round. Treatment of eosinophils with TAT-Pyk2-CT had no effect on the initial cell polarization, but blocked the formation of fillopodia and lamellipodia in adherent cells. Migration of eosinophils through Transwell plates caused by IL-5, PAF, or fMLP was blocked significantly after inhibition of Pyk2. These data indicate that Pyk2, although not involved in β2-integrin adhesion, causes eosinophil spreading and regulates subsequent chemotactic migration after β2-integrin ligation to endothelial counter ligands. We conclude that Pyk2 is activated by β2-integrin adhesion and is a required signal for eosinophil spreading and subsequent chemotactic migration.
Keywords: eosinophils, chemotaxis, adhesion molecules, signal transduction
CLINICAL RELEVANCE
This research focuses on the mechanisms by which the protein tyrosine kinase, proline-rich tyrosine kinase 2, regulates eosinophil spreading and chemotaxis. Data derived from this study should suggest novel approaches for antiinflammatory therapies in asthma and allergic diseases.
Eosinophils are the predominant granulocytes in asthmatic airway inflammation and are recruited into sites of chronic allergic inflammation (1–3). An initial step in this process is eosinophil adhesion to receptors on the luminal surface of vascular endothelial cells. The β2-integrin subfamily of the CD11b (Mac-1)/CD18 molecule and the CD49d (VLA-4)/CD29 of the β1-integrin subfamily have both been reported to contribute to eosinophil adhesion to endothelial cells through binding to the endothelial ligands, intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1 (4–6). Once they are bound to venular endothelium, eosinophils subsequently strengthen their adhesive interaction by spreading, thus resisting detachment by disruptive shear flow. The migration of eosinophils from the endothelial cells into tissues depends solely on β2-integrins (7, 8). Prior studies have demonstrated that transmigration of adherent eosinophils across cultured human umbilical vein endothelial cells requires interaction of CD11a and CD11b with the counter ligand, ICAM-1 (9–11). Although the role of β1- and β2-integrin has been defined as initial events in eosinophil migration, the signaling mechanisms after integrin engagement are largely undefined.
Focal adhesion kinases (Faks) are responsible for transferring signals from integrins to downstream signaling cascades that regulate cell behavior (12). There are two members of the Fak family: Fak and proline-rich tyrosine kinase (Pyk) 2. Fak often coordinates integrin-mediated cell migration in nonhematopoetic cells (13, 14). However, at present, there is no evidence that Fak plays a role in activation of specific adhesion-dependent function in human granulocytes (15, 16). Pyk2 expression is restricted to the central nervous system, cells of hematopoietic lineage, and epithelial cells (17). Fak and Pyk2 appear to have distinct roles. Fak-null genetic mutation displays a general defect of mesoderm development, and is lethal in mice (14). In contrast to Fak, Pyk2 deletion causes no overt impairment in the development of Pyk2-deficient mice (18, 19). However, defects in cell migration were found in the macrophages and marginal zone B cells from Pyk2-deficient mice (18, 19). This suggests that Pyk2 may play a key role in the migration of specific cell types. Recent studies have suggested that Pyk2 plays a critical role in the respiratory burst activity of adherent human neutrophils (20, 21). However, the role of Pyk2 in eosinophil spreading and migration has not been studied previously.
In this study, we investigated the role of Pyk2 in eosinophil spreading and chemotaxis. Because specific chemical inhibitors have not been developed previously for Pyk2, we developed a highly selective TAT-mediated protein transduction of dominant-negative C-terminal Pyk2 (TAT-Pyk2-CT), enabling us to block the activation of Pyk2 in human eosinophils. Using this dominant-negative TAT-Pyk2-CT, we have defined a distinct function for Pyk2 in eosinophils. We find that, although Pyk2 is activated during β2-integrin ligation in vitro, it has no role in eosinophil adhesion; rather, Pyk2 selectively causes cell spreading and subsequent migration.
MATERIALS AND METHODS
Materials
IL-5 was purchased from R&D Systems (Minneapolis, MN). Formyl-met-leu-phe (fMLP), platelet-activating factor (PAF) (C16), anti-C terminal Pyk2 antibody (Ab), and BSA were purchased from Sigma Chemical Co. (St. Louis, MO). Polyclonal anti-Pyk2 and anti-Y402 phosphorylation–specific Pyk2 Abs were purchased from Cell Signaling (Beverly, MA). Eosinophil isolation materials were obtained from StemCell Technologies (Vancouver, BC, Canada). Polystyrene 96-well microtiter plates were obtained from Costar (Cambridge, MA). Mouse IgG1 and anti-CD11b monoclonal Ab (clone Bear 1) were purchased from BD Biosciences (San Jose, CA). Anti-CD18 Ab (clone 7E4) was purchased from Beckman Coulter (Fullerton, CA). Anti-paxillin Ab was purchased from Upstate (Lake Placid, NY).
Generation of TAT-Pyk2-CT Fusion Protein
Plasmid encoding TAT-Pyk2-CT (pTAT-Pyk2-CT) was provided by Dr. C. Nathan (Cornell University, New York, NY). pTAT-green fluorescent protein (GFP) plasmid was provided by Dr. S. Dowdy (University of California, San Diego, San Diego, CA). TAT-Pyk2-CT is a fusion protein in which TAT peptide is fused to the N terminus of the proline-rich C-terminal domain of Pyk2 (amino acid residues 680–1009). BL21(DE3)pLysS-competent Escherichia coli was transformed with either pTAT-Pyk2-CT or pTAT-GFP constructs, and fusion proteins were produced using a native isolation method (22, 23). Briefly, E. coli cells were sonicated in Tris-buffered saline (TBS) plus protease inhibitor cocktail (Roche, Indianapolis, IN) and centrifuged, and the resulting supernatant was loaded onto a Ni-ProBond resin column (Invitrogen, Carlsbad, CA). Pure TAT fusion proteins with N-terminal hexa-His tags were eluted with an imidazole gradient, washed, and transferred into 20 mM Hepes buffer, pH 8.0, for storage and use.
Isolation of Human Blood Eosinophils
Eosinophils were isolated by a modification of the negative immunomagnetic selection technique (24). The method is based on percoll centrifugation (density, 1.089 g/ml) to isolate granulocytes, hypotonic lysis of red blood cells, and, finally, immunomagnetic depletion of neutrophils by the magnetic cell separation system using anti-CD16–coated magnetic cell sorting (MACS) particles. Eosinophil purity of greater than or equal to 98% was routinely obtained. Cells were kept on ice until use.
Measurement of Eosinophil Adhesion
Eosinophil adhesion was assessed as residual eosinophil peroxidase (EPO) activity of adherent cells (25). Briefly, 96-well microplates were coated with 10 μg/ml BSA overnight and washed with HBSS. Eosinophils (1 × 104/100 μl HBSS/0.1% gelatin) were preincubated with different concentrations of TAT fusion proteins for 15 minutes at 37°C. Cells then were added to each well of BSA-coated microplates with 10 ng/ml IL-5, 1 μM fMLP, or 10 mM Mn2+ and incubated 37°C for 30 minutes. After three washes with HBSS, 100 μl of HBSS/0.1% gelatin was added to the reaction wells, and serial dilutions of original cell suspension were added to the empty wells to generate a standard curve. A 100 μl aliquot of EPO substrate (1 mM H2O2, 1 mM OPD, and 0.1% Triton X-100 in Tris buffer, pH 8.0) was then added to each well. After 30-minute incubation at room temperature, 50 μl of 4 M H2SO4 was added to stop the reaction. Absorbance was measured at 490 nm in a microplate reader (Thermomax; Molecular Devices, Menlo Park, CA). All assays were performed in duplicate. Data storage and analysis were facilitated by the use of computer software interfaced with the reader (Softmax, Molecular Devices).
Microscopy Analysis of F-Actin in Eosinophils
Eosinophils (105) were pretreated with 2 μM TAT-Pyk2-CT or 2 μM TAT-GFP for 15 minutes and eosinophil adhesion was performed in 8-chamber Labtech glass slides (Nalgene Nunc, Naperville, IL). After nonadhering eosinophils were washed off, attached cells were fixed with 1% paraformaldehyde for 15 minutes. For F-actin staining, the cells were stained with 1 μg/ml FITC- or rhodamine-conjugated phalloidin in PBS containing 0.1% lysophosphatidylcholine and 0.5% BSA for 30 minutes at 4°C. In TAT-GFP–treated eosinophils, rhodamine phalloid was chosen to label actin to distinguish it from GFP fluorescence. Samples were mounted and observed with an LSM 510 laser scanning microscope equipped with Axiovert 100 M-BP (Zeiss, Milan, Italy). Excitation wavelengths of 488 nm and emission filters were used to detect FITC (515–520 nm) and rhodamine (625 nm). Attached cells that were two to four times larger in diameter than spherical cells, irregularly shaped, had multiple fillopodia or lamellipodia and phase-dark under transmitting light were considered spreading (26). Cells that were larger than spherical cells, but smaller than fully spreading cells, had uneven cortical actin distribution, and irregularly shaped were considered to be in a stage of initial polarization.
Chemotaxis Assay
Eosinophils (105) were incubated with TAT-Pyk2-CT or TAT-GFP at 37°C for 15 minutes, after which chemotaxis assays were performed in a 96-Transwell chemotaxis system (Neuroprobe, Inc., Gaithersburg, MD). The cells were added to the upper compartment of Transwell plates with 5 μm polycarbonate membrane filters. HBSS/0.2% BSA with or without activating agents (IL-5 or fMLP) was added to the lower compartment. After 1 hour incubation in 5% CO2 at 37°C, the cells on the top of plates were removed and replaced with PBS plus 3 mM EDTA, and the migration system was incubated at 4°C for 30 minutes. The PBS then was removed and the migration system was centrifuged at 400 × g for 10 minutes. Serial dilutions of original cell suspension were added to the empty wells to generate a standard curve. Migrated cells were detected by standard EPO activity assay, as described for eosinophil adhesion.
Western Blot Analysis of Pyk2 Tyrosine Phosphorylation
Eosinophils (106) were pretreated with TAT-Pyk2-CT or TAT-GFP for 15 minutes at 37°C. The cells then were added to plated BSA in the presence of buffer control, 10 ng/ml IL-5, 1 μM fMLP, 1 μM PAF, or 10 mM Mn2+, and incubated for 15 minutes. For eosinophils in suspension, cells were added to polystyrene round-bottom tubes with shaking. In experiments involving Ab-mediated β2-integrin engagement, eosinophils were incubated with 10 μg/ml anti-CD18 (clone 7E4) for 30 minutes at 4°C, washed once with PBS, and then stimulated with soluble goat anti-mouse IgG (1.5 μg/106) for 2 to 20 minutes at 37°C. Eosinophils were lysed in 100 μl of lysis buffer (50 mM Tris-HCl, 2 mM EDTA, 2 mM EGTA, 2 mM Na3VO4, 50 mM NaF, 2.5 mM DFP, 1% SDS, and proteinase inhibitor cocktail) and then mixed with loading buffer and boiled for 5 minutes. Samples then were subjected to SDS-PAGE, using 7.5% acrylamide gels under reducing condition (15 mA/gel). Electrotransfer of proteins from the gels to nitrocellulose membrane was achieved using a semidry system (400 mA, 60 min). The membrane was blocked with 1% BSA for 60 minutes, then incubated with 1:1,000 anti-Pyk2 or anti-Y402 phospho-Pyk2, diluted in TBS plus 0.05% Tween 20 (TBS-T) overnight. The membranes were then washed three times for 20 minutes with TBS-T. Anti-rabbit IgG conjugated with horseradish peroxidase was diluted 1:3,000 in TBS-T and incubated with nitrocellulose membrane for 60 minutes. The membrane was again washed three times with TBS-T and assayed by an enhanced chemiluminescence system (Amersham, Arlington Heights, IL).
Immunoprecipitation of Pyk2
Eosinophils (4 × 106) were pretreated with 2 μM TAT-Pyk2-CT or 2 μM TAT-GFP for 15 minutes and adhered to plated BSA in a six-well plate for 15 minutes in the presence of IL-5. Cells were lysed for 30 minutes at 4°C in ice-cold immunoprecipitation buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and protease inhibitor cocktail (Roche). The lysates were centrifuged at 11,000 × g for 10 minutes, and 5 μl anti-Pyk2 Ab was added to the supernatants and shaken at 4°C overnight. The lysates were further incubated with protein A/G agrose beads for 1 hour, washed four times with lysis buffer, and the pellets were resuspended in loading buffer and boiled for 5 minutes. The immunoprecipitated proteins were separated on 7.5% SDS-PAGE, and transferred to membranes by semidry transfer. After incubation with 1:1,000 anti-paxillin Ab or anti-Pyk2 Ab, blots were amplified and visualized using horseradish peroxidase–conjugated anti-rabbit or mouse IgG and enhanced chemiluminescence.
Statistical Analysis
Data are expressed as mean ± SEM. Comparison among experimental groups was assessed by ANOVA. When ANOVA indicated a significant difference between groups, an independent t test was done to determine which intergroup differences were significant. Statistical significance was claimed whenever P < 0.05.
RESULTS
Effect of Eosinophil Adhesion and Soluble Agonist on Activation of Pyk2
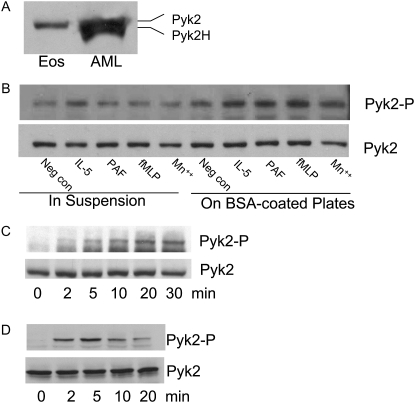
We first identified the isoform of Pyk2 expressed in eosinophils (Figure 1). Previous studies have found two isoforms of Pyk2 expressed in various cells (27–29). The full-length form of Pyk2 (110 kD) is expressed in neuronal cells, platelets, mast cells, and epithelial cells. The lower molecular weight form of Pyk2 (105 kD), identified as the splice variant of Pyk2, is restricted to specific hematopoietic cells, such as T cell, B cells, and monocytes (therefore, named Pyk2-H) (27). Western blot analysis demonstrated that human blood eosinophils expressed the high molecular weight full-length Pyk2 isoform (Figure 1A). By contrast, eosinophilic leukemic AML14.3D10 cells expressed both full-length Pyk2 and Pyk2-H.
Figure 1.
(A) Western blot analysis of proline-rich tyrosine kinase (Pyk) 2 isoform expression in human blood eosinophils. Lysates from 106 human blood eosinophils and AML14.3D10 cells were separated on 10% SDS-PAGE, transferred, and probed with anti-Pyk2 antibody (Ab). (B) Western blot analysis of Pyk2 tyrosine phosphorylation induced by eosinophil adhesion to plated BSA and soluble agonists. Eosinophils were either in suspension or added to plated BSA, and then stimulated with 10 ng/ml IL-5, 1 μM platelet-activating factor (PAF), 1 μM formyl-met-leu-phe (fMLP), or 10 mM Mn2+ for 15 minutes at 37°C. Pyk2 activation was measured by its autophosphorylation detected by pY402 phosphorylation–specific Ab (upper panel). Total Pyk2 expression was measured by Western blot using anti-Pyk2 Ab (lower panel). (C) Time course of Pyk2 phosphorylation during adhesion. Eosinophils were added to a BSA-coated plate in the presence of 10 mM Mn2+ for various times, and Pyk2 phosphorylation and expression were measured as above. (D) Time course of β2-integrin–stimulated Pyk2 tyrosine phosphorylation. Eosinophils were left in buffer or incubated with anti-CD18 (clone 7E4) monoclonal Ab for 30 minutes at 4°C, and then cross-linked with goat anti-mouse for 2–20 minutes at 37°C. Cell lysates were analyzed by Western blots using anti-Y402 phosphorylation–specific Pyk2 Ab (upper panel). Equal protein loading was detected by anti-Pyk2 Ab (lower panel). These results are representative of three different experiments. Pyk2H = hematopoietic cell–specific Pyk2; Pyk2-P = phosphorylation-specific Pyk2.
We next assessed activation of Pyk2 caused by IL-5, PAF, fMLP, and Mn2+ for eosinophil in suspension or adhered to plated BSA. Pyk2 activation was measured by its phosphorylation at Y402 (17). Prior studies in our laboratory have defined a role for β2-integrin CD11b/CD18 in eosinophil adhesion to plated ICAM-1, and that BSA is a full surrogate for plated ICAM-1 (25, 30). In suspension eosinophils—only IL-5, but not PAF, fMLP, or Mn2+—caused slight Pyk2 phosphorylation. Nonspecific adhesion alone also caused slight Pyk2 phosphorylation. Using optimal concentrations for eosinophil adhesion (25, 31), 10 ng/ml IL-5, 1 μM fMLP, 1 μM PAF, or 10 mM Mn2+ enhanced Pyk2 phosphorylation in eosinophils adherent to plated BSA as compared with nonstimulated cells in BSA-coated plates and stimulated cells in suspension (Figure 1B).
We then examined the kinetics of Pyk2 activation during eosinophil adhesion. Mn2+ was chosen in this study to determine the intracellular signals caused solely by integrin engagement without exogenous cytokine/chemokine addition (32). In Mn2+-stimulated eosinophil adhesion, Pyk2 phosphorylation occurred at 2 minutes and peaked at 10 to 30 minutes (Figure 1C). We also obtained similar kinetics of Pyk2 phosphorylation in IL-5– or PAF-stimulated eosinophils adhered to plated BSA (data not shown).
We examined the effect of binding of anti-β2 integrin monoclonal Ab in causing Pyk2 phosphorylation in human eosinophils to determine further whether β2-integrin activates Pyk2. Induction of Pyk2 phosphorylation by this method is putative confirmation that β2-integrin is an activator of Pyk2 during cell adhesion (33). Eosinophils were incubated with 10 μg/ml saturating concentration of anti-CD18 Ab (clone 7E4) for 30 minutes at 4°C and then stimulated by goat anti-mouse IgG for 2–20 minutes at 37°C. Cross-linking of CD18 Ab caused the time-dependent phosphorylation of Pyk2, which peaked at 2 to 5 minutes and declined at 10 to 20 minutes (Figure 1D). These results support the supposition that β2-integrin engagement activates Pyk2 in human blood eosinophils.
Efficacy of TAT-Pyk2-CT Protein Transduction in Eosinophils
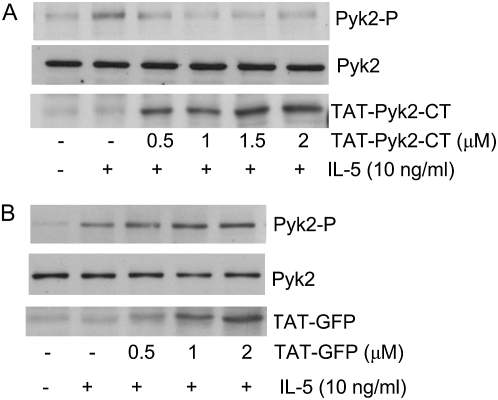
Because eosinophils are terminally differentiated cells, traditional methods, such as transfection and small interfering RNA, cannot be used to study the signaling pathway related to eosinophil adhesion process. Therefore, we used TAT-mediated protein transduction techniques to study the role of Pyk2 in eosinophil function. Eosinophils were preincubated with increasing concentrations of TAT-Pyk2-CT (0.5–2.0 μM) for 15 minutes and adhered to plated BSA in the presence of 10 ng/ml IL-5. Figure 2A demonstrates that 0.5–2.0 μM TAT-Pyk2-CT inhibited augmentation of Pyk2 phosphorylation caused by IL-5 in adherent eosinophils. TAT-Pyk2-CT was transduced into eosinophils in a concentration-dependent manner (Figure 2A, lower panel), and plateaued at 1.5–2.0 μM. Figure 2B demonstrates that 0.5–2 μM TAT-GFP was transduced into eosinophils in concentration-dependent fashion (Figure 2B, lower panel); however, TAT-GFP had no inhibitory effect on phosphorylation of Pyk2 caused by IL-5 in adherent eosinophils (Figure 2B, upper panel versus middle panel). These data suggest that TAT-Pyk2-CT acted as a dominant-negative protein to inhibit endogenous Pyk2 activation caused by IL-5 in adhering eosinophils.
Figure 2.
(A) Inhibitory effect of TAT-mediated protein transduction of dominant-negative C-terminal Pyk2 (TAT-Pyk2-CT) on the phosphorylation of Pyk2 in adherent eosinophils stimulated by IL-5. Eosinophils were preincubated with indicated concentrations of TAT-Pyk2-CT for 15 minutes at 37°C and adhered to plated BSA for 15 minutes in the presence of IL-5. Eosinophil lysates were separated on 10% SDS-PAGE, transferred and probed by anti-Y402 phosphorylation–specific Pyk2 Ab (upper panel), anti-Pyk2 Ab (middle panel), and anti-C terminal Pyk2 Ab (lower panel). (B) Absence of inhibitory effect of TAT-green fluorescent protein (GFP) on the phosphorylation of Pyk2. Eosinophils were preincubated with indicated concentrations of TAT-GFP for 15 minutes at 37°C, and adhered to plated BSA for 15 minutes in the presence of IL-5. Eosinophil lysates were separated on 10% SDS-PAGE, transferred, and probed by anti-Y402 phosphorylation specific Pyk2 Ab (upper panel), anti-Pyk2 Ab (middle panel), and anti-GFP Ab (lower panel). These results are representative of three different experiments.
Effect of TAT-Pyk2-CT on Eosinophil Adhesion and Spreading
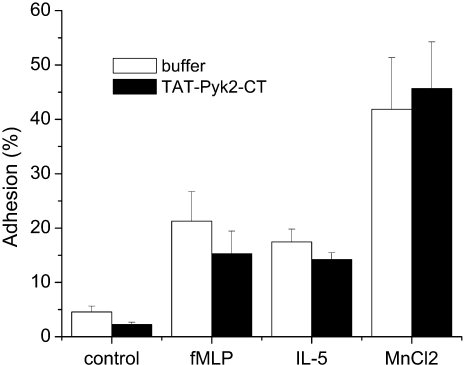
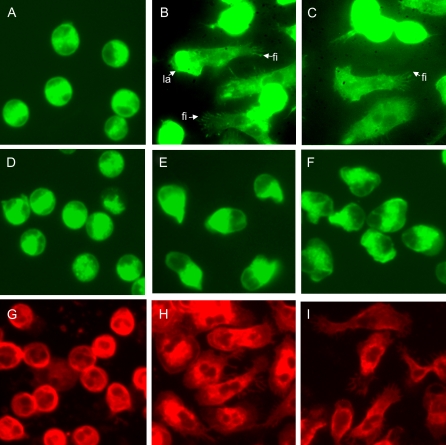
We further examined the effect of TAT-Pyk2-CT on eosinophil adhesion to plated BSA. Eosinophil adhesion to plated BSA caused by fMLP, IL-5, or Mn2+ was not attenuated by TAT-Pyk2-CT pretreatment (Figure 3). However, when analyzed by fluorescent microscopy, we found that the morphologic changes caused by IL-5 or fMLP during eosinophil adhesion were blocked by TAT-Pyk2-CT, but not by TAT-GFP treatment. Unstimulated eosinophils with typical, unpolarized, round appearance were found on plated BSA (Figure 4A). Adherent eosinophils activated by IL-5 (Figure 4B) or fMLP (Figure 4C) showed spreading, long, thin fillopodia (thin protrusions from a cell) or lamellipodia (sheet-like protrusions from a cell). TAT-Pyk2-CT had no effect on morphology of unstimulated cells (Figure 4D), but blocked the spreading of adherent eosinophils (Figures 4E and 4F). Although treatment of eosinophils with IL-5 or fMLP showed initial polarization, after pretreatment with TAT-Pyk2-CT, the great majority of cells were smooth and smaller than fully spreading cells (Figures 4E and 4F). By contrast, TAT-GFP had no effect on morphology of unstimulated eosinophils (Figure 4G) or morphologic change after stimulation with IL-5 (Figure 4H) or fMLP (Figure 4I). These results suggest that, although Pyk2 does not cause eosinophil adhesion or initial polarization (see definition in Materials and Methods), it is critical for eosinophils to spread fully after eosinophil adhesion.
Figure 3.
No inhibitory effect of TAT-Pyk2-CT on eosinophil adhesion. Eosinophils were pretreated with 2 μM TAT-Pyk2-CT for 15 minutes and added to plated BSA with buffer, 1 μM fMLP, 10 ng/ml IL-5, or 10 mM MnCl2 for 30 minutes. After washing out the nonadherent cells, eosinophil adhesion to plated BSA was measured by residual eosinophil peroxidase activity, as described in Material and Methods. Results are presented as the mean ± SEM from five separate experiments.
Figure 4.
Effect of TAT-Pyk2-CT on eosinophil spreading and morphological changes. Eosinophils were preincubated with buffer (A–C), 2 μM TAT-Pyk2-CT (D–F), or 2 μM TAT-GFP (G–I) for 15 minutes, and added to BSA-coated plates with buffer (A, D, G), IL-5 (B, E, H), or fMLP (C, F, I) for 15 minutes. Eosinophils were stained with FITC- (A–F) or rodamine- (G–I) conjugated phalloidin, and the morphology of adhered eosinophils was observed under fluorescent microscope. Fi = fillopodia; la = lamellipodia. A representative image is shown (n = 3).
Inhibition of Eosinophil Migration by TAT-Pyk2-CT
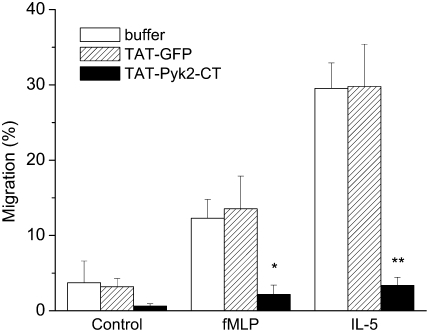
As eosinophil spreading is required for subsequent eosinophil migration, we next examined the effect of TAT-Pyk2-CT on eosinophil chemotactic migration. Spontaneous migration was 3.7 ± 2.9% and eosinophil migration was induced by IL-5 or fMLP. TAT-Pyk2-CT blocked eosinophil migration in response to IL-5 or fMLP (Figure 5). Eosinophil migration caused by IL-5 was reduced from 29.5 ± 3.4% to 3.3 ± 1.1% upon treatment with 2 μM TAT-Pyk2-CT (P < 0.01). Similarly, eosinophil migration caused by fMLP was reduced from 12.3 ± 2.5% to 2.2 ± 1.2% (P < 0.05). By contrast, 2 μM TAT-GFP had no effect on eosinophil chemotaxis induced by IL-5 or fMLP. These data indicate that Pyk2 controls the signaling pathways leading to eosinophil chemotaxis.
Figure 5.
Effect of TAT-Pyk2-CT on eosinophil migration. Eosinophils were preincubated with buffer, 2 μM TAT-Pyk2-CT, or 2 μM TAT-GFP for 15 minutes and added on the top plate of the migration system. Eosinophil migration in response to fMLP and IL-5 was measured after 60 minutes. Results are presented as the mean ± SEM from six separate experiments. *P < 0.05 vs. fMLP only; **P < 0.01 vs. IL-5 only.
Effect of TAT-Pyk2-CT on the Physical Association of Pyk2 with Paxillin
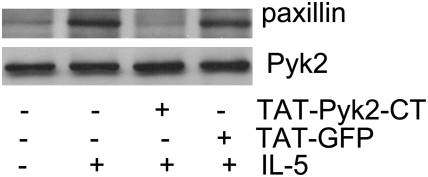
As paxillin has been suggested to be involved in the rearrangement of the cytoskeleton (34), we then determined whether TAT-Pyk2-CT decreased the physical association of paxillin with Pyk2 in adherent eosinophils. Eosinophils were treated with TAT-Pyk2-CT or TAT-GFP for 15 minutes and then adhered to plated BSA in the presence of 10 ng/ml IL-5 for another 15 minutes. Eosinophil lysates were immunoprecipitated with anti-Pyk2, and blotted sequentially with anti-Paxillin and anti-Pyk2. A small amount of paxillin was present in Pyk2-immunocomplexes in nonadhering cells. Adhesion to plated BSA increased the magnitude of paxillin in Pyk2 immunoprecipitates (Figure 6, upper panel). Preincubation of eosinophils with 2 μM TAT-Pyk2-CT, but not TAT-GFP, decreased the coimmunoprecipitation of paxillin with Pyk2. The amount of Pyk2 precipitated in these cell lysates was comparable for treatment groups (Figure 6, lower panel). These results suggest that Pyk2 activation caused by β2-integrin adhesion increased its binding to paxillin.
Figure 6.
Effect of TAT-Pyk2-CT on the physical association of paxillin with Pyk2 in adherent eosinophils. Eosinophils were preincubated with 2 μM TAT-Pyk2-CT or TAT-GFP for 15 minutes at 37°C and adhered to plated BSA in the presence of IL-5 for 15 minutes. The eosinophil lysates were immunoprecipitated with anti-Pyk2 and immunoblotted with anti-paxillin (upper panel) or anti-Pyk2 Ab (lower panel). These results are representative of three different experiments.
DISCUSSION
The objective of this investigation was to determine the role of Pyk2 in eosinophil spreading and subsequent migration after β2-integrin–mediated cell adhesion. We found that: (1) Pyk2 is activated during eosinophil adhesion; (2) however, Pyk2 does not facilitate β2-integrin adhesion of eosinophils to endothelial counter-ligands (i.e., inhibition of Pyk2 by TAT-Pyk2-CT does not block cell adhesion); (3) Pyk2 phosphorylation is essential for eosinophil spreading and migration after β2-integrin ligation; and (4) inhibition of Pyk2 by TAT-Pyk2-CT attenuates eosinophil migration caused by IL-5 or fMLP. Our data indicate a distinct postadhesive function of Pyk2 in eosinophils. The prolonged kinetics (15–30 min) of Pyk2 tyrosine phosphorylation during eosinophil adhesion is consistent with a downstream role in adhesion signaling, which could facilitate cytoskeletal reorganization and morphologic change. The association with paxillin is a potential manifestation of its execution of this function.
Previous studies have found that engagement of β2-integrin induced tyrosine phosphorylation of several proteins around 100 to115 kD in eosinophils (16). However, the identity of these proteins was not fully defined. In the current studies, we found that β2-integrin ligation caused tyrosine phosphorylation of 110 kD Pyk2. Pyk2 is a cytoplasmic protein tyrosine kinase that is closely related to the p125 Fak. Fak has been detected in human eosinophils (16); however, it does not appear to be activated by cell adhesion, suggesting that Fak has no role in integrin signaling in eosinophils. Here we report that the full-length isoform of Pyk2 is present in human eosinophils, and stimulation of eosinophils with fMLP, IL-5, PAF, or Mn2+ in BSA-coated plates induced the tyrosine phosphorylation of Pyk2 (Figure 1). Adhesion of human eosinophils to plated BSA has been demonstrated to be mediated by β2-integrin (25); therefore, β2-integrin signaling likely accounts for Pyk2 phosphorylation. Direct engagement of β2-integrin with anti-CD18 Ab that cross-linked with the secondary Ab caused Pyk2 phosphorylation (Figure 2), supporting this conclusion.
Integrin-mediated adhesion interactions play a critical mechanical role in cell spreading and migration by linking the extracellular matrix proteins to the intracellular cytoskeleton. In this study, Pyk2 inhibition by TAT-Pyk2-CT did not inhibit β2-integrin–mediated adhesion of eosinophils to plated BSA, but dramatically reduced eosinophil spreading and chemotaxis to various chemoattractants, suggesting that Pyk2 activation plays a key role in the morphologic change and migratory behavior after eosinophil adhesion.
The molecular mechanisms leading to eosinophil spreading and formation of focal adhesion–like structures are not yet clearly defined. In adherent cells, Fak and paxillin have been suggested to govern integrin-mediated cell spreading and focal adhesion assembly by their ability to interact with several cytoskeletal and signaling molecules (35). It is therefore conceivable that β2-integrin–activated Pyk2 and paxillin may serve a similar function in eosinophils by regulating some of the morphological changes occurring during their migration. We reasoned that, if Pyk2 was not associated to paxillin in adherent eosinophils, Pyk2 could not be implicated in cell spreading. In this study, we demonstrated that the association of paxillin with Pyk2 was increased in adherent eosinophils. Pyk2 inhibition by TAT-Pyk2-CT prevented the increase in association of Pyk2 with paxillin (Figure 6) and substantially attenuated the spreading of eosinophils (Figure 4). These results suggest that Pyk2 tyrosine phosphorylation increases its binding to paxillin, which likely promotes eosinophil spreading and subsequent migration. However, the mechanisms by which paxillin regulates cytoskeletal rearrangement after integrin ligation require further study.
It is important to recognize specific limitations of our findings. Our data were obtained in vitro, with eosinophil adhesion, spreading, and migration assays performed under static condition. The regulatory role of Pyk2 in eosinophil migratory behavior cannot be extrapolated to the in vivo state, in which eosinophils flow in the blood vessels. Nonetheless, the substantial differences in the ability of Pyk2 inhibitor to block eosinophil migration versus adhesion under our experimental conditions suggest a possible role of Pyk2 in postadhesive functions of eosinophils.
In summary, we find that β2-integrin adhesion of eosinophils induces activation of Pyk2. Specific inhibition of Pyk2 by TAT-Pyk2-CT causes a substantial attenuation of eosinophil spreading and migration, but has no effect on eosinophil adhesion or initial polarization, suggesting a critical role for Pyk2 in eosinophil spreading and migratory behavior.
Acknowledgments
The authors thank Dr. Carl Nathan (Cornell University) for providing pTAT-Pyk2-CT plasmid and Dr. Steven Dowdy (University of California, San Diego) for providing pTAT-GFP expression plasmid.
This work was supported by National Heart, Lung, and Blood Institute grants HL-92328 (X.Z.) and HL-85779 (A.R.L.), and by a grant from the GlaxoSmithKline Center of Excellence.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0047OC on March 26, 2008
Conflict of Interest Statement: A.R.L. has received $3000 in the past 3 years for a GlaxoSmithKline advisory board, $13,000/year for participation in a Merck advisory board, $24,000/year for participation in a Broncus/Asthmatx advisory board, and $3,500 for participation in a Pfizer advisory board. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol 2007;119:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol 1990;85:422–436. [DOI] [PubMed] [Google Scholar]
- 3.Leff AR, Hamann KJ, Wegner CD. Inflammation and cell–cell interactions in airway hyperresponsiveness. Am J Physiol 1991;260:L189–L206. [DOI] [PubMed] [Google Scholar]
- 4.Walsh GM, Hartnell A, Wardlaw AJ, Kurihara K, Sanderson CJ, Kay AB. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin (CD11/18)–dependent manner. Immunology 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 5.Weber C, Katayama J, Springer TA. Differential regulation of beta 1 and beta 2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA 1996;93:10939–10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wegner CD, Gundel RH, Reilly P, Haynes N, Letts LG, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) in the pathogenesis of asthma. Science 1990;247:456–459. [DOI] [PubMed] [Google Scholar]
- 7.Moser R, Fehr J, Olgiati L, Bruijnzeel PL. Migration of primed human eosinophils across cytokine-activated endothelial cell monolayers. Blood 1992;79:2937–2945. [PubMed] [Google Scholar]
- 8.Das AM, Williams TJ, Lobb R, Nourshargh S. Lung eosinophilia is dependent on IL-5 and the adhesion molecules CD18 and VLA-4, in a guinea-pig model. Immunology 1995;84:41–46. [PMC free article] [PubMed] [Google Scholar]
- 9.DiScipio RG, Daffern PJ, Jagels MA, Broide DH, Sriramarao P. A comparison of C3a and C5a-mediated stable adhesion of rolling eosinophils in postcapillary venules and transendothelial migration in vitro and in vivo. J Immunol 1999;162:1127–1136. [PubMed] [Google Scholar]
- 10.Ebisawa M, Bochner BS, Georas SN, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. I. Role of endothelial and eosinophil adhesion molecules in IL-1 beta–induced transendothelial migration. J Immunol 1992;149:4021–4028. [PubMed] [Google Scholar]
- 11.Yamamoto H, Sedgwick JB, Busse WW. Differential regulation of eosinophil adhesion and transmigration by pulmonary microvascular endothelial cells. J Immunol 1998;161:971–977. [PubMed] [Google Scholar]
- 12.Hauck CR, Klingbeil CK, Schlaepfer DD. Focal adhesion kinase functions as a receptor-proximal signaling component required for directed cell migration. Immunol Res 2000;21:293–303. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 2000;19:5606–5613. [DOI] [PubMed] [Google Scholar]
- 14.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from Fak-deficient mice. Nature 1995;377:539–544. [DOI] [PubMed] [Google Scholar]
- 15.Fuortes M, Jin WW, Nathan C. Beta 2 integrin–dependent tyrosine phosphorylation of paxillin in human neutrophils treated with tumor necrosis factor. J Cell Biol 1994;127:1477–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato M, Abraham RT, Okada S, Kita H. Ligation of the β2 integrin triggers activation and degranulation of human eosinophils. Am J Respir Cell Mol Biol 1998;18:675–686. [DOI] [PubMed] [Google Scholar]
- 17.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal 2000;12:123–133. [DOI] [PubMed] [Google Scholar]
- 18.Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci USA 2003;100:10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2–deficient mice defines their role in the humoral response. Nat Immunol 2000;1:31–36. [DOI] [PubMed] [Google Scholar]
- 20.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase Pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest 1999;104:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med 2003;197:63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myou S, Leff AR, Myo S, Boetticher E, Meliton AY, Lambertino AT, Liu J, Xu C, Munoz NM, Zhu X. Activation of group IV cytosolic phospholipase A2 in human eosinophils by phosphoinositide 3-kinase through a mitogen-activated protein kinase–independent pathway. J Immunol 2003;171:4399–4405. [DOI] [PubMed] [Google Scholar]
- 23.Sano M, Leff AR, Myou S, Boetticher E, Meliton AY, Learoyd J, Lambertino AT, Munoz NM, Zhu X. Regulation of interleukin-5–induced β2-integrin adhesion of human eosinophils by phosphoinositide 3-kinase. Am J Respir Cell Mol Biol 2005;33:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods 1991;145:105–110. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Subbaraman R, Sano H, Jacobs B, Sano A, Boetticher E, Munoz NM, Leff AR. A surrogate method for assessment of beta(2)-integrin–dependent adhesion of human eosinophils to ICAM-1. J Immunol Methods 2000;240:157–164. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez R, Boxer LA, Suchard SJ. Beta2 integrins are not required for tyrosine phosphorylation of paxillin in human neutrophils. J Immunol 1997;159:5568–5575. [PubMed] [Google Scholar]
- 27.Dikic I, Schlessinger J. Identification of a new Pyk2 isoform implicated in chemokine and antigen receptor signaling. J Biol Chem 1998;273:14301–14308. [DOI] [PubMed] [Google Scholar]
- 28.Xiong WC, Macklem M, Parsons JT. Expression and characterization of splice variants of PYK2, a focal adhesion kinase–related protein. J Cell Sci 1998;111:1981–1991. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Hunter D, Morris J, Haskill JS, Earp HS. A calcium-dependent tyrosine kinase splice variant in human monocytes: activation by a two-stage process involving adherence and a subsequent intracellular signal. J Biol Chem 1998;273:9361–9364. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin–dependent adhesion of human eosinophils. J Immunol 1999;163:3423–3429. [PubMed] [Google Scholar]
- 31.Xu X, Hakansson L. Regulation of the release of eosinophil cationic protein by eosinophil adhesion. Clin Exp Allergy 2000;30:794–806. [DOI] [PubMed] [Google Scholar]
- 32.Yan SR, Huang M, Berton G. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J Immunol 1997;158:1902–1910. [PubMed] [Google Scholar]
- 33.Gismondi A, Jacobelli J, Strippoli R, Mainiero F, Soriani A, Cifaldi L, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase 2 and Rac activation by chemokine and integrin receptors controls NK cell transendothelial migration. J Immunol 2003;170:3065–3073. [DOI] [PubMed] [Google Scholar]
- 34.Yan SR, Berton G. Antibody-induced engagement of beta2 integrins in human neutrophils causes a rapid redistribution of cytoskeletal proteins, Src-family tyrosine kinases, and p72syk that precedes de novo actin polymerization. J Leukoc Biol 1998;64:401–408. [DOI] [PubMed] [Google Scholar]
- 35.Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci 1999;4:D102–D113. [DOI] [PubMed] [Google Scholar]