Abstract
Inherited mutations in the human α1-antitrypsin (AAT) gene lead to deficient circulating levels of AAT protein and a predisposition to developing emphysema. Gene therapy for individuals deficient in AAT is an attractive goal, because transfer of a normal AAT gene into any cell type able to secrete AAT should reverse deficient AAT levels and attenuate progression of lung disease. Here we present an approach for AAT gene transfer based on the transplantation of lentivirally transduced hematopoietic stem cells (HSCs). We develop a novel dual-promoter lentiviral system to transfer normal human AAT cDNA as well as a fluorescent tracking “reporter gene” into murine HSCs. After transplantation of 3,000 transduced HSCs into irradiated mouse recipients, we demonstrate simultaneous and sustained systemic expression of both genes in vivo for at least 31 weeks. The stem cells transduced with this protocol maintain multipotency, self-renewal potential, and the ability to reconstitute the hematopoietic systems of both primary and secondary recipients. This lentiviral-based system may be useful for investigations requiring the systemic secretion of anti-proteases or cytokines relevant to the pathogenesis of a variety of lung diseases.
Keywords: gene therapy, bone marrow, α1-antitrypsin, lung, stem cells
CLINICAL RELEVANCE
This study shows that lentivirally transduced hematopoietic stem cells can be used to deliver a gene systemically, either as a cell-based gene therapy or for laboratory research purposes.
α1-antitrypsin (AAT) deficiency is one of the most common hereditary diseases worldwide (1, 2). A variety of inherited mutations in the AAT gene have been shown to cause decreased levels of circulating AAT resulting in a predisposition to developing panacinar emphysema and cirrhosis. The pulmonary disease phenotype is thought to result directly from unbalanced protease activity in the lung and subsequent destruction of elastin, extracellular matrix, and basement membrane components (3–5). The standard of care for patients with AAT deficiency and impaired lung function is weekly infusion of AAT protein derived from pooled human plasma (6). The high cost, inconvenient route of administration, risk of bloodborne infection, and time-consuming nature of this therapy provide an impetus to develop alternative treatment modalities such as gene therapy.
Because AAT deficiency results from inherited AAT mutations, transfer of a normal AAT gene into any cell type able to secrete AAT should reverse deficient AAT levels and attenuate progression of lung disease. Over the past two decades, numerous attempts to apply gene therapy to AAT deficiency have been made (7–16); however, the majority have been unsuccessful due to transduction of insufficient target cell numbers (7, 9, 14) or failure to produce sustained gene expression (10, 15).
Stem cell–based therapy has been proposed as a novel approach to treating degenerative or genetic diseases such as AAT deficiency. Stem cells undergo indefinite self-renewal and maintain broad differentiative capacity, making them ideal vehicles for permanent delivery of normal genes to those affected by loss-of-function genetic mutations. For example, hematopoietic stem cells (HSCs), which give rise indefinitely to all blood cells of an organism, have been employed as gene delivery vehicles in clinical trials (17). Development of an HSC-based therapy for AAT deficiency, in particular, is attractive for several reasons. First, engraftment of genetically altered HSCs potentially establishes a self-renewing supply of virtually unlimited numbers of circulating cells secreting normal AAT protein into the bloodstream. Second, transplantation of HSCs, the stem cell source of alveolar macrophages, results in a permanent supply of local resident cells potentially able to secrete AAT directly into the epithelial lining fluid bathing all alveoli. Such a local source of AAT would efficiently reach its site of action in the lung in contrast to circulating blood AAT, only a small percentage of which diffuses into the epithelial lining fluid (ELF) (18, 19). As a result, lower levels of AAT would need to be produced than with other systemic approaches.
Because HSCs are quiescent, gene transfer into these cells has been inefficient, and most approaches have required use of stimulating culture conditions that result in differentiation and loss of multipotency (20). The capacity of lentiviruses to transduce quiescent cells, such as stem cells, offers a potential solution. We have previously developed a method whereby small numbers of hematopoietic stem cells (200–2,000) can be infected ex vivo before transplantation into recipient animals, where they undergo clonal expansion and life-long reconstitution of all hematopoietic lineages (20). This results in life-long propagation of the integrated transgene in all blood cells, leading to significant increases in overall gene expression.
Here, we present a lentiviral system engineered for the sustained expression of normal human AAT together with a tracking “reporter gene.” We target transplantable HSCs to establish a self-renewing stem cell source of circulating cells expressing both transgenes. The approach presented may also be useful for laboratory investigations requiring the systemic secretion of anti-proteases, cytokines, or other transgenes relevant to the pathogenesis of a variety of lung diseases.
MATERIALS AND METHODS
Generation of Dual Promoter and Internal Ribosome Entry Site–Containing Lentiviral Constructs for Dual Transgenesis
Lentiviral constructs utilized the third generation, self-inactivating, replication incompetent lentiviral backbone vector originally published as pHR′ (21) and subsequently modified into the “pHAGE” vector (22), a generous gift of Dr. Richard C. Mulligan (Harvard Medical School, Boston, MA). The pHAGE vector was modified for dual transgenesis as follows: cDNA encoding a variant of the red fluorescent protein adapted from Discosoma sp. (DsRed-Express; Clontech, Mountain View, CA) was amplified by PCR attaching NotI and BamH1 restriction sites to 5′ and 3′ ends, respectively. This amplicon was cloned into the pHAGE backbone in the first gene expression position by ligation to NotI/BamH1 cohesive ends. Next, enhanced green fluorescence protein (GFP; Clontech) cDNA was generated by PCR attaching NdeI and ClaI sites to the 5′ and 3′ ends, respectively, for ligation into the second gene position of pHAGE. Immediately upstream of the dsRed or GFP ATG start site, the indicated promoter fragment (cytomegalovirus [CMV], 584 bp; phosphoglycerate kinase [PGK], 464 bp; ubiquitin C [UBC], 397 bp; or elongation factor 1 α [EF1α], 228 bp [22]) was inserted by standard cloning techniques as illustrated in Figure 1. For bicistronic vectors, the internal ribosome entry site (IRES) from the encephalomyocarditis virus was inserted (Figure 1; sequences available for download at www.kottonlab.com) immediately upstream of the second cistron's ATG start site.
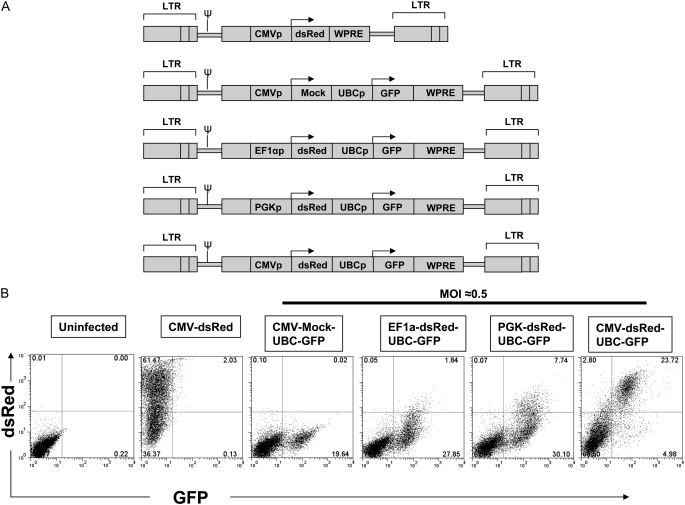
Figure 1.
Testing of dual promoter lentiviral constructs for simultaneous expression of two genes. (A) Schematic of five lentiviral constructs used to infect 293 cells. The activity of three different promoters (EF1a, PGK, or CMV) is compared based on expression levels of gene position 1 (dsRed). Gene position 2 expression (green fluorescence protein [GFP]) is driven by the human ubiquitin C (UBC) promoter in each vector. (B) Flow cytometry analysis of 293 cells 3 days after infection with the lentiviruses shown in A. Multiplicity of infection (MOI) = 0.5 is employed to achieve an average single copy integration of each vector in approximately 1/3 of cells; at this MOI, approximately 2/3 of cells are predicted to remain untransduced (29). Only the combination of a cytomegalovirus (CMV) promoter in position 1 followed by a UBC promoter in position 2 effectively expresses both dsRed and GFP reporter genes in transduced cells. Cells transduced with CMV-dsRed alone or CMV-Mock-UBC-GFP are used as single color controls. Results are representative of experiments repeated three times at varying MOI.
Generation of AAT-Containing Lentiviral Constructs and Viral Packaging
Lentiviral constructs for human AAT expression were generated using full-length (1,257-bp) human AAT (hAAT) cDNA cloned from the c-AT plasmid, a generous gift of Drs. Terry R. Flotte and Sihong Song (University of Florida, Gainesville, FL; Figure 1) (8). PCR was used to add NotI and BglII restriction sites upstream and downstream of the 5′ hAAT ATG start site and 3′ TAA stop site, respectively. This amplicon was ligated into the first gene position of the indicated single or dual transgenesis pHAGE, lentiviral construct (Figure 1) by standard directional cloning into compatible NotI and BamH1 sites.
Vesicular stomatitis virus (VSV) pseudotyped lentivirus was generated by quintuple transfection of 293T cells with the lentiviral backbone construct together with four helper plasmids encoding the viral genes Gag-Pol, Tat, Rev, and VSV-G (20, 21). Cell supernatants containing virus were concentrated by centrifugation (90 min; 48,960 × g). Titers of fluorochrome-expressing lentiviruses were calculated as “293-transducing units” per ml (TU/ml) based on flow cytometry of infected 293 cells. Titers of viruses lacking a fluorochrome were determined by p24 enzyme-linked immunosorbent assay (ELISA) kit (Cell Biolabs, San Diego, CA) according to the manufacturer's instructions and equivalent TU/ml were calculated.
HSC Isolation, Transduction, and Transplantation
Donor 11-week old B6.SJL-PtprcaPep3b/BoyJ (CD45.1; Jackson Labs, Bar Harbor, ME) mice expressing the Ly5.1 (CD45.1) isoform of CD45 were killed and their bone marrow extracted from tibias, femurs, and iliac crests using a mortar and pestle. HSCs were purified from marrow by selection of Hoechst effluxing “side population” (SP) cells by flow cytometry as published by our lab and others (23–26). Sorted HSCs were incubated overnight in culture (37°C, 5%CO2) with lentivirus at a multiplicity of infection (MOI) of 200 under “minimally stimulating conditions” (20) defined as serum-free Stempro 34 media (Invitrogen) supplemented with 10 ng/ml murine stem cell factor (SCF; R&D Systems, Minneapolis, MN), 100 ng/ml human thrombopoietin (TPO; R&D Systems), and 5 μg/ml polybrene (Sigma, St. Louis, MO). Immediately after overnight infection, 3,000 HSCs were transplanted without selection of transduced cells into 8-week-old C57BL/6J (CD45.2; Jackson Labs) recipient mice by retroorbital intravenous injection. All recipients were prepared for transplant by exposure to 9.5 Gy of ionizing radiation 24 hours before HSC injection. Twenty-four weeks after primary transplant, mice were killed and blood, bone marrow, bronchoalveolar lavage (BAL), and lung tissues were harvested for analysis. Bone marrow was harvested from one mouse in each group for secondary transplantation. Ten million unfractionated whole bone marrow cells from each of these mice were secondarily transplanted into four 9-week-old irradiated (9.5 Gy) C57BL/6J recipients. Peripheral blood chimerism and gene expression levels in secondarily transplanted recipients were followed for 7 weeks after transplant. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Boston University.
Measurement of In Vitro Gene Expression
In vitro gene expression was measured after lentiviral infection of either the FG293 cell line or primary murine HSCs in culture at the MOI indicated. Cultured cells were harvested and stained with propidium iodide (PI) to exclude dead cells. PI fluorescence and reporter fluorochrome transgene expression (dsRed, GFP, or ZsGreen) were assessed by flow cytometry (BD FACScan; BD Biosciences, San Jose, CA, and FlowJo analysis software, Treestar, Ashland, OR). Cell supernatants were collected after 6 days in culture to measure human AAT protein expression by ELISA as detailed below. The presence of hAAT in cell supernatants was further demonstrated by immunoblot as follows: 2.5 μg of total protein was loaded per sample and proteins were separated by 10% sodium dodecyl sulfate/polyacrylaimde gel electrophoresis (SDS-PAGE). hAAT was detected by Western Blot analysis using a rabbit anti-hAAT primary antibody (RDI, Concord, NH; protocol generously provided by Drs. Yuanqing Lu and Sihong Song, University of Florida, Gainsville, FL). Serial dilutions of cell supernatants were incubated with human neutrophil elastase (Calbiochem, San Diego, CA) in the presence of methoxysuccinyl-ala-ala-pro-val-paranitroanilide (Sigma) to measure bioactivity. Colorimetric change in the presence of substrate was measured using a 96-well plate reader set at 405 nm (protocol generously provided by Drs. McGarry Houghton and Steven Shapiro, University of Pittsburgh, Pittsburgh, PA).
Measurement of In Vivo Gene Expression
At 6-week intervals, blood cells and plasma samples were obtained from the retroorbital venous plexus of anesthetized recipient mice using heparinized capillary tubes (Drummond Scientific, Broomall, PA). After exposure to red blood cell lysis buffer (Sigma), blood samples were stained with phycoerythrin (PE)-conjugated anti-CD45.1 (BD Biosciences #553776) and biotinylated anti-CD45.2 monoclonal antibodies (BD Biosciences #553771) followed by streptavidin-labeled peridinin-chlorophyll-protein complex (Per-CP; BD Biosciences #554064) and 2 μg/ml PI before flow cytometry analysis. BAL was performed on the right lung of each animal with two consecutive 500-μl aliquots of PBS. BAL fluid (BALF) was stored at −80°C before analysis of urea and human AAT protein content. The contralateral lung from each animal was then harvested for enzyme digestion with Collagenase A and Dispase II (Roche, Basel, Switzerland) as previously published (27) to prepare lung single-cell suspensions. Cell suspensions were stained with PE-conjugated rat anti-mouse CD45 monoclonal antibody (BD Biosciences) or nonspecific isotype control IgG and 2 μg/ml PI for flow cytometry (27).
Measurement of hAAT Protein Levels
hAAT protein expression was measured by dual Ab, sandwich ELISA specific for human AAT (protocol generously provided by Drs. Roberto Calcedo and Joanita Figueredo, University of Pennsylvania, Philadelphia, PA). A detailed ELISA protocol is included in the online supplement. The lower limit of detection for hAAT was 0.39 ng/ml. Calculation of the hAAT level in the ELF of selected animals was performed using the urea dilution method (Quantichrom Urea Assay; Bioassay Systems, Hayward, CA) (28).
Splenocyte Stimulation and Intracellular Cytokine Staining
Splenocytes were harvested from individual mice 18 weeks after HSC transplant. T cells were stimulated by incubation with anti-CD28 (2 μg/ml), anti-CD49 d (2 μg/ml), and hAAT whole protein (4 μg/ml). Unstimulated cells were incubated with all the above reagents except for the protein. As a positive control, splenocytes were incubated for 6 hours with Phorbol 12-myristate 13-acetate (PMA, 2 μg/ml; Sigma) and ionomycin (10 μg/ml). After stimulation, standard intracellular cytokine staining was performed by exposure to Golgiplug (BD Biosciences) followed by cell fixation/permeabilization and staining with fluorescence-conjugated monoclonal antibodies specific to CD4, CD8, IFN-γ, and IL-2 (29, 30). Data were collected on an LSR II instrument and analyzed using FlowJo software to quantify the percentage of CD4+ and CD8+ lymphocytes producing IFN-γ and IL-2 in response to hAAT stimulation. T cell proliferation in response to hAAT stimulation was quantified by standard CFSE staining (CFSE Cell Proliferation Kit; Molecular Probes) of stimulated and unstimulated splenocytes and further culturing of stained cells for 4 days after stimulation. Additional details of the T- cell stimulation protocol are available in the online supplement.
Anti-hAAT Serology
Blood serum was collected from HSC recipient mice via RV puncture 18 weeks after HSC transplant. Anti-hAAT-specific IgG antibodies were measured by ELISA for each mouse. Absorption was read at 405 nm using an ELISA reader (SPECTRA max PLUS). A detailed ELISA protocol is included in the online supplement.
RESULTS
Generation of Lentiviral Vectors for Dual Transgenesis
To develop a stem cell–based therapy for AAT deficiency we co-expressed a normal hAAT gene together with a fluorescent reporter molecule that would allow tracking of transplanted stem cells and their progeny. Although transferring multiple genes into the same cell is necessary for combined genetic correction, tracking, and selection of transduced cells, most existing gene transfer technologies do not reliably accomplish this in HSCs (31). For this reason, our initial studies focused on in vitro screens of novel promoter combinations that might allow effective in vivo dual transgene expression from a single lentiviral vector. Vectors consisting of combinations of the viral CMV promoter and mammalian UBC, PGK, and EF1α promoters were generated to express combinations of the dsRed and GFP reporter genes in tandem (vector sequences available for download at www.kottonlab.com). As shown in Figure 1, only a combination of an internal CMV promoter in the first gene position followed by the UBC promoter in the second gene position resulted in co-expression of both reporter genes in the majority of transduced cells. Hence this vector was selected for further testing, and the dsRed cassette was replaced with human AAT cDNA (Figure 2C).
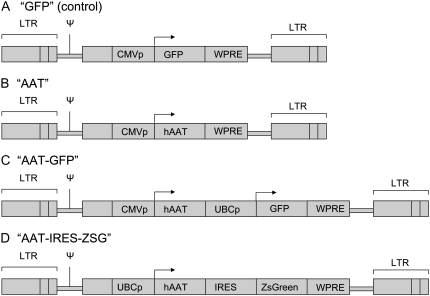
Figure 2.
Schematic of lentiviral constructs employed for stem cell transduction. (A) Negative control lentiviral vector (“GFP”) expresses only the GFP reporter gene driven by an internal CMV promoter (CMVp). (B) The “AAT” vector expresses the human α1-antitrypsin gene (hAAT) driven by CMVp. (C) Dual promoter vector “AAT-GFP” expresses hAAT driven by CMVp as well as the GFP gene driven by a human UBC promoter (UBCp). (D) A bicistronic vector “AAT-IRES-ZsG” expresses a single mRNA encoding hAAT and ZsGreen genes linked by an internal ribosome entry site (IRES). All vectors include the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) to augment gene expression levels. LTR: long terminal repeat. Ψ: Psi packaging signal.
Next we tested our dual promoter vector (AAT-GFP), a bicistronic vector (AAT-IRES-ZSGreen), and a single promoter vector (AAT) by infecting 293 cells and freshly purified murine HSCs in vitro (Figures 2 and 3). The ZsGreen fluorochrome rather than GFP was utilized for the bicistronic IRES-containing vector based on brighter FL1 channel fluorescence (data not shown). Two additional bicistronic vectors (CMV-dsRed-IRES-GFP and CMV-dsRed-IRES-ZsGreen) were not adapted for AAT expression based on low level in vivo expression from the second cistron in HSCs (data not shown). Cells infected with each vector were analyzed by flow cytometry, fluorescence microscopy, and ELISA of cell supernatants to assess relative levels of hAAT and reporter gene expression (Figure 3). Supernatants of infected cells were further analyzed by Western Blot and assay for anti-elastase activity to evaluate hAAT protein size and functional capacity (Figures 3D and 3E). GFP or ZsGreen expression was easily detected after transduction of both the 293 cell line and primary HSCs, and was used to demonstrate effective transduction of the majority of cells (Figures 3A and 3B). High levels of hAAT protein were documented in all cell supernatants, providing in vitro confirmation that dual trangenesis (i.e., expression of both hAAT protein and the fluorescent reporter) was achieved using dual promoter constructs as well as the bicistronic IRES-containing construct (Figure 3C). hAAT protein secreted by transduced cells was the same size as hAAT from human serum, indicating that proper post-translational processing of the transgene occurred (Figure 3D). Supernatants of cells transduced with AAT vectors had the capacity to inactivate human neutrophil elastase in contrast to supernatants from cells transduced with control (GFP-expressing) vectors (Figure 3E).
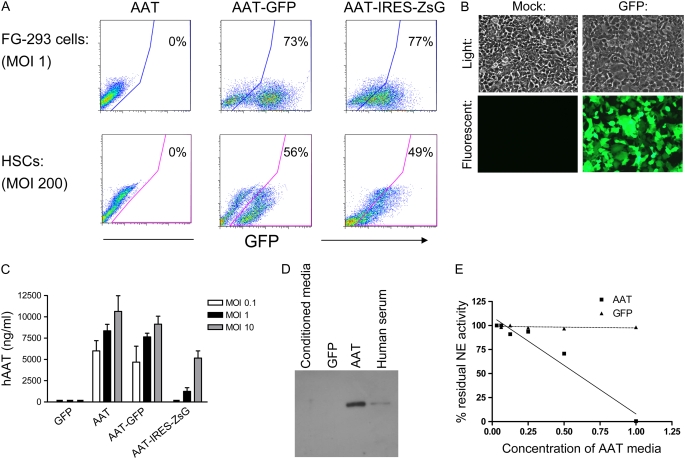
Figure 3.
In vitro testing of AAT-expressing lentiviral constructs. Cells infected overnight with each vector from Figure 2 were cultured further for 72 hours before analysis of gene expression. Separate aliquots were cultured in serum-free conditions for 48 hours before immunoblotting or anti-elastase assay. (A) Flow cytometry reveals approximately 75% of 293 cells and 50% of hematopoietic stem cells (HSCs) were transduced (GFP+ or ZsGreen+) with each vector. (B) Representative light and fluorescence microscopy shows GFP+ transduced 293 cells versus mock-infected controls. (C) Enzyme-linked immunosorbent assay (ELISA) of 293 cell supernatants reveals that cells transduced with “AAT,” “AAT-GFP,” or “AAT-IRES-ZsG” lentiviruses produce human AAT protein while cells transduced with virus encoding GFP alone do not. Increasing the MOI results in increased AAT protein levels. Error bars represent SEM. (D) Western blot of 293 cell supernatants after transduction with no virus (conditioned media), or lentivirus “AAT” versus “GFP.” hAAT secreted from transduced cells is the same size as hAAT in human serum. (E) Assay of anti-elastase activity in supernatants of 293 cells transduced with “AAT” vector versus “GFP” vector. “AAT” supernatant inactivated human neutrophil elastase (NE) in a dose-dependent fashion, while control “GFP” supernatant did not demonstrate anti-elastase activity.
Transplantation of Lentivirally Transduced HSCs Results in Long-Term Expression of hAAT In Vivo
Next, we tested in vivo gene expression after transplantation of 3,000 HSCs infected with each AAT-expressing lentiviral vector. Because growth factors present in serum are known to stimulate differentiation of HSCs, serum-free culture conditions supplemented with low levels of SCF and TPO were used during overnight lentiviral infection before transplantation (20). Transduced HSCs reconstituted the hematopoietic systems of all recipients and expression of reporter genes as well as hAAT protein was tracked in the peripheral blood of each recipient for 24 weeks (Figure 4). At all time points after transplant, 60 to 90% of peripheral blood cells were donor-derived. The dual promoter construct developed for these experiments accomplished stable and efficient transduction of 87 ± 10% (avg. ± SD) of donor-derived cells as determined by flow cytometry for GFP expression (Figure 4B). In addition, circulating hAAT protein levels were easily detected for all vectors tested for this 24-week period (Figure 4C). Although IRES-containing vectors achieved stable hAAT levels in the peripheral blood, fluorescent reporter gene expression from the second cistron in circulating cells was low or below detectable threshold (Figure 4), consistent with prior reports of inefficient activity of IRES-containing lentiviral vectors in hematopoietic cells (31, 32).
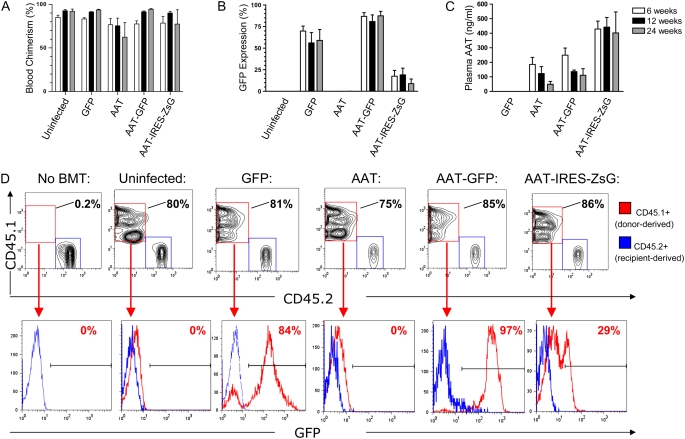
Figure 4.
Peripheral blood chimerism and in vivo gene expression after HSC infection and transplant. HSCs were infected overnight with each indicated lentiviral vector, and 3,000 HSCs were transplanted into each irradiated mouse recipient (n = 4 mice per group). Six, 12, and 24 weeks after transplantation, peripheral blood from each recipient was analyzed by flow cytometry to detect the percentage of donor-derived cells (blood chimerism; A) and the percentage of donor-derived cells expressing GFP or ZSGreen reporter genes (B). ELISA of blood plasma was used to quantify circulating human AAT protein levels (C). Error bars represent SEM. (D) Representative flow cytometry analysis from one mouse in each group, 24 weeks after HSC transplant: nucleated peripheral blood cells are analyzed after staining with antibodies that identify donor-derived cells (CD45.1) versus recipient-derived cells (CD45.2). A subgate of only donor-derived blood cells (CD45.1+/CD45.2−; represented as a red histogram) illustrates the percentage of donor-derived cells expressing the GFP or ZSGreen tracking reporter genes. The blue histogram overlay illustrates the absence of GFP expression in recipient cells (CD45.1−/CD45.2+) in each sample.
Maintenance of Self-Renewal and Multipotency in Transduced Stem Cells
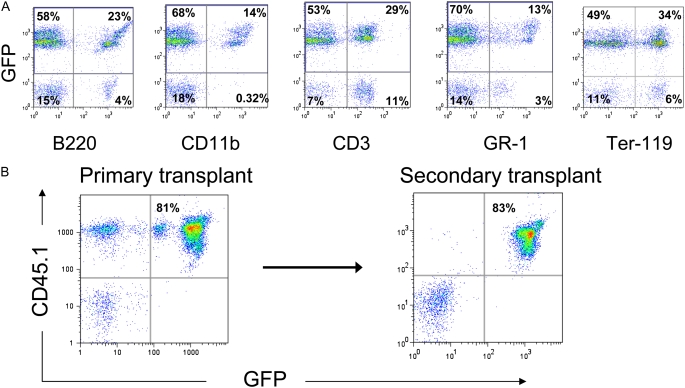
Hematopoietic stem cells are defined by a remarkable capacity for indefinite self-renewal as well as differentiation into all blood cell lineages. Although we have previously demonstrated that our lentiviral transduction protocol does not diminish self-renewal or differentiation capacities of stem cells (20), we specifically tested whether stem cells transduced to overexpress AAT still possessed these defining stem cell characteristics. For this reason the ability of transduced HSCs to contribute to myeloid, erythroid, and lymphoid lineages in the peripheral blood of all recipients was followed for 14 weeks after transplantation. Recipients of HSCs transduced with either AAT-GFP or AAT-IRES-ZsG vectors demonstrated robust multilineage reconstitution of circulating myeloid (CD11b, GR-1), lymphoid (B220, CD3), and erythroid (TER-119) blood lineages (Figure 5). In AAT-GFP–transduced recipients, reporter transgene expression (percent GFP+ and mean fluorescence intensity) was maintained in all peripheral blood lineages and did not appear to differ from recipients of HSCs transduced with control GFP vectors.
Figure 5.
Assay of HSC functional capacity after lentiviral transduction. (A) Multilineage blood differentiation is illustrated by representative flow cytometry analysis of peripheral blood 14 weeks after transplantation of 3,000 HSCs infected with “AAT-GFP” lentivirus. Robust engraftment of differentiated lymphoid (CD3+ or B220+), myeloid (CD11b+ or GR-1+), and erythroid (Ter-119+) hematopoietic lineages derives from the transduced, transplanted donor cells (GFP+). Peripheral blood ELISA at this time point demonstrated an hAAT level of 149 ng/ml. (B) Analysis of peripheral blood drawn 13 weeks after primary transplant reveals 80% of all peripheral blood cells are donor-derived cells transduced with the “AAT-GFP” lentiviral construct (CD45.1+/GFP+; hAAT = 245 ng/ml). Twenty-four weeks after the initial transplant, 10 × 106 bone marrow cells from this primary recipient were transplanted into an irradiated secondary recipient. Seven weeks later, 83% of peripheral blood cells are derived from the original donor stem cells and continue to express both the GFP reporter gene (CD45.1+/GFP+).
Maintenance of self-renewing undifferentiated HSCs was demonstrated by performing secondary transplantation. For these experiments, 24 weeks after primary transplantation of transduced HSCs, bone marrow from each individual recipient was transplanted into four secondary recipients and peripheral blood reconstitution was followed for 7 weeks. In each case the original transduced HSCs or their progeny were able to robustly reconstitute the peripheral blood of secondary recipients (Figure 5B, and Figure E1 in the online supplement). Importantly, expression of hAAT in addition to reporter transgenes was detectable in secondary recipients 31 weeks after lentiviral transduction of the original HSCs.
Repopulation of Lung Alveolar Macrophages and Secretion of AAT in ELF
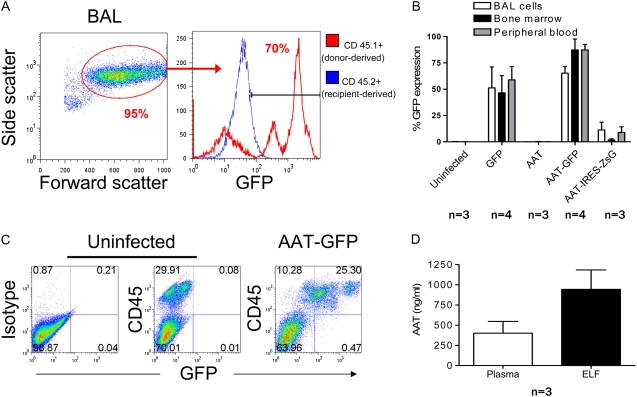
We considered the possibility that repopulation of alveolar macrophages with the progeny of transduced HSCs would establish a local population of cells able to secrete human AAT into the airspaces of the lung. For this reason, we performed BAL in each recipient to measure macrophage engraftment and levels of hAAT in the ELF. In accord with the known contribution of circulating bone marrow–derived monocytes to the alveolar macrophage compartment of the lung (33), we found that 24 weeks after transplant, chimerism of resident alveolar macrophages reflected the reconstituted peripheral blood and bone marrow compartments of each recipient (data not shown). Furthermore, transgene expression appeared to be stable in each compartment, as the percentage of cells expressing GFP was similar in bone marrow, peripheral blood, and BAL populations (Figures 6A and 6B). As was observed in transduced blood and bone marrow populations, reporter gene expression in BAL cells transduced with IRES-containing constructs was 10- to 100-fold lower than in experiments employing the dual promoter (AAT-GFP) vector. Further analysis of BALF by ELISA revealed detectable hAAT only in animals with higher levels of serum hAAT. In these animals, estimated ELF hAAT protein levels were noted to be in excess of serum levels (Figure 6D).
Figure 6.
Analysis of lung tissue 24 weeks after transplantation of HSCs infected with AAT-GFP lentivirus. (A) Flow cytometry analysis of cells obtained by BAL. Alveolar macrophages (AMs), representing 95% of BAL cells, are identified by their characteristic side scatter/forward scatter profile. Approximately 70% of AMs are GFP positive. (B) Percentage of cells expressing GFP/ZsGreen in the BAL, bone marrow, and peripheral blood compartments of recipient mice. No significant difference was found between tissue compartments in the percentage of cells expressing the tracking reporter gene (one way ANOVA > 0.05 for each vector). Error bars represent SEM. (C) Flow cytometry analysis of cells obtained by whole lung digestion. GFP+ cells are CD45+, indicating that transduced cells present in lung tissue are predominantly of hematopoietic phenotype. For negative controls, samples were prepared from a mouse transplanted with unmanipulated (uninfected) HSCs. Specificity of anti-CD45 staining is indicated by a sample exposed to nonspecific control antibody of identical isotype. (D) AAT expression in the plasma and epithelial lining fluid of recipients transplanted with HSCs infected with AAT-IRES-ZsG lentivirus.
Because it is possible that other lung cell types contributed to AAT levels in the epithelial lining fluid, we evaluated the phenotype of all reporter gene–expressing cells present in lung tissue after transplantation of transduced HSCs. Single-cell suspensions prepared from whole lung digests were analyzed by flow cytometry for expression of reporter transgenes and the pan-hematopoietic marker, CD45 (Figure 6C). In each recipient (n = 11), more than 99% of cells in the lung expressing reporter transgenes also expressed CD45, demonstrating that stem cells transduced with either GFP, GFP-AAT, or AAT-IRES-ZsGreen constructs had predominantly given rise to hematopoietic derivatives rather than epithelial or endothelial cell types after transplantation.
Absence of Murine Immune Response to Circulating Human Transgene
To determine whether expression of hAAT protein resulted in activation of a cellular or humoral immune response, we screened for the presence of circulating anti-hAAT antibodies, and analyzed CD4+ and CD8+ T cells isolated from the spleens of experimental recipient animals (Figure E2). There were no detectable circulating anti-hAAT IgG antibodies in any of the samples tested. Similarly, ex vivo stimulation of splenocytes with hAAT protein did not reveal evidence of a cell-mediated response as determined by intracellular cytokine staining (ICS) for IFN-γ and IL-2 in CD4+ or CD8+ lymphocytes. Furthermore, stimulation with hAAT protein did not result in T cell proliferation by CFSE assay (data not shown).
DISCUSSION
The concept of cell-based therapy for AAT deficiency was initially demonstrated by Garver and coworkers, who employed a retrovirus to introduce human AAT cDNA into murine fibroblasts in culture prior to intraperitoneal transplantion into nude mice (7). Recent advances in stem cell biology and the development of lentiviral vectors allow us to extend this approach to transplantable stem cells with well-characterized self-renewal and differentiation capacities.
Our results support a novel approach to the systemic delivery of AAT. This approach harnesses the considerable capacity of HSCs to expand in vivo and uses lentiviruses to integrate genes into the HSC genome. In doing so, we adapt our previously reported method for stem cell transduction to accomplish prolonged expression of circulating human AAT in vivo. This work suggests stem cells in general and HSCs in particular as potential targets for gene therapy of AAT deficiency.
HSCs transduced to overexpress AAT appear to retain their capacity for engraftment and long-term multilineage blood reconstitution. The ability of transduced HSCs to contribute to all peripheral blood cell lineages is particularly significant given recent publications regarding the putative role of AAT in the regulation of bone marrow cell populations (34). AAT is produced by a variety of hematopoietic cell types in the bone marrow, and down-regulation of AAT expression in the marrow to allow protease-based cleavage of matrix anchors may be important for the release of hematopoietic progenitors into the peripheral blood (34). If true, overexpression of AAT in the marrow could interfere with progenitor cell mobilization. We found that HSCs transduced to overexpress AAT contributed to all peripheral blood lineages, and the proportion of cells that were GFP+ remained constant as cells were mobilized from bone marrow to blood compartments. Although transduced (GFP+) cells competed well with untransduced (GFP-) cells in their ability to contribute to the peripheral blood over time, cytokine mobilization experiments would be required to properly test for any effect of AAT overexpression on the kinetics of blood progenitor mobilization from marrow.
Despite accomplishing transduction of the majority of the hematopoietic system, our method did not result in circulating hAAT levels in mice above the theoretical “protective threshold” of 800 μg/ml in human serum, the level thought to protect patients from developing emphysema (18, 35–37). This contrasts with previous studies in which transduction of muscle fibers with adeno-associated viral vectors (AAV) was able to achieve “protective” levels of circulating hAAT in mice (8). The levels achieved may reflect intrinsic secretory limitations of hematopietic cell types compared with hepatocytes and myocytes, or may be a consequence of limited promoter activity levels in our studies. Low circulating levels do not appear to have resulted from either a cell-mediated or from a humoral immune response directed against the hAAT protein, in contrast to prior gene therapy approaches (8). This result is consistent with the observation that HSC transplantation and resultant immune reconstitution typically abrogates humoral and cellular immunity against heterologous proteins expressed by the donor-derived stem cells.
Future studies employing lentiviral transduction of other progenitor cell populations, such as hepatic oval cells, muscle satellite cells, or embryonic stem cell–derived hepatocytes may reveal better targets for cell-based therapies of AAT deficiency and result in higher levels of AAT expression. Alternatively, evaluation of additional nonviral promoters may reveal candidates able to drive higher levels of gene expression. Indeed, in our studies, the highest level of hAAT expression in the plasma resulted from HSCs transduced with AAT under control of the human UBC promoter.
It is notable that estimated hAAT expression in the ELF exceeded plasma expression in some animals. This result raises the possibility that a local pool of transduced cells, such as alveolar macrophages, contributed to hAAT production in these recipients. A lung-localized hAAT-producing cell could be more effective than a distant one in suppressing protease activity in the lung. It is known that only a fraction of circulating AAT reaches the lung interstitium, where it purportedly exerts its protective benefit (18). While the serum AAT threshold necessary to provide protection from lung damage is widely accepted to be approximately 800 μg/ml, the level required in the lung itself to provide this benefit is likely to be much lower (18, 19).
A significant potential limitation to our approach is the use of bone marrow transplant (BMT), a procedure that carries with it the risk of infection as well as complications resulting from chemo- or radiotherapy. However, nonmyeloablative BMTs using Busulfan or direct modulation of endogenous HSCs may offer a less-toxic alternative for patients undergoing HSC transplantation (22, 38). The use of integrating vectors, such as lentiviruses, to transduce stem cell populations raises the additional concern of the potential for oncogenesis. The likelihood of lentiviral-induced insertional mutagenesis may be reduced by decreasing the number of target cells subjected to proviral integration and the number of integrating events per cell. Previously published work (20) has determined that HSC transduction with our protocol results in one to five proviral insertions per transduced cell. In contrast to protocols employing simple retroviral vectors in which millions of cells are typically transplanted (17), our approach targets low numbers of cells (3,000), minimizing the statistical chance of insertional mutagenic events. Although experimental mice in this study and others had normal life spans and exhibited no evidence of leukemia (20, 39), the potential for lentiviruses and other complex retroviruses to cause insertional mutagenesis is an area that warrants further study. Finally, this method and others aimed solely at increasing circulating wild-type hAAT levels do not address the hepatotoxic effects of misfolded mutant AAT proteins. Therapies that incorporate silencing of mutant AAT genes are lacking and will need to be developed as the field progresses.
In summary, these findings demonstrate that lentiviral transduction of small numbers of transplantable stem cells can achieve sustained, systemic in vivo hAAT expression in mice. In addition to potential use as a technique for gene therapy, this approach could also serve as a valuable laboratory tool to study the putative biological role of anti-proteases in mobilizing blood progenitors from their marrow niche as well as their ability to prevent emphysema in mouse models. Further studies, including the evaluation of alternative, nonviral promoters; targeting of resident lung cells; evaluation of safety concerns; and the use of regulatable lentiviral vectors, need to be performed before experiments in human subjects can be considered.
Supplementary Material
Acknowledgments
The authors thank Dr. Gustavo Mostoslavsky and Dr. Richard C. Mulligan for reagents and generous assistance, and Dr. Mary C. Williams for assistance with manuscript editing.
This work was funded by: American Lung Association of Maine/Alpha-1 Foundation RT-22452-N; Flight Attendant Medical Research Institute 062572_YCSA; American Thoracic Society/Alpha-1 Foundation A-05-005; NIH/NHLBI: K08HL71640 (to D.N.K.); and NIH/NHLBI: 5R21HL086414-02 (to D.N.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0133OC on March 6, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.de Serres FJ. Worldwide racial and ethnic distribution of alpha-1 antrypsin deficiency:summary of an analysis of published genetic epidemiologic surveys. Chest 1986;89:370. [DOI] [PubMed] [Google Scholar]
- 2.Brantly M, Nukiwa T, Crystal RG. Molecular basis of alpha-1 antitrypsin deficiency. Am J Med 1988;84:13–31. [DOI] [PubMed] [Google Scholar]
- 3.Stockley RA. The pathogenesis of chronic obstructive lung diseases: implications for therapy. QJM 1995;88:141–146. [PubMed] [Google Scholar]
- 4.Sandhaus RA Elastase May Play a Central Role in the Neutrophil Migration Through Connective Tissue. In: Taylor JC, Mittman C, editors. Pulmonary emphysema and proteolysis Orlando, FL: Academic Press; 1997. p. 227.
- 5.Larsson C. Natural history and life expectancy in severe alpha-1-antitrypsin deficiency, PiZ. Acta Med Scand 1978;204:345–351. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society. Guidelines for the approach to the patient with severe hereditary alpha-1-antitrypsin deficiency. Am Rev Respir Dis 1989;140:1494–1497. [DOI] [PubMed] [Google Scholar]
- 7.Garver RI Jr, Chytil A, Courtney M, Crystal RG. Clonal gene therapy:transplanted mouse fibroblast clones express human alpha 1-antitrypsin gene in vivo. Science 1987;237:762–764. [DOI] [PubMed] [Google Scholar]
- 8.Song S, Morgan M, Ellis T, Poirier A, Chesnut K, Wang J, Brantly M, Muzyczka N, Byrne BJ, Atkinson M, et al. Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc Natl Acad Sci USA 1998;95:14384–14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferkol T, Mularo F, Hilliard J, Lodish S, Perales JC, Ziady A, Konstan M. Transfer of the human alpha1-antitrypsin gene into pulmonary macrophages in vivo. Am J Respir Cell Mol Biol 1998;18:591–601. [DOI] [PubMed] [Google Scholar]
- 10.Kay MA, Baley P, Rothenberg S, Leland F, Fleming L, Ponder KP, Liu T, Finegold M, Darlington G, Pokorny W, et al. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci USA 1992;89:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan YY, Wu J, Zhu JL, Liu SL, Ozaki I, Strayer DS, Zern MA. Gene therapy for human alpha1-antitrypsin deficiency in an animal model using SV40-derived vectors. Gastroenterology 2004;127:1222–1232. [DOI] [PubMed] [Google Scholar]
- 12.Song S, Embury J, Laipis PJ, Berns KI, Crawford JM, Flotte TR. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (RAAV) vectors. Gene Ther 2001;8:1299–1306. [DOI] [PubMed] [Google Scholar]
- 13.Lemarchand P, Jaffe HA, Danel C, Cid MC, Kleinman HK, Stratford-Perricaudet LD, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. Adenovirus-mediated transfer of a recombinant human alpha 1-antitrypsin CDNA to human endothelial cells. Proc Natl Acad Sci USA 1992;89:6482–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld MA, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier LE, Paakko PK, Gilardi P, Stratford-Perricaudet LD, Perricaudet M, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 1991;252:431–434. [DOI] [PubMed] [Google Scholar]
- 15.Setoguchi Y, Jaffe HA, Chu CS, Crystal RG. Intraperitoneal in vivo gene therapy to deliver alpha 1-antitrypsin to the systemic circulation. Am J Respir Cell Mol Biol 1994;10:369–377. [DOI] [PubMed] [Google Scholar]
- 16.Stecenko AA, Brigham KL. Gene therapy progress and prospects: alpha-1 antitrypsin. Gene Ther 2003;10:95–99. [DOI] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S, le Deist F, Carlier F, Bouneaud C, Hue C, de Villartay JP, Thrasher AJ, Wulffraat N, Sorensen R, Dupuis-Girod S, et al. Sustained correction of X–linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002;346:1185–1193. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard RC, Crystal RG. Augmentation therapy of alpha 1-antitrypsin deficiency 4. Eur Respir J Suppl 1990;9:44s–52s. [PubMed] [Google Scholar]
- 19.Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987;316:1055–1062. [DOI] [PubMed] [Google Scholar]
- 20.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Efficiency of transduction of highly purified murine hematopoietic stem cells by lentiviral and oncoretroviral vectors under conditions of minimal in vitro manipulation. Mol Ther 2005;11:932–940. [DOI] [PubMed] [Google Scholar]
- 21.Naldini L, Blomer U, Gallay P, Ory D, Mulligan RC, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996;272:263–267. [DOI] [PubMed] [Google Scholar]
- 22.Mostoslavsky G, Fabian AJ, Rooney S, Alt FW, Mulligan RC. Complete correction of murine artemis immunodeficiency by lentiviral vector-mediated gene transfer. Proc Natl Acad Sci USA 2006;103:16406–16411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summer R, Kotton DN, Sun X, Fitzsimmons K, Fine A. Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L477–L483. [DOI] [PubMed] [Google Scholar]
- 24.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotton DN, Fabian AJ, Mulligan RC. A novel stem cell population in adult liver with potent hematopoietic reconstitution activity. Blood 2005;106:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balazs AB, Fabian AJ, Esmon CT, Mulligan RC. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone Marrow. Blood 2006;107:2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60:532–538. [DOI] [PubMed] [Google Scholar]
- 29.Hovav AH, Panas MW, Rahman S, Sircar P, Gillard G, Cayabyab MJ, Letvin NL. Duration of antigen expression in vivo following DNA immunization modifies the magnitude, contraction, and secondary responses of CD8+ T lymphocytes. J Immunol 2007;179:6725–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hovav AH, Panas MW, Osuna CE, Cayabyab MJ, Autissier P, Letvin NL. The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells. J Virol 2007;81:12793–12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol 2005;23:108–116. [DOI] [PubMed] [Google Scholar]
- 32.Borman AM, Le MP, Girard M, Kean KM. Comparison of picornaviral IRES-driven internal initiation of translation in cultured cells of different origins. Nucleic Acids Res 1997;25:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 1976;192:1016–1018. [DOI] [PubMed] [Google Scholar]
- 34.Winkler IG, Hendy J, Coughlin P, Horvath A, Levesque JP. Serine protease inhibitors serpina1 and serpina3 are down-regulated in bone marrow during hematopoietic progenitor mobilization. J Exp Med 2005;201:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse JO. Alpha1-antitrypsin deficiency (first of two parts). N Engl J Med 1978;299:1045–1048. [DOI] [PubMed] [Google Scholar]
- 36.Morse JO. Alpha1-antitrypsin deficiency (second of two parts). N Engl J Med 1978;299:1099–1105. [DOI] [PubMed] [Google Scholar]
- 37.Kueppers F, Black LF. Alpha1-antitrypsin and its deficiency. Am Rev Respir Dis 1974;110:176–194. [DOI] [PubMed] [Google Scholar]
- 38.Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, Rees PA, Cheung MC, Mittelstaedt S, Bingaman AW, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol 2001;167:1103–1111. [DOI] [PubMed] [Google Scholar]
- 39.Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM. Gene therapy: therapeutic gene causing lymphoma. Nature 2006;440:1123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.