Abstract
Migration of fibroblasts plays an essential role in tissue repair after injury. Sphingosine 1-phosphate (S1P) is a multifunctional mediator released by many cells that can be released in inflammation and after injury. This study evaluated the effect of S1P on fibroblast chemotaxis toward fibronectin. S1P alone did not affect fibroblast migration, but S1P enhanced fibronectin-directed chemotaxis in a concentration-dependent manner. The effect of S1P was not mimicked by dihydro (dh) S1P or the S1P1 receptor agonist SEW2871. S1P augmentation of fibroblast chemotaxis, however, was completely blocked by JTE-013, an S1P2 antagonist, but not by suramin, an S1P3 antagonist. Suppression of the S1P2 receptor by small interfering (si)RNA also completely blocked S1P augmentation of fibroblast chemotaxis to fibronectin. S1P stimulated Rho activation and focal adhesion kinase (FAK) phosphorylation, and these were also significantly inhibited by the S1P2 receptor antagonist (JTE-013) or by S1P2 siRNA. Further, the potentiation of S1P signaling was blocked by the Rho-kinase inhibitor Y-27632 in a concentration-dependent manner. Inhibition of FAK with siRNA reduced basal chemotaxis toward fibronectin slightly but significantly, and almost completely blocked S1P augmented chemotaxis. These results suggest that S1P-augmented fibroblast chemotaxis toward fibronectin depends on the S1P2 receptor and requires Rho and Rho-kinase, and FAK phosphorylation. By augmenting fibroblast recruitment, S1P has the potential to modulate tissue repair after injury. The pathways by which S1P mediates this effect, therefore, represent a potential therapeutic target to affect tissue repair and remodeling.
Keywords: sphingosine 1-phosphate, fibroblasts, migration, fibronectin
CLINICAL RELEVANCE
Sphingosine 1-phosphate could be one possible therapeutic target for fibrosis.
Cell migration plays a fundamental role in many biological processes, such as embryonic development, tissue repair and regeneration, tissue remodeling, immune surveillance, tumor cell invasion, and metastasis (1, 2). Many cells migrate directionally in response to a variety of extracellular signals, including gradients of chemokines, growth factors, or extracellular matrix molecules such as fibronectin, a potent chemoattractant for fibroblasts. In this context, the migration of fibroblasts from neighboring connective tissue into sites of inflammation is essential for tissue repair and wound healing (3, 4).
Sphingosine 1-phosphate (S1P) is a sphingolipid metabolite that has a variety of biological effects on cells, including modulation of proliferation, differentiation, cytoskeleton organization, motility, apoptosis, neurite retraction, contraction, angiogenesis, and platelet activation (5–8). S1P is produced by various cell types, including platelets, in response to diverse stimuli. S1P functions as a specific ligand for a family of G protein–coupled receptors (GPCRs) named S1P1, S1P2, S1P3, S1P4, and S1P5 (previously termed Edg 1, 5, 3, 6, and 8, respectively) (9). The majority of the biological functions of S1P are mediated through these receptors (10); however, considerable evidence also indicates that S1P can act as an intracellular second messenger (11). S1P generated extracellularly may also act through these intracellular pathways to regulate intracellular calcium homeostasis, cell growth, and cell survival (6, 10, 12–14).
Effects of S1P on cell migration have been reported previously; these largely depend on cell type, the isoforms of S1P receptors expressed, the concentration of S1P, and the specific chemoattractants. The mechanisms by which S1P exerts its effects on the migration of cells, however, have not been fully determined. S1P negatively modulates platelet-derived growth factor (PDGF)-induced migration and proliferation in mouse embryonic fibroblasts and human vascular smooth muscle cells (15, 16). In human endothelial cells, S1P stimulates phosphorylation of focal adhesion kinase (FAK) and cell migration through Gi-coupled receptors and the PLC pathway (17). S1P also stimulates Rho-mediated tyrosine phosphorylation of FAK and paxillin in Swiss 3T3 fibroblasts (18).
The effects of S1P on human lung fibroblast migration mediated by fibronectin, however, have not been investigated, but they are likely to be of relevance to the airway remodeling and fibrosis that characterize multiple airway diseases. In the current study, therefore, we investigated the effect of S1P on chemotaxis of human fetal lung fibroblasts (HFL-1 cells) toward fibronectin. In addition, we defined the pathway for S1P modulation of HFL-1 chemotaxis, which involves the S1P2 receptor, Rho and Rho-kinase, and FAK.
MATERIALS AND METHODS
Materials
S1P was obtained from Sigma (St. Louis, MO), dissolved in methanol as a stock solution of 10−2 M, and further diluted in medium to the designated concentrations. (2S,3R)-Dihydrosphingosine 1-phosphate dhS1P was from ALEXIS Biochemicals (San Diego, CA). SB203580, PD98059, JNK inhibitor II, PP2, and Y-27632 were from Calbiochem (San Diego, CA). LY294002 was obtained from Promega (Madison, WI). Mouse monoclonal anti-FAK, clone 4.47, anti-phosphotyrosine, clone 4G10, and Rho Activation Assay Kit (Cat# 17-294) were purchased from Millipore-Upstate Cell Signaling Solutions (Lake Placid, NY). Phosphorylation site-specific mouse monoclonal antibody against FAK (pY397) was from Millipore-Chemicon International (Temecula, CA). Phosphorylation site-specific rabbit polyclonal antibodies against FAK (pY407, pY576, pY577, and pY861) were obtained from BioSource International (Camarillo, CA). Phosphorylation site-specific rabbit polyclonal antibody against FAK (pY925) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-S1P1, anti-S1P2, anti-S1P5, and anti–β-actin antibodies were purchased from Sigma. Human fibronectin was prepared from human plasma by gelatin-sepharose affinity chromatography as described (19). After elution with 4 M urea, the fibronectin was further purified by heparin-agarose affinity chromatography and eluted with 500 mM NaCl.
Cells and Cell Culture
HFL-1 cells were purchased from American Type Culture Collection (Rockville, MD). The cells were cultured in 100-mm tissue culture dishes (FALCON, Becton-Dickinson Labware, Lincoln Park, NJ) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 U/ml penicillin G sodium, 50 μg/ml streptomycin sulfate, and 0.25 μg/ml fungizone (all from Invitrogen, Carlsbad, CA) and maintained at 37°C in a humidified 5% CO2 incubator. Fibroblasts were routinely passaged every 4 or 5 days, and the cells were used between passages 13 and 18 in all experiments. Confluent fibroblasts were detached from culture dishes by treatment with 0.05% trypsin in 0.53 mM EDTA and resuspended in serum-free DMEM.
Cell Migration Assays
Migration of HFL-1 cells was assessed by using Boyden chambers (Nucleopore, Cabin John, MD) as previously described (20, 21). Briefly, human fibronectin (20 μg/ml unless otherwise indicated) in serum-free DMEM was placed in the bottom chamber as the chemoattractant. The wells of the chamber were divided by an 8-μm pore filter (Nucleopore, Pleasanton, CA) coated with 0.1% gelatin (Bio-Rad, Hercules, CA). Fibroblasts (1 × 106 cells/ml in serum-free DMEM) were loaded into the upper well of the chamber with S1P and/or other additives. The chamber was incubated at 37°C in a humidified 5% CO2 incubator for 6 hours and adherent cells on the upper surface of the membrane were removed by scraping. Migratory cells attached to the bottom of the membrane were fixed and stained with PROTOCOL (Biochemical Science, Swedesboro, NJ). Membranes were then mounted on a glass microscope slide and the number of migrated cells was counted in five high-power fields (5HPF) under a light microscope. All chemotaxis experiments were repeated on separate occasions at least three times, each with at least triplicate wells per condition.
RT-PCR
Total RNA was extracted using Trizol reagent. Reverse transcription (RT) and PCR were performed using 1 μg total RNA and GeneAmp kit (Applied Biosystems cat#: N808-0017) following the manufacturer's instruction. β-actin was used as an internal control. Primer sequences were as follows. S1P1 (384 bp): forward, 5′-CCTCTTCCTGCTAATCAGCG-3′; reverse, 5′-ACAGGTCTTCACCTTGCAGC-3′. S1P2 (397 bp): forward, 5′-CGGAGACTGACTGTCAGCAC-3′; reverse, 5′-GGTCCAGAACTATGCCGAGA-3′. S1P3 (370 bp): forward, 5′-TCAGCCTGTCTCCCACGGTC-3′; reverse, 5′-ACGGCTGCTGGACTTCACCA-3′. S1P4 (319 bp): forward, 5′-ACGGGAGGGCCTGCTCTTCA-3′; reverse, 5′-AAGGCCAGCAGGATCATCAG-3′. S1P5 (278 bp): forward, 5′-GTGAGCGAGGTCATCGTCCTG-3′; reverse, 5′-GTCTGCAGCCGGTTCTGATAC-3′. β-actin (800 bp): forward, 5′-AGCCATGTACGTTGCTA-3′; reverse, 5′-AGTCCGCCTAGAAGCA-3′.
RNA Interference
RNA interference was performed using commercially available SMARTpool small interfering RNA (siRNA) specific for FAK and for S1P2 (Dharmacon, Lafayette, CO). Transfection reagent–siRNA complexes were prepared by using Lipofectamine 2000 and Opti-MEM I (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. HFL-1 cells were plated into 100-mm tissue culture dishes and cultured for 24 hours. Cells were 30 to 50% confluent at the time of the siRNA transfection. Cells were incubated with the siRNA (50 nM) in serum-free DMEM for 6 hours. The medium was then changed to 10% FBS in DMEM, and the cells were cultured for an additional 48 hours. Cells treated with Liptofectamine 2000 alone were used as control.
Immunoprecipitation and Western Blotting
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation assay (RIPA) buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethane sulfonyl fluoride, 100 μg/ml aprotinin, 1 mM sodium orthovanadate). The lysates were briefly sonicated and then centrifuged for 15 minutes to remove debris. The protein concentration in the supernates was determined using the Bio-Rad Protein Assay. For immunoprecipitations, lysates were incubated with anti-FAK conjugated to agarose (Upstate Biotechnology) at 4°C overnight according to the manufacturer's instructions. The agarose beads were washed three times with ice-cold PBS and the precipitates were eluted in 2× Laemmli sample buffer (125 mM Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue) by boiling for 5 minutes.
For Western blotting, protein samples were separated by 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Immuno-Blot PVDF Membrane (Bio-Rad) with a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad). The membranes were blocked with 5% skim milk or 1% BSA in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) for 1 hour at room temperature and incubated with primary antibodies at 4°C overnight. Horseradish peroxidase (HRP)-conjugated IgG secondary antibodies and ECL Western blot detection system (Amersham Pharmacia Biotech, Uppsala, Sweden) were used for visualization. Band intensities were measured by the public image analysis program Image J obtained from the National Institutes of Health.
Rho Activation Assay
GTP-bound active Rho was assessed by pull-down assays following the manufacturer's instructions (Upstate, cat# 17-294). Briefly, nearly confluent HFL-1 cells were stimulated with S1P or LPA. After washing with ice-cold PBS, cells were lysed with cold MLB buffer (500 μl/100-mm dish). Cell lysates were lysed, and the supernates were subjected to Rho pull-down using agarose beads conjugated to binding domain of 7 to 89 residues of mouse Rhotekin Rho, which selectively bind GTP-bound active Rho, not GDP-Rho from cell lysates. The beads were treated with Mg2+ Lysis/Wash Buffer (supplied in the kit) to solubilize GTP-bound Rho, and this was subjected to SDS-PAGE. After transferring to PVDF membrane and blocking, the membranes were incubated with anti-Rho (-A, -B, -C), clone 55, overnight at 4°C. After washing and applying anti-mouse HRP-conjugated antibody, ECL reagent was used for visualization as described above.
Statistical Analysis
Grouped data were evaluated by one-way or two-way ANOVA. Differences between series of data that appeared statistically different were corrected by Tukey-Kramer test. In all cases, comparisons were considered statistically significant if P < 0.05. Data are expressed as means ± SEM.
RESULTS
Effect of S1P on Chemotactic Response of Human Fibroblasts to Fibronectin
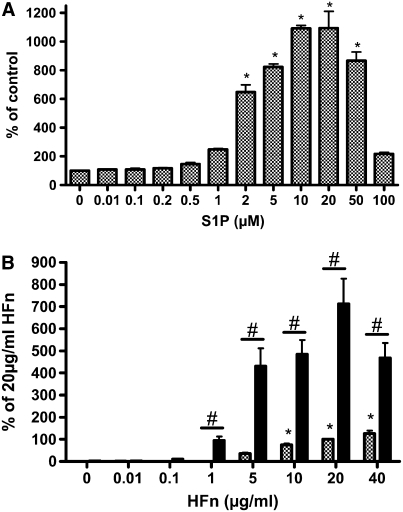
S1P stimulated HFL-1 cell migration toward human fibronectin in a concentration-dependent manner (Figure 1A). S1P at concentrations less than 1 μM had no effect on fibronectin-induced chemotaxis, whereas S1P concentrations greater than 1 μM markedly augmented the chemotaxis. S1P had a similar effect on human foreskin fibroblast chemotaxis toward fibronectin (data not shown). At concentrations of S1P above 20 μM, chemotaxis was inhibited. To determine whether the stimulatory effect was due to only S1P or the combination of S1P and fibronectin, varying concentrations of fibronectin were placed in the bottom wells of the chemotactic chamber. The number of migrated cells increased as the concentration of fibronectin increased (Figure 1B). In the absence of chemoattractant (fibronectin), S1P had little effect on HFL-1 cell migration (data not shown), indicating that S1P augmented fibronectin-directed chemotactic activity rather than enhancing chemokinetic activity. The S1P metabolites sphingosine and ceramide did not affect the HFL-1 cell chemotaxis (data not shown).
Figure 1.
S1P stimulates human lung fibroblast migration toward human fibronectin (HFn). (A) S1P concentration dependence. Chemotaxis was performed with human fetal lung fibroblasts (HFL-1 cells) in the blindwell chamber assay with fibronectin (20 μg/ml) as the chemoattractant. S1P was added in various concentrations to the top wells. Vertical axis: migrated cells. Horizontal axis: S1P concentration. (B) S1P potentiation of chemotaxis, fibronectin concentration dependence (n = 3). S1P (10 μM, solid bars), or methanol control (shaded bars) was added to fibroblasts in the top wells and fibronectin was used in various concentrations in the lower wells as a chemoattractant. Vertical axis: migrated cells. Horizontal axis: fibronectin concentration. *P < 0.05 compared with 0 μM S1P (A) or 0 μg/ml HFn (B). #P < 0.01 compared with or without S1P. Each panel represents one experiment performed in triplicate that was repeated three times.
Expression of S1P Receptors in HFL-1 Cells
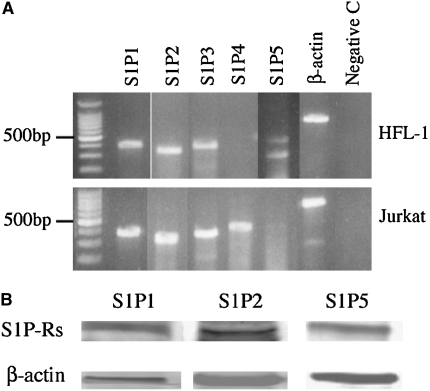
To determine the expression of S1P receptors by HFL-1 cells, two methods were used. First, mRNA expression for S1P1, S1P2, S1P3, S1P4, and S1P5 was examined by RT-PCR. HFL-1 cells expressed S1P1, S1P2, S1P3, and S1P5, but not S1P4 (Figure 2A). A similar expression pattern was observed in human bronchial fibroblasts and human airway smooth muscle cells (data not shown). In contrast, Jurkat cells expressed S1P1, S1P2, S1P3, and S1P4, but not S1P5 (Figure 2A). Second, to confirm protein expression, immunoblots were performed, and immunoreactive protein was detected for S1P1, S1P2, and S1P5 in HFL-1 cell lysates (Figure 2B).
Figure 2.
Expression of S1P receptors in human lung fibroblasts. (A) mRNA expression. Total RNA was extracted from HFL-1 cells or Jurkat cells. RT-PCR was performed as described in Materials and Methods. (B) Immunoblots for S1P1, S1P2, and S1P5. Cell lysate from HFL-1 cells was subjected to immunoblotting for S1P1, S1P2, and S1P5. β-actin was used as a loading control.
Role of Extracellular Receptors in S1P-Stimulated HFL-1 Cell Chemotaxis
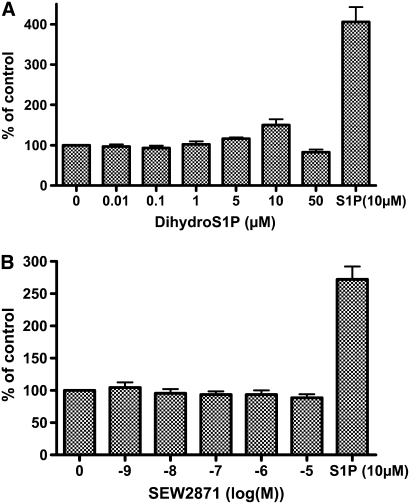
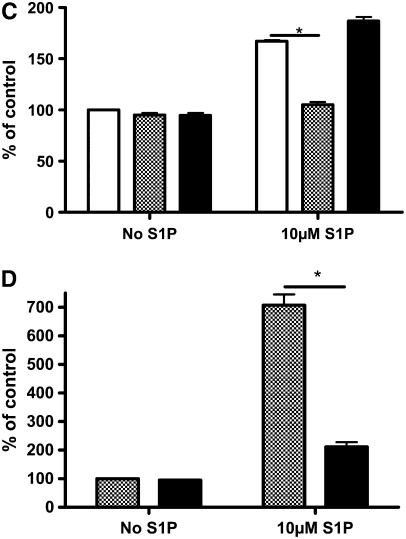
The role of extracellular receptors in mediating S1P-augmented HFL-1 cell chemotaxis was first assessed with pharmacologic reagents. The nonspecific S1P agonist dihydrosphingosine 1-phosphate (dihydroS1P) activates all extracellular S1P receptors, but does not enter cells to activate intracellular receptors and has markedly less activity on S1P2. This analog was without effect on HFL-1 chemotaxis (Figure 3A). Similarly, neither the S1P1 agonist SEW2871 nor the S1P3 antagonist (100 μM suramin) affected HFL-1 chemotaxis toward human fibronectin at any concentrations tested (Figures 3B and 3C). In contrast, the S1P receptor antagonist JTE-013 (1 μM) completely blocked S1P-augmented HFL-1 cell chemotaxis (Figure 3C). To confirm the role of the S1P2 receptor, RNAi was used to inhibit expression of the S1P2 receptor. This also resulted in complete blockade of S1P-augmented HFL-1 cell chemotaxis (Figure 3D), indicating that S1P2 mediates S1P augmentation of HFL-1 cell chemotaxis. Control lipofectamine was without effect.
Figure 3.
Role of S1P receptors in mediating S1P-augmented fibroblast chemotaxis. (A) Effect of Dihydro S1P. Chemotaxis to fibronectin was performed in the presence of varying concentrations of dihydro S1P. Vertical axis: migrated cells per five high-power fields expressed as percentage of HFn only (20 μg/ml). Horizontal axis: Dihydro S1P concentration. (B) Effect of the S1P1 agonist SEW2871. Chemotaxis was performed to fibronectin in the presence of varying concentrations of SEW2871. Vertical axis: migrated cells per five high-power fields expressed as percentage of HFn only (20 μg/ml). Horizontal axis: SEW2871 concentration. (C) Effect of S1P2 and S1P3 antagonists on S1P potentiated chemotaxis. HFL-1 cells were trypsinized and pre-treated with the S1P2 antagonist (JTE-013) or an S1P3 antagonist (suramin) for 10 minutes, after which chemotaxis was assessed in the presence or absence of S1P. Vertical axis: migrated cells per five high-power fields. Horizontal axis: S1P concentration. S1P receptor antagonists: open bars, control; shaded bars, JTE-013; solid bars, suramin. (D) Effect of S1P2-siRNA. HFL-1 cells were transfected with control (shaded bars) or S1P2-siRNA (solid bars) followed by chemotaxis in the presence or absence of S1P as described in Materials and Methods. Vertical axes: migrated cell number per five high-power fields expressed as percentage of HFn only (20 μg/ml). *P < 0.01 by two-way ANOVA.
Effect of Rho and Rho Kinase in Mediating S1P Augmentation of HFL-1 Cell Chemotaxis
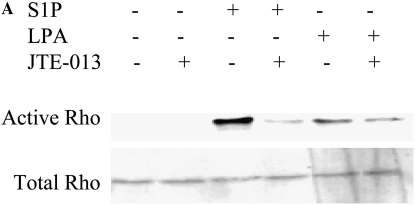
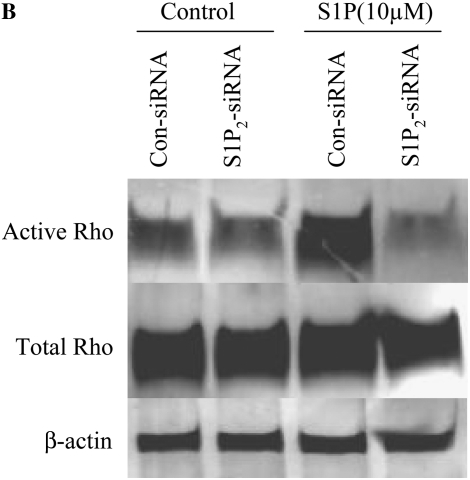
Since S1P has been reported to activate Rho and Rho kinase in a variety of cell types (22, 23), their role in mediating S1P-stimulated HFL-1 chemotaxis was evaluated. S1P (2 μM) significantly stimulated Rho activation, as evidenced by GTP binding to Rho, and this was completely blocked by the S1P2 antagonist (JTE-013; Figure 4A). LPA (2 μM) slightly increased Rho activation and this was not affected by JTE-013 (Figure 4A), indicating that S1P activates Rho through the S1P2 receptor, but LPA was activated through other mechanisms. Activation of Rho through the S1P2 receptor was further confirmed by showing that suppression of the S1P2 receptor with siRNA blocked S1P-induced Rho activation (Figure 4B).
Figure 4.
Role of the S1P2 receptor in mediating S1P-stimulated Rho activation. (A) Inhibition of S1P-induced Rho activation by an S1P2 antagonist. HFL-1 cells were treated with S1P (2 μM) or LPA (2 μM) for 15 minutes in the presence or absence of JTE-013 (1 μM). Rho activation was then assessed by immunoblotting GTP-bound Rho. (B) Inhibition of S1P-stimulated Rho activation by S1P2-siRNA. S1P2 receptor expression was suppressed with siRNA as described in Materials and Methods. On the next day, cells were stimulated with S1P for 15 minutes and Rho activation was assessed.
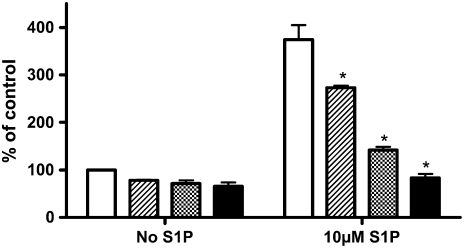
Consistent with Rho activation by S1P through the S1P2 receptor mediating S1P augmentation of HFL-1 chemotaxis, the Rho kinase inhibitor, Y27632, inhibited S1P-augmented HFL-1 cell chemotaxis (Figure 5). Treatment with Y-27632 for 1 hour before the assay resulted in a modest 32 ± 13% (P > 0.05) inhibition of chemotaxis toward fibronectin in the absence of S1P. In contrast, Y-27632 resulted in a concentration-dependent inhibition of S1P-augmented chemotaxis (P < 0.05, Figure 5), with essentially complete inhibition of the S1P augmentation by 10 μM Y-27632.
Figure 5.
Effect of Rho kinase inhibitor on S1P-stimulated cell migration. Fibroblast chemotaxis was performed to fibronectin (20 μg/ml) with varying concentrations of Y27632 added to the cells in the top wells. Vertical axis: migrated cell number per five high-power fields expressed as percentage of control (20 μg/ml HFn only). Horizontal axis: S1P concentration. Open bars, control; striped bars, 2 μM Y27632; hatched bars, 5 μM Y27632; solid bars, 10 μM Y27632. *P < 0.01 by two-way ANOVA.
Other Potential Signaling Pathways for S1P-Augmented HFL-1 Cell Chemotaxis
To further investigate the mechanism by which S1P stimulates HFL-1 chemotaxis, pharmacologic inhibitors of other potential signal transduction pathways were used. To examine whether the effect of S1P on cell migration is mediated through Gi, an established target for S1P2, cells were pretreated with the Gi inactivator pertussis toxin (PTX, 1 μg/ml) followed by the chemotaxis assay. PTX did not abrogate the stimulatory effect of S1P on HFL-1 cell migration (Table 1). Pretreatment of HFL-1 cells with the protein kinase C (PKC) inhibitor calphostin C (100 nM) for 1 hour partially inhibited HFL-1 cell chemotaxis toward fibronectin, reducing the number of migrated cells by 53 ± 2% (P < 0.05), and it caused a similar 34 ± 9% (P < 0.05) reduction in the S1P-augmented chemotaxis (n = 3, P < 0.05, Table 1). The PI 3-kinase inhibitor LY294002 significantly inhibited chemotaxis of HFL-1 cells toward fibronectin by 74 ± 3% (P < 0.05). However, S1P-stimulated chemotaxis was not significantly inhibited by LY294002 (Table 1). PP2 (50 μM), a selective Src kinase inhibitor, significantly decreased the baseline HFL-1 chemotaxis toward fibronectin by 48 ± 2% (P < 0.05). In the presence of S1P, however, PP2 did not inhibit, but rather further augmented HFL-1 chemotaxis by 16 ± 5% (n = 4, P > 0.05; Table 1). In addition, treatment of HFL-1 cells with PD98059, an inhibitor of Erk (100 μM), SB203580, an inhibitor of p38 (10 μM), and JNK inhibitor II, an inhibitor of JNK (2 μM) for 30 minutes before the chemotaxis assays did not affect HFL-1 chemotaxis toward fibronectin in either the presence or absence of S1P (data not shown).
TABLE 1.
EFFECT OF PHARMACOLOGIC INHIBITORS ON S1P-AUGMENTED FIBROBLAST CHEMOTAXIS
| Control
|
S1P (10 μM)
|
|||
|---|---|---|---|---|
| − | + | − | + | |
| PTX (1 μg/ml) | 39 ± 2 | 52 ± 2 | 340 ± 21 | 389 ± 20 |
| Calphostin C (0.1 μM) | 165 ± 6 | 78 ± 6* | 687 ± 51 | 447 ± 31* |
| Y294002 (50 μM) | 114 ± 5 | 29 ± 2* | 328 ± 34 | 295 ± 13 |
| PP2 (50 μM) | 157 ± 5 | 80 ± 5* | 341 ± 17 | 389 ± 12 |
Definition of abbreviations: Calphostin C, PKC inhibitor; PP2, Src kinase inhibitor; PTX, pertussis toxin; S1P, sphingosine 1-phosphate; Y294002, PI3-kinase inhibitor.
P < 0.05 by Student's t test compared with control.
Role of FAK in S1P-Stimulated HFL-1 Cell Chemotaxis
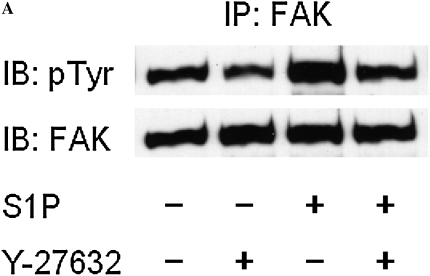
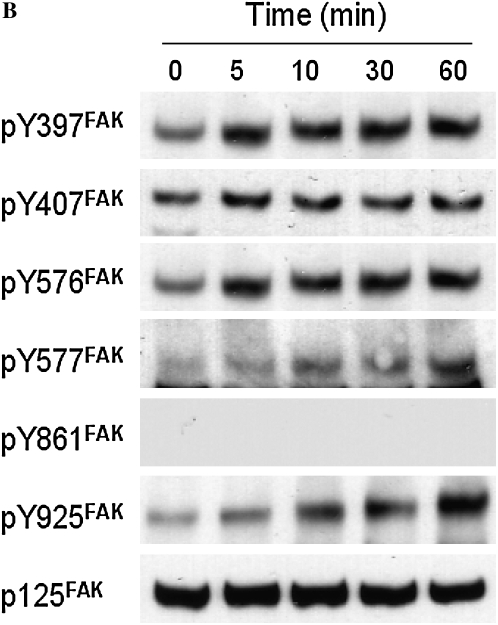
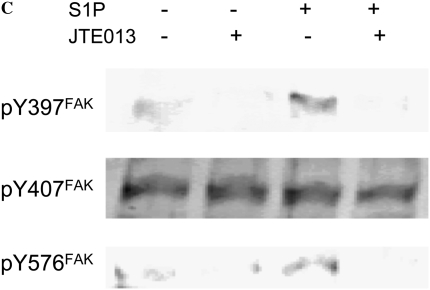
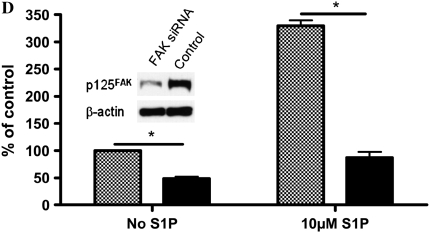
To evaluate whether FAK is involved in the stimulatory effect of S1P on HFL-1 cell chemotaxis, we assessed FAK phosphorylation in response to S1P. We used FAK siRNA to suppress FAK expression in HFL-1 cells and assessed its ability to attenuate S1P augmentation of chemotaxis. Consistent with previous studies (18, 24), S1P markedly increased FAK phosphorylation in HFL-1 cells, and Y-27632 inhibited this S1P-induced FAK phosphorylation (Figure 6A), indicating FAK phosphorylation is mediated by Rho kinase. High concentrations of S1P rapidly activated Tyr-397 and Tyr-576 phosphorylation within 5 minutes (Figure 6B). Tyr-577 and Tyr-925 of FAK were more gradually phosphorylated within 60 minutes. In contrast, FAK Tyr-861 was not phosphorylated by S1P (Figure 6B). Phsophorylation of Tyr-397 and Tyr-576 by S1P was blocked by the S1P2 antagonist, JTE013 (Figure 6C), suggesting that S1P2 mediates S1P-induced FAK phosphorylation. FAK siRNA treatment resulted in a marked reduction of total FAK protein (Figure 6D, inset), a significant but partial decrease of baseline chemotaxis toward fibronectin of (51 ± 4%), and a more complete inhibition of the S1P-augmented chemotaxis 74 ± 4%, (n = 3, *P < 0.05; Figure 6D).
Figure 6.
Role of focal adhesion kinase (FAK) in S1P-stimulated HFL-1 cell migration. (A) Effect of S1P and Y-27632 on FAK tyrosine phosphorylation. IP, immunoprecipitation; IB, immunoblot. (B) Time dependency of S1P-induced tyrosine phosphorylation at varying sites. (C) Effect of S1P2 antagonist on S1P-induced FAK phosphorylation. (D) Effect of FAK siRNA on HFL-1 cell migration in the presence or absence of S1P. Hatched bar: transfection control (reagent only) treated cells; solid bar: FAK siRNA-treated cells. Inset: total FAK protein 48 hours after FAK siRNA transfection. *P < 0.05.
DISCUSSION
The current study demonstrates that human lung fibroblasts (HFL-1) express S1P1, S1P2, S1P3, and S1P5 receptors, and that S1P augments chemotactic migration of HFL-1 cells toward fibronectin through the S1P2 receptor. S1P similarly stimulated chemotaxis of human foreskin fibroblasts toward fibronectin. The stimulatory effect of S1P on HFL-1 chemotaxis was not mimicked by dhS1P or by SEW2871, an S1P1 receptor agonist. S1P augmented chemotaxis was blocked by an S1P2 antagonist (JTE-013) and by S1P2 siRNA, but not by the S1P3 antagonist suramin. S1P also activated Rho and FAK, activities which were also blocked by the S1P2 antagonist or by S1P2 siRNA. Furthermore, both S1P-activated FAK phosphorylation and S1P-augmented chemotaxis were blocked by the Rho-kinase inhibitor Y-27632 in a concentration-dependent manner. FAK siRNA partially inhibited baseline chemotaxis to fibronectin and more completely blocked the S1P-augmented chemotaxis. S1P-augmented fibroblast chemotaxis was not sensitive to pertussis toxin, the protein kinase C inhibitor calphostin C, the PI3 kinase inhibitor Y-29402, or the Src inhibitor PP2. These data suggest that S1P augments fibroblast chemotaxis toward fibronectin by acting through the S1P2 receptor to activate a Rho/Rho-kinase/FAK pathway. This pathway for S1P modulation of fibroblast chemotaxis may allow S1P to regulate the reparative functions of lung fibroblasts.
The migration of cells to sites of injury is believed to play a key role in tissue repair and remodeling. Consistent with the importance of this process, a number of mediators likely generated at sites of inflammation and injury have been demonstrated to stimulate the migration of fibroblasts, which are believed to be the main cells responsible for the production of interstitial extracellular matrix macromolecules. Among the chemotactic factors for fibroblasts is fibronectin, a multi-functional macromolecule that is believed to mediate many interactions between cells and extracellular matrix (25). S1P is a mediator that also can be released by a variety of cells. The present study demonstrates that S1P is not directly active as a chemotactic factor for fibroblasts. S1P, however, greatly enhances the chemotactic response of fibroblasts toward fibronectin. Thus, in an inflammatory milieu in which fibronectin may function as a fibroblast chemoattractant, the generation of S1P could play an important role in enhancing fibroblast recruitment.
S1P is a lipid mediator that can be generated by several pathways, most prominently from the cleavage of sphingomyelin to ceramide and sphingosine (26). S1P can also be converted into sphingosine and hence into ceramide. Because these lipid mediators each have their own diverse biological effects, the balance between S1P and its related metabolites has been suggested to play a key role in modulating a variety of cellular processes, including apoptosis and proliferation. In the current study, both sphingosine and ceramide were found to be without chemotactic activity and failed to potentiate fibronectin-driven chemotaxis (data not shown). Concentrations of S1P in the micromolar range were required to enhance chemotaxis. However, because authentic sphingosine and ceramide were without activity, the activity of S1P is not likely due to contamination with, or conversion to, these related lipid mediators.
S1P functions both as an extracellular lipid mediator and an intracellular signaling molecule (6, 10, 12–14). Five G protein–coupled receptors have been identified as high-affinity S1P receptors. These couple to a variety of G proteins that regulate diverse downstream signaling pathways. In WI-38 cells, Urata and coworkers found expression of mRNA for S1P1, S1P2, and S1P3, but not for S1P4 or S1P5 (27), results that differ from the current study with HFL-1 cells, which express S1P1, S1P2, S1P3, and S1P5, but not S1P4. Antibody reagents confirmed expression for S1P1, S1P2, and S1P5 by immunoblot. In the current study, both S1P-augmented fibroblast chemotaxis and S1P-induced Rho activation were completely blocked by an S1P2 antagonist and by S1P2-specific siRNA. While a limited number of reagents that activate or inhibit other S1P receptors are available, an S1P1 agonist failed to augment chemotaxis and an S1P3 antagonist similarly failed to inhibit the effect of S1P, consistent with an action mediated by S1P2. The nonselective agonist dihydro-S1P also failed to mimic the action of S1P. While this agonist is approximately 10-fold less potent at S1P2 than is S1P, the concentration tested is used more than the 10−7 M required to activate S1P2 in other cells (28). Unlike S1P, which can be taken up by cells and can act on internal S1P targets, dihydro S1P is not active on intracellular receptors. Thus, it is possible that intracellularly located S1P2 receptors account for the effects observed in the current study. Consistent with such a possibility, the stimulatory effects of S1P on chemotaxis were observed at micromolar concentrations of S1P. This is consistent with other intracellular actions of S1P on cell growth and growth arrest, survival, and migration (12, 29–31) that are also elicited by micromolar concentrations. Moreover, it contrasts with the action of S1P on cell surface GPCRs where S1P is usually active in nanomolar concentrations, consistent with the reported Kd values of these receptors. Furthermore, recent studies are increasingly citing evidence for important roles of intracellular receptors, particularly for lipid-derived mediators such as S1P, LPA, and prostaglandins (11, 32). The findings from the current study, however, do not delineate whether S1P potentiates chemotaxis through an intracellular or a cell surface S1P2 receptor.
The levels of S1P required to potentiate fibronectin-mediated chemotaxis are within the range observed biologically. Concentrations in the micromolar range have been observed in serum (33, 34) and in ascitic fluid (35). Higher concentrations may occur in tissues where S1P is being actively released in response to injury or other challenges. Thus, the S1P-driven enhancement of chemotaxis, which required S1P levels greater than 1 μM, could occur in these in vivo settings, precisely the settings in which augmented chemotaxis would be relevant.
Whether S1P-augmented chemotaxis is mediated by cell surface or intracellular S1P2 receptors is not defined in the current study. However, the downstream signaling pathways appear to involve both Rho-kinase and FAK in that blockade of S1P2 by specific antagonist or siRNA result in inhibition of Rho and FAK activation. While Y-27632 slightly decreased the basal activity of cell migration, it markedly abrogated the stimulatory effect of S1P in a concentration-dependent manner. This suggests that the mechanism by which fibronectin itself induces chemotaxis is not as dependent on Rho as is the S1P potentiation of this chemotaxis. The results with both the PI3-kinase inhibitor L-294002 and the Src inhibitor PP2 also support S1P acting through pathways different from those that modulate basal chemotaxis to fibronectin. In contrast to Rho-kinase inhibition, which showed a greater effect on S1P-augmented chemotaxis, both L294002 and PP2 inhibited basal chemotaxis, but had no effect or slightly increased the S1P augmentation.
FAK siRNA blocked both basal and S1P-augmented HFL-1 chemotaxis, although the effect was somewhat greater on the S1P-potentiated chemotaxis. A role for FAK is consistent with its previously defined role in cell motility (36), and suggests that FAK is not specific for S1P-mediated chemotaxis. Moreover, FAK can modulate chemotaxis in multiple ways. However, S1P leads to FAK phosphorylation on multiple tyrosine residues in HFL-1 cells. This raises the possibility that some phosphorylation sites may enhance and some may inhibit chemotactic migration. Interestingly, inhibition of Src, which can also phosphorylate FAK, failed to abrogate the S1P-potentiated chemotaxis, suggesting that Src-mediated phosphorylation of FAK plays no role in mediating the S1P effect. This contrasts with the inhibition of basal chemotaxis toward fibronectin, which was inhibited by the Src inhibitor PP2. Inhibition of chemotaxis by FAK phosphorylation at Tyr-397 has been reported in human breast cancer cells (37). The mechanistic basis for the inhibition of breast cancer cell migration, which contrasts markedly with the enhancement of chemotaxis observed in normal lung fibroblasts in the current study, remains undefined, but effects due to differential phosphorylation of FAK are a possible mechanism.
Many chronic diseases are associated with fibrosis that results in alteration of tissue architecture and disrupts function. Prevention of the fibroblast recruitment and accumulation that characterizes fibrosis, therefore, could be an important therapeutic goal in these conditions, including lung diseases such as interstitial lung disease, asthma, and chronic obstructive lung disease. Inhibition of fibroblast recruitment, however, could also impair normal tissue healing. The current study suggests that the pathway mediating S1P-potentiated chemotaxis toward fibronectin is distinct from that for basal fibronectin-mediated chemotaxis and can be specifically targeted. As S1P is likely to be present in an inflammatory milieu and could be a driver of excessive fibrosis, the current results suggest a therapeutic strategy to block fibrosis without impairing normal tissue repair.
In summary, the current study demonstrates that S1P at micromolar concentrations can enhance the directed migration of fibroblasts toward fibronectin. This action occurs through S1P2 receptors and is mediated through a pathway involving Rho, Rho-kinase, and FAK, a pathway that is distinct from that for the basal response to fibronectin. As concentrations of S1P that could act through this pathway may be present in vivo in pathologic settings, targeting S1P may be an appealing strategy to modify fibroblast recruitment and subsequent fibrosis.
This work was supported by National Institutes of Health grant HL64088-04 (to S.R.).
Originally Published in Press as DOI: 10.1165/rcmb.2006-0427OC on March 26, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 2003;302:1704–1709. [DOI] [PubMed] [Google Scholar]
- 2.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 1996;84:359–369. [DOI] [PubMed] [Google Scholar]
- 3.Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol 2003;121:695–705. [DOI] [PubMed] [Google Scholar]
- 4.Clark RA. Potential roles of fibronectin in cutaneous wound repair. Arch Dermatol 1988;124:201–206. [PubMed] [Google Scholar]
- 5.Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res 1999;253:230–238. [DOI] [PubMed] [Google Scholar]
- 6.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J 2000;349:385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res 2003;58:170–177. [DOI] [PubMed] [Google Scholar]
- 8.Yatomi Y, Yamamura S, Ruan F, Igarashi Y. Sphingosine 1-phosphate induces platelet activation through an extracellular action and shares a platelet surface receptor with lysophosphatidic acid. J Biol Chem 1997;272:5291–5297. [DOI] [PubMed] [Google Scholar]
- 9.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev 2002;54:265–269. [DOI] [PubMed] [Google Scholar]
- 10.Panetti TS. Differential effects of sphingosine 1-phosphate and lysophosphatidic acid on endothelial cells. Biochim Biophys Acta 2002;1582:190–196. [DOI] [PubMed] [Google Scholar]
- 11.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett 2002;531:54–57. [DOI] [PubMed] [Google Scholar]
- 12.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, et al. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol 1998;142:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel S, Kolesnick R. Sphingosine 1-phosphate as a therapeutic agent. Leukemia 2002;16:1596–1602. [DOI] [PubMed] [Google Scholar]
- 14.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 2002;277:25851–25854. [DOI] [PubMed] [Google Scholar]
- 15.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol 2005;25:4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu Y, Takuwa N, Sugimoto N, Sakurada S, Usui S, Okamoto H, Matsui O, Takuwa Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ Res 2002;90:325–332. [DOI] [PubMed] [Google Scholar]
- 17.Lee OH, Lee DJ, Kim YM, Kim YS, Kwon HJ, Kim KW, Kwon YG. Sphingosine 1-phosphate stimulates tyrosine phosphorylation of focal adhesion kinase and chemotactic motility of endothelial cells via the G(i) protein-linked phospholipase C pathway. Biochem Biophys Res Commun 2000;268:47–53. [DOI] [PubMed] [Google Scholar]
- 18.Wang F, Nobes CD, Hall A, Spiegel S. Sphingosine 1-phosphate stimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem J 1997;324:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer 1977;20:1–5. [DOI] [PubMed] [Google Scholar]
- 20.Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am J Physiol Lung Cell Mol Physiol 2001;281:L1257–L1263. [DOI] [PubMed] [Google Scholar]
- 21.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 1962;115:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouach N, Pebay A, Meme W, Cordier J, Ezan P, Etienne E, Giaume C, Tence M. S1P inhibits gap junctions in astrocytes: involvement of G and Rho GTPase/ROCK. Eur J Neurosci 2006;23:1453–1464. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeldt HM, Amrani Y, Watterson KR, Murthy KS, Panettieri RA Jr, Spiegel S. Sphingosine-1-phosphate stimulates contraction of human airway smooth muscle cells. FASEB J 2003;17:1789–1799. [DOI] [PubMed] [Google Scholar]
- 24.Ohmori T, Yatomi Y, Okamoto H, Miura Y, Rile G, Satoh K, Ozaki Y. G(i)-mediated Cas tyrosine phosphorylation in vascular endothelial cells stimulated with sphingosine 1-phosphate: possible involvement in cell motility enhancement in cooperation with Rho-mediated pathways. J Biol Chem 2001;276:5274–5280. [DOI] [PubMed] [Google Scholar]
- 25.Rennard SI, Hunninghake GW, Bitterman PB, Crystal RG. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci USA 1981;78:7147–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hla T. Signaling and biological actions of sphingosine 1-phosphate. Pharmacol Res 2003;47:401–407. [DOI] [PubMed] [Google Scholar]
- 27.Urata Y, Nishimura Y, Hirase T, Yokoyama M. Sphingosine 1-phosphate induces alpha-smooth muscle actin expression in lung fibroblasts via Rho-kinase. Kobe J Med Sci 2005;51:17–27. [PubMed] [Google Scholar]
- 28.Arikawa K, Takuwa N, Yamaguchi H, Sugimoto N, Kitayama J, Nagawa H, Takehara K, Takuwa Y. Ligand-dependent inhibition of B16 melanoma cell migration and invasion via endogenous S1P2 G protein-coupled receptor: requirement of inhibition of cellular RAC activity. J Biol Chem 2003;278:32841–32851. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 1991;114:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogler R, Sauer B, Kim DS, Schafer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol 2003;120:693–700. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor: potential involvement in angiogenesis. J Biol Chem 1999;274:35343–35350. [DOI] [PubMed] [Google Scholar]
- 32.Goetzl EJ, Wang W, McGiffert C, Liao JJ, Huang MC. Sphingosine 1-phosphate as an intracellular messenger and extracellular mediator in immunity. Acta Paediatr Suppl 2007;96:49–52. [DOI] [PubMed] [Google Scholar]
- 33.Murata N, Sato K, Kon J, Tomura H, Okajima F. Quantitative measurement of sphingosine 1-phosphate by radioreceptor-binding assay. Anal Biochem 2000;282:115–120. [DOI] [PubMed] [Google Scholar]
- 34.Boguslawski G, Grogg JR, Welch Z, Ciechanowicz S, Sliva D, Kovala AT, McGlynn P, Brindley DN, Rhoades RA, English D. Migration of vascular smooth muscle cells induced by sphingosine 1-phosphate and related lipids: potential role in the angiogenic response. Exp Cell Res 2002;274:264–274. [DOI] [PubMed] [Google Scholar]
- 35.Hong G, Baudhuin LM, Xu Y. Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Lett 1999;460:513–518. [DOI] [PubMed] [Google Scholar]
- 36.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 2001;1540:1–21. [DOI] [PubMed] [Google Scholar]
- 37.Wang F, Nohara K, Olivera A, Thompson EW, Spiegel S. Involvement of focal adhesion kinase in inhibition of motility of human breast cancer cells by sphingosine 1-phosphate. Exp Cell Res 1999;247:17–28. [DOI] [PubMed] [Google Scholar]