Abstract
HIV-1 infection impairs alveolar macrophage immune function and renders patients susceptible to pneumonia by poorly understood mechanisms. Alveolar macrophage maturation and function depends on granulocyte-macrophage colony–stimulating factor (GM-CSF), which is produced and secreted by the alveolar epithelium. Macrophages respond to GM-CSF through the GM-CSF receptor (GM-CSFR), which has a binding subunit (GM-CSFRα) and a signaling subunit (GM-CSFRβ). In this study, we measured GM-CSFR expression and alveolar macrophage function in a transgene HIV-1 rat model (NL4-3Δ gag/pol); this construct bears a pro-virus with gag and pol deleted, but other HIV-1–related proteins, such as gp120 and Tat, are expressed, and the rats develop an AIDS-like phenotype as they age. We first determined that HIV-1–transgenic expression selectively decreased alveolar macrophage expression of GM-CSFRβ and impaired bacterial phagocytosis in vitro. Next, we examined the role of zinc (Zn) deficiency as a potential mechanism underlying these effects, and determined that HIV-1–transgenic rats have significantly lower levels of Zn in the alveolar space and macrophages. To test the direct effect of Zn deficiency on macrophage dysfunction, we treated rat alveolar macrophage cell line with a Zn chelator, N,N,N′,N′-tetrakis-(2-pyridyl-methyl) ethylenediamine, and this decreased GM-CSFRβ expression and phagocytosis. In parallel, treatment with Zn acetate in vitro for 48 hours restored intracellular Zn levels and phagocytic function in alveolar macrophages from HIV-1–transgenic rats. Taken together, these data suggest that pulmonary Zn deficiency could be one of the mechanisms by which chronic HIV-1 infection impairs alveolar macrophage immune function and renders these individuals susceptible to serious lung infections.
Keywords: AIDS, lung, monocyte/macrophages, phagocytosis, rodent
CLINICAL RELEVANCE
This study demonstrates that pulmonary zinc status may be important in HIV-1 infection, and that pulmonary zinc deficiency could be one of the mechanisms by which HIV-1 infection impairs alveolar macrophage immune function.
Individuals infected with HIV-1 are susceptible to bacterial and viral infections of the airways leading to pneumonia (1–4). Despite major advances in HIV-1 treatment, opportunistic infections of the lung, such as tuberculosis and pneumocystis, remain the leading cause of death in these individuals (5). In addition, more typical bacterial pathogens, such as Streptococcus pneumoniae, are also increased in patients with HIV/AIDS, reflecting a significant problem with airway host defense. The precise mechanisms underlying HIV-1–induced immune dysfunction within the lung are not known; however, HIV-1 can infect alveolar macrophages (6), and evidence suggests that phagocytosis and other innate immune functions are impaired in monocytes/macrophages from individuals infected with HIV-1 (7, 8). Therefore, there are compelling reasons to identify the mechanisms by which alveolar macrophage immune function is impaired in chronic HIV-1 infection so that novel therapies designed to augment pulmonary host defenses can be developed.
More recently, it has become recognized that not all HIV-1 disease manifestations can be attributed to viral infection of target cells alone, and there is evidence that HIV-1–related proteins, including gp120 and Tat, can independently cause organ dysfunction. The recently developed HIV-1–transgenic mouse and rat models provide powerful tools for studying the impact of viral proteins, independently of viral infection, on different organs, including the lung (9–11). In both the rat and the mouse construct, the HIV-1 gag and pol genes have been deleted and, therefore, this is a pro-virus insertion in which there is no viral replication or infection, but in which other HIV-1–related proteins are expressed (12). The advantage of the rat model, when compared with the mouse model, is that the HIV-1–transgenic rat has a functional Tat and efficient viral gene expression in many organs. These rats have circulating gp120 in their blood, and tissue mRNA expression of gp120, Nef, Tat, and Rev. Furthermore, they develop muscle wasting, cataracts, nephropathy, and immune deficiencies that are all consistent with an AIDS-like phenotype (12). Therefore, although primate models remain the best means of studying viral infection and replication, the transgenic rodent models are considerably less expensive and offer the unique opportunity to study the effects of HIV-1–related proteins independently of viral infection per se.
Granulocyte-macrophage colony–stimulating factor (GM-CSF) is a 23-kD glycosylated peptide that is secreted by many cells, including alveolar epithelial cells (13). It is known that GM-CSF is essential for maturation and function of alveolar macrophages. Alveolar macrophages respond to GM-CSF through GM-CSF receptor (GM-CSFR), which is present on the membrane. The GM-CSFR shares a common β-chain (GM-CSFRβ) with the IL-3 and IL-5 receptors that initiates intracellular signaling, and a unique α chain (GM-CSFRα), which acts as the binding subunit (14, 15). We have shown recently that alveolar macrophages from alcohol-fed rats have significantly fewer GM-CSFR, and have decreased GM-CSF signaling (16). This dampening of GM-CSF signaling resulted in decreased immune function of macrophages (16). As both chronic alcohol abuse and chronic HIV-1 infection predispose to a variety of pulmonary infections, we speculated that alterations in GM-CSFR expression might likewise explain, at least in part, the alveolar macrophage immune defects associated with HIV-1 infection. Furthermore, if this proved to be the case, then strategies found to increase GM-CSFR expression could be an effective adjunctive therapy to enhance pulmonary host defense in this setting.
Recently, we focused on one such strategy; specifically, the trace element, zinc (Zn), which is essential for host defense, but, to our knowledge, has not been examined in experimental models of HIV-1. Micronutrient Zn is implicated in many aspects of immune function, including function of T and B lymphocytes and innate immunity (17). In addition, Zn has structural and catalytic roles in more than 300 metalloenzymes, and regulates many cellular processes, including signal transduction and gene expression (18). It also plays a role in the regulation of cellular glutathione (19) and protects cell membranes from oxidative damage (20). Chronic Zn deficiency can cause further damage when combined with other insults. For example, in Zn-deprived individuals, smoking can result in replacement of absent Zn with toxic cadmium from cigarette smoke, and this may contribute to tobacco-related lung diseases.
Although considerable information is available on the role of Zn in the brain (21, 22), liver (23, 24), immune system (17), and bones (25, 26), very few studies have focused on its role in the lungs. In addition to our recent reports on alcohol-induced dampening of GM-CSFR expression and signaling in the alveolar macrophage (16), we have investigated the potential role of Zn deficiency within the alveolar space as a contributing factor, and shown an immunostimulatory role of Zn in the alveolar space in our rat model of chronic alcohol ingestion (unpublished data). To our knowledge, there are no reports on the effects of HIV-1–related proteins on Zn levels within the alveolar space or on alveolar macrophage GM-CSFR expression and signaling. Therefore, in this study, we examined GM-CSFR expression and bacterial phagocytic function in alveolar macrophages from HIV-1–transgenic rats. We then examined the levels of Zn in the alveolar space and whether Zn supplementation could improve GM-CSFR expression and immune function in alveolar macrophages from HIV-1–transgenic rats.
MATERIALS AND METHODS
HIV-1–Transgenic Rats
Age-matched HIV-1–transgenic or wild-type female Fischer rats were either purchased from Harlan or produced by a breeding colony that we recently established. This rat model is genetically engineered to contain the entire genome of the AIDS virus HIV-1 except for gag and pol that make the virus infectious (12). Mating of male HIV-1 rats with wild-type females produced several transgenic and normal offspring. The transgenic rats are born with cataracts and can be clearly identified from wild-type normal rats, and were positive for transgene as determined by tail-snip DNA analysis (12). The female transgenic rats used in this study were always matched for age and gender with normal wild-type rats. All the work was performed under the approval of the Institutional Animal Care and Use Committee at the Atlanta Veterans Affairs Medical Center. In most experiments, 8- to 9-month-old rats were used. To investigate age-related issues, younger rats were used in some experiments, as HIV-1–transgene expression has a relatively mild phenotype in young animals, but, as animals age, they develop a profound phenotype (12).
Bronchoalveolar Lavage and Isolation of Alveolar Macrophages
After pentobarbital anesthesia (100 mg/kg intraperitoneally), a tracheotomy tube was placed and rat lungs were first lavaged with 5 ml sterile, cold PBS (pH 7.4). The recovered fluid (∼3.5 ml) was stored in aliquots at −70°C for measurement of Zn levels as described below. To isolate macrophages, lungs were lavaged four times with 10 ml sterile, cold PBS (pH 7.4). The recovered lavage solution was centrifuged at 1,500 rpm for 7 minutes, and the cell pellet resuspended in sterile media for functional studies. This procedure routinely yields about two million cells per rat. These cells are over 98% viable by trypan blue exclusion test. Diff-Quick (IMEB, Inc., San Marcos, CA) and CD-32 (a marker for alveolar macrophages) staining showed that more than 95% of cells were alveolar macrophages. These cells did not have any neutrophils, and more than 95% of cells adhered to coverslips after 30-minute incubation at 37°C. In some experiments, a rat alveolar macrophage cell line NR8383 (American Type Culture Collection, Manassas, VA) was used to investigate the effect of Zn deficiency on GM-CSFR expression and phagocytosis.
Measurement of Zn Levels
Zn levels in the bronchoalveolar lavage fluid and in the isolated alveolar macrophages and epithelial cells were measured using a Zn-specific dye (Invitrogen, Carlsbad, CA). FluoZin-3 is a recently developed fluorochrome with a high affinity for Zn (dissociation constant, 15 nM) and little affinity for Ca or Mg. This free-acid form is not membrane permeable, and is mainly used to detect Zn release into the extracellular space or in solutions such as lung lavage fluid, as employed here. FluoZin-3 (500 nM) was added to the first aliquot of lavage fluid, and resulting fluorescence was measured in a fluorescence plate reader. The excitation and emission wavelengths for FluoZin-3 are 494 and 516 nm, respectively. Standard curves for Zn acetate were generated with each run, and Zn values in the unknown samples were extrapolated from this curve. To avoid Zn contamination, sterile tubes and MiliQ water were used. The membrane-permeable form, FluoZin-3AM, was used for staining alveolar macrophages for 1 hour at room temperature. The labeled cells were analyzed by FACScan flow cytometer (Becton Dickinson, San Jose, CA). Data are expressed both as percent of positive cells as well as the mean channel fluorescence (MCF) for positive cells in each group. To correlate cellular function with Zn levels, GM-CSFR expression and phagocytosis were measured in the alveolar macrophages.
Flow Cytometric Detection of Membrane and Intracellular Receptor Expression
Membrane and intracellular expression of GM-CSFRs on alveolar macrophages were measured by an established protocol (27). Briefly, cells were incubated for 30 minutes at room temperature with rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) to either the rat GM-CSFRα or -β subunit, or to an isotype-matched control antibody and anti-gp120 antibody. Cells were washed to remove unbound antibody, followed by 30-minute incubation at room temperature with secondary anti-rabbit antibody conjugated to FITC. For intracellular staining of the receptors, cells were permeabilized with 0.1% saponin in PBS, followed by staining with the antibody. Cells were washed with PBS-saponin before adding FITC-conjugated secondary antibody (Santa Cruz Biotechnology). Cells were washed with PBS and kept in the dark at 4°C until being analyzed. The labeled cells were analyzed by FACScan flow cytometer (Becton Dickinson). Data are expressed both as percent of positive cells as well as the MCF for positive cells in each group. Negative controls (cells stained with isotype-matched control antibody) were routinely less than 1% for percentage of positive cells (data not shown), and were subtracted from the positive control.
Enzyme-Linked Immunosorbent Assay
The expression of gp120 protein in serum and lavage was measured by gp120 capture enzyme-linked immunosorbent assay (ELISA) kit (Immunodiagnostics Inc., Bedford, MA). The lower limit of detection was 100 pg/ml.
RNA Isolation and Real-Time PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and treated with Qiagen DNase I to eliminate contaminating genomic DNA. Reverse transcription was performed with 1 μg of total RNA in a total volume of 25 μl per reaction in an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR was carried on the Bio-Rad iCycler. Amplification was preformed in Bio-Rad iQ SYBR green supermix containing specific primers and with denaturing at 95°C for 20 seconds, annealing at 58°C for 20 seconds, and extension at 20 seconds. Aliquots of cDNA were diluted 10- to 10,000-fold to generate relative standard curves to which sample cDNA was compared. Standards and samples were run in triplicate. The primers and product sizes were as follows: 5′-GCA TAC ATC GTC GTC ACT GG-3′ and 5′-ACT CGA TCT CAA AGC GGA AA-3′ for GMCSFR-α (202 bp); 5′-AGA GCA GGC TTC TTG CTG AG-3′ and 5′-ATT GGG AAG TTG CTC TGT GG-3′ for GMCSFR-β (164 bp). QuantumRNA class II 18S primers were purchased from Ambion (Austin, TX). PCR amplicons from all species were normalized for the amount of 18S in the same cDNA sample, which was also standardized on a dilution curve from the cDNA samples. A dilution curve showed that the real-time PCR efficiency was greater than 95% for all genes analyzed. Real-time SYBR green dissociation curves showed one species of amplicon for each primer set.
Alveolar Macrophage Bacterial Phagocytosis
Alveolar macrophages were isolated from wild-type and HIV-1–transgenic rats and incubated for 1 hour with FITC-labeled Staphylococcus aureus (Wood strain without protein A; Molecular Probes, Eugene, OR) in a 10:1 ratio; after incubation, cells were vigorously washed several times with PBS. Extracellular fluorescence was quenched by addition of trypan blue, as previously described (28), and FITC-labeled bacteria containing cells were measured by flow cytometry. Phagocytic index was calculated as follows: (% positive cells × MCF)/100. Phagocytosis images were obtained using an epifluorescence microscope (Leica Microsystems, Wetzlar, Germany). In some experiments, alveolar macrophages from wild-type and HIV-1–transgenic rats were incubated with or without Zn acetate (1 and 10 μM) for 48 hours. To one portion of the cells, FITC-labeled S. aureus was added in a 10:1 ratio and incubated at 37°C in vitro for 1 hour. Phagocytosis was measured as described above. Other cells were used to measure intracellular Zn levels by flow cytometry as described above.
Statistical Analysis
Data are presented as mean (± SEM). Data analysis was done by ANOVA with Student-Newman-Keuls test for group comparison and were considered statistically significant at a P value of less than 0.05.
RESULTS
Characterization of the HIV-1–Transgenic Rat Model
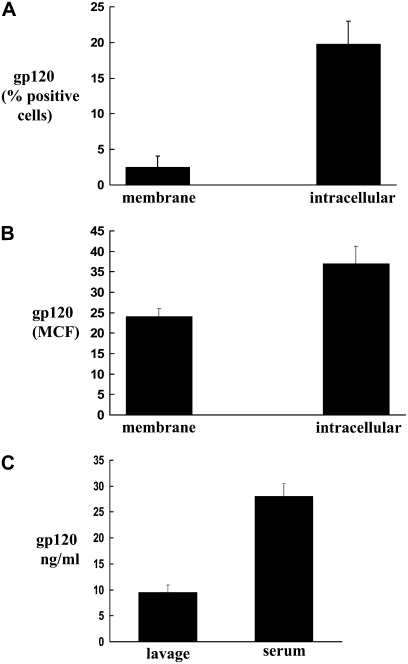
These animals are known to express the HIV-1 envelop protein, gp120. Therefore, we examined gp120 expression in the peripheral blood white cells of HIV-1–transgenic rats by flow cytometry. As shown in Figures 1A and 1B, cells from transgenic rats expressed membrane and intracellular gp120. Both the percentages of cells positive for gp120, as well as the MCF, were significantly higher in the HIV-1–transgenic rats. In parallel, gp120 was detected in the lung lavage fluid and serum of HIV-1–transgenic female rats by ELISA (Figure 1C). These levels were not significantly different than those from age-matched HIV-1–transgenic male rats (data not shown); however, age-dependent differences in the expression of these proteins may be possible. The nonspecific low background levels in the wild-type rats were subtracted from the gp120 levels from transgenic rats.
Figure 1.
Expression of protein gp120 in transgenic HIV-1 rats. (A and B) Membrane and intracellular expression of gp120 on white blood cells of HIV-1–transgenic rats (solid bars), as determined by flow cytometry. Percentages of positive cells are shown in A, and mean channel fluorescence (MCF) in B. (C) Levels of gp120 in the bronchoalveolar lavage and serum of transgenic rats as measured by ELISA. Each value in A–C represents the mean ± SEM of four rats. The nonspecific low background levels in the wild-type rats (open bars) were subtracted from the gp120 levels from transgenic rats.
Down-Regulation of GM-CSFRβ and Decreased Phagocytosis by Alveolar Macrophages from HIV-1–Transgenic Rats
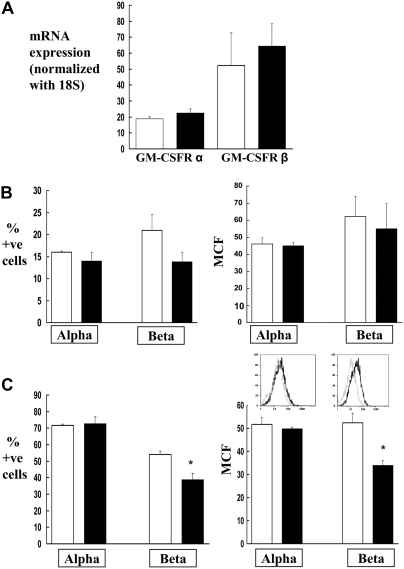
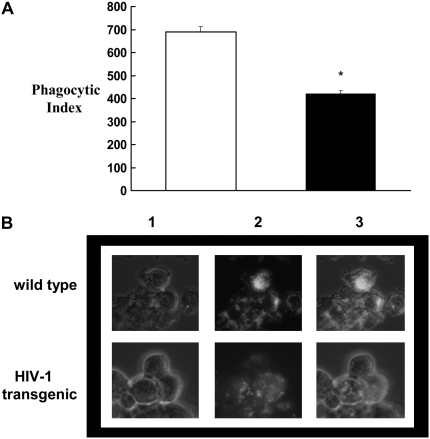
As we had shown recently that alveolar macrophages from rats that are chronically fed alcohol have significantly fewer GM-CSFR and display decreased GM-CSF signaling and immune function (16), we examined the relative expression of the GM-CSFRα and -β subunits by real-time PCR and flow cytometry in the alveolar macrophages from wild-type and HIV-1–transgenic rats. As shown in Figure 2A, HIV-1–transgene expression had no effect on the gene expression of GM-CSFRα and -β. However, HIV-1–transgene expression decreased the membrane localization of GM-CSFRβ (Figure 2B), and, to an even greater extent, decreased the intracellular expression of this signaling subunit (Figure 2C). This effect on receptor protein expression was not observed with the α subunit (Figures 2B and 2C) that binds GM-CSF. These results suggest that HIV-1–transgene expression affects the expression of GM-CSFR at a post-transcriptional level. Whether this effect is at the translational level or at a post-translational stage, such as trafficking and insertion into the plasma membrane, was not addressed in this study. These findings parallel our recent studies in which GM-CSFR protein expression, but not gene expression, was significantly decreased in alveolar macrophages by chronic alcohol ingestion (16). GM-CSF is produced by the alveolar epithelium and binds to specific GM-CSF receptors on the plasma membrane of the alveolar macrophage, which, in turn, activates intracellular signaling pathways. GM-CSF signaling is complex and involves Janus kinases, signal transducers and activators of transcription (STAT), and the GM-CSF master transcription factor, PU.1. The specific modulation of this signaling cascade by HIV-1–transgene expression was beyond the scope of this study. However, we find it interesting and noteworthy that the intracellular pool of the signaling unit (GM-CSFRβ) is significantly decreased. This finding suggests that the capacity of the alveolar macrophage to maintain a robust signaling response to GM-CSF is dampened by chronic HIV-1–related protein expression through a mechanism that appears to involve Zn deficiency. Again, these findings are remarkably similar to our findings in the alcohol model, and raise the intriguing possibility that seemingly disparate chronic stresses—namely, alcohol abuse and HIV-1—could have common pathophysiological effects on alveolar macrophage function that are mediated, at least in part, by Zn deficiency. We also explored the potential functional consequences of this decrease in the expression of the GM-CSFR signaling unit. As shown in Figure 3, phagocytosis of FITC-labeled bacteria by alveolar macrophages from HIV-1–transgenic rats was significantly decreased as compared with phagocytosis by alveolar macrophages from wild-type rats. These findings support the hypothesis that down-regulation of the GM-CSFRβ signaling unit in HIV-1–transgenic subjects may be responsible for the decreased phagocytosis and resulting immune dysfunction.
Figure 2.
Expression of GM-CSFRα and GM-CSFRβ gene and protein on alveolar macrophages. (A) The gene expression of GM-CSFRα and GM-CSFRβ by real-time PCR. Each value represents the mean ± SEM of three rats in each group. (B) Flow cytometric analysis of membrane expression of GM-CSFRα and GM-CSFRβ on alveolar macrophages from wild-type and HIV-1–transgenic rats. (C) Flow cytometric analysis of intracellular expression of GM-CSFRα and GM-CSFRβ on alveolar macrophages from wild-type and HIV-1–transgenic rats. In both panels, data are represented as percentage of positive cells and MCF. Insets in C show representative histograms of MCF for GM-CSFRα and β. Histograms for receptors from wild-type and transgenic rats are represented by black and gray lines, respectively. Negative control cells (cells stained with isotype-matched control antibody) were routinely less than 1% for percentage of positive cells (data not shown) and were subtracted from the positive control. Each value in B and C represents the mean ± SEM of six to eight rats in each group. *P < 0.05 compared to wild-type rats. Both HIV-1–transgenic and wild-type rats used in these experiments were 8 to 9 months old.
Figure 3.
Phagocytosis by alveolar macrophages from wild-type and HIV-1–transgenic rats. (A) Phagocytic index of alveolar macrophages from HIV-1 rats (solid bar), as determined by flow cytometry. Open bar, wild type. Phagocytic index was calculated as described in Materials and Methods. (B) Representative phase contrast (1), fluorescence (2), and merged (3) images of alveolar macrophages with ingested FITC-labeled, inactivated Staphylococcus aureus. Each value in (A) represents the mean ± SEM of six rats in each group. *P < 0.05 compared to wild-type rats. Both HIV-1–transgenic and wild-type rats used in these experiments were 8 to 9 months old.
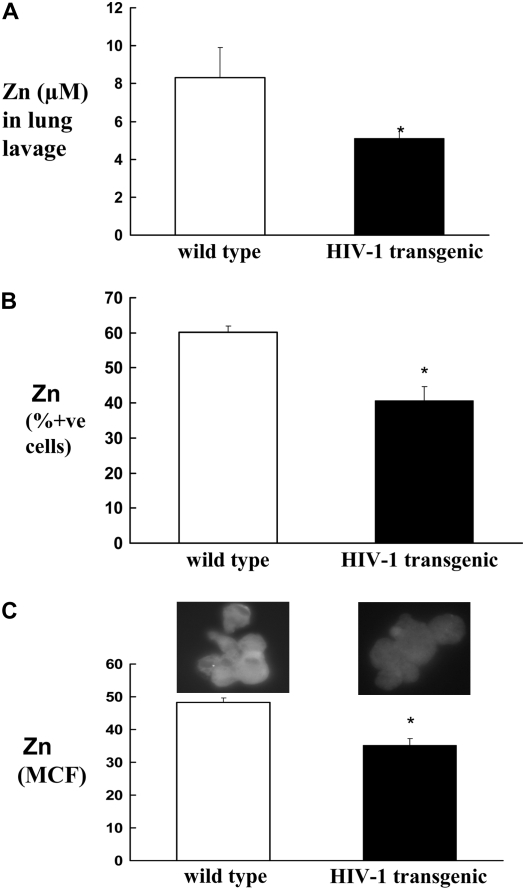
Decreased Zn Levels in the Bronchoalveolar Lavage and Alveolar Macrophages from HIV-1–Transgenic Rats
To examine a putative mechanism underlying HIV-1–related protein expression and alveolar macrophage dysfunction, we focused on Zn availability, as it is known to be deficient in some patients with HIV-1 as the disease progresses (29). In addition, supplementation of Zn in the diets of alcohol-fed rats improves alveolar macrophage function (unpublished data). Therefore, we examined Zn levels in the alveolar space of HIV-1–transgenic rats. We used FluoZin-3 (Invitrogen) to measure Zn in the bronchoalveolar lavage, and FluoZin-3AM (membrane-permeable form) to evaluate intracellular Zn in alveolar macrophages, from wild-type and transgenic rats. As shown in Figure 4A, Zn levels in the bronchoalveolar lavage fluid from HIV-1–transgenic rats were significantly lower when compared with levels in wild-type rats. In parallel, intracellular Zn levels of alveolar macrophages from HIV-1 rats were significantly decreased, as reflected both by percent positive cells (Figure 4B) and by MCF (Figure 4C).
Figure 4.
Zinc (Zn) levels in the bronchoalveolar lavage and alveolar macrophages from wild-type and HIV-1–transgenic rats. Zinc was measured by a Zn-specific dye, FluoZin-3 (Invitrogen). Soluble form of this dye was used to measure Zn in the lavage (A), as described in Materials and Methods. (B and C) Flow cytometric determination of intracellular Zn by using a membrane-permeable form of FluoZin-3AM. Each value in A–C represents the mean ± SEM of six to eight rats in each group. *P < 0.05 compared to wild-type rats. Both HIV-1–transgenic and wild-type rats used in these experiments were 8 to 9 months old.
Age-Related Effect on Zn Levels
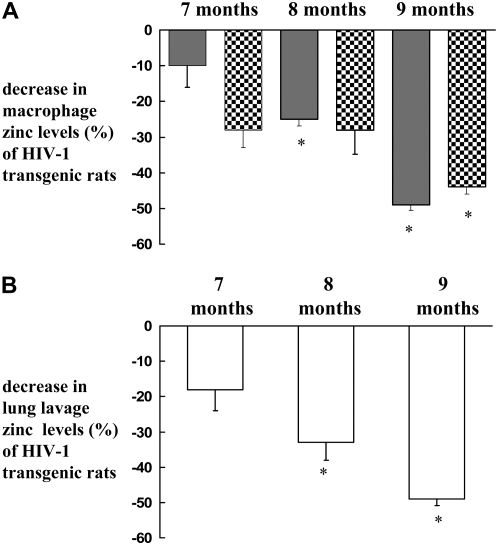
In the original paper describing the HIV-1–transgenic rat model, the resulting AIDS-like pathologies, such as muscle wasting, lymphoid hyperplasia, and renal failure, were much more dramatic in rats that were 5 to 12 months of age (12). This implies that there is a progressive burden of HIV-1–related protein expression and consequent tissue injury over time. In addition, some reports have documented a correlation between Zn deficiency and progression of HIV-1/AIDS (30). Therefore, we sought to determine if there was an age-dependent effect on alveolar macrophage Zn levels in the HIV-1–transgenic rats. As shown in Figure 5A, there was a progressive decrease in the intracellular Zn levels of alveolar macrophages from HIV-1–transgenic rats as they aged; in contrast, no age-related change in the Zn levels were evident in wild-type rats (data not shown). In parallel, lung lavage Zn levels also followed the same pattern by showing a progressive decline as the HIV-1–transgenic rats aged (Figure 5B). In Figure 5, Zn levels in HIV-1–transgenic rats are expressed as the percent of those in wild-type rats.
Figure 5.
Age-dependent modulation in Zn levels. Zn levels in the alveolar macrophages and lavage of HIV-1–transgenic and age-matched wild-type rats were compared. (A) Percent decrease in the intracellular Zn in the alveolar macrophages from HIV-1–transgenic rats as compared with wild-type rats. Data are presented as percentage of positive cells (shaded bars) and MCF (checkered bars), as measured by flow cytometry. (B) Percent decrease in Zn in the lavage from HIV-1–transgenic rats as compared with the lavage from wild-type rats. Zn was measured by a fluorescence plate reader as described in Materials and Methods. Each value in A and B represents the mean ± SEM of three to four rats in each group. Zn levels in HIV-1–transgenic rats are expressed as percent of those in wild-type rats. *P < 0.05 compared to younger rats with HIV-1.
In Vitro Treatment of Normal Alveolar Macrophages with the Zn Chelator N,N,N′,N′-Tetrakis-(2-Pyridyl-Methyl) Ethylenediamine Decreased GM-CSFR Expression and Bacterial Phagocytosis
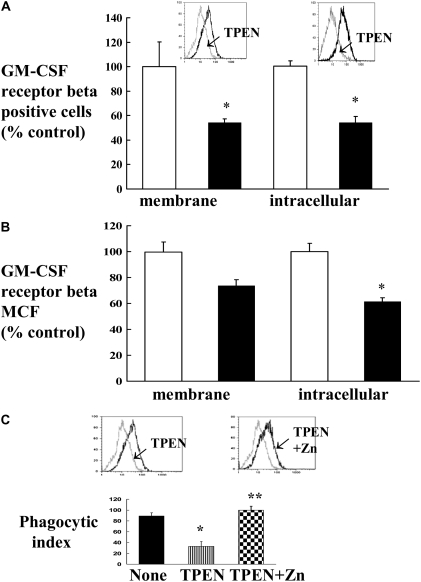
To examine a direct connection between Zn deficiency and GM-CSFR expression and phagocytosis in the HIV-1–transgenic rats, we examined whether or not a Zn chelator, N,N,N′,N′-tetrakis-(2-pyridyl-methyl) ethylenediamine (TPEN), could reproduce these defects in normal macrophages in vitro. We used a rat alveolar macrophage cell line for these experiments. Cells were treated with or without 5 μM TPEN before staining with appropriate antibodies against GM-CSFR subunits. As shown in Figure 6, flow cytometric analysis showed a decrease in the expression of GM-CSFRβ (Figure 6A shows % positive cells, and Figure 6B shows the MCF). As shown, GM-CSFRβ intracellular expression was significantly decreased, and there was also a strong trend toward a decrease in the membrane expression. In contrast, there was no effect on either membrane or intracellular GM-CSFRα expression (data not shown). In parallel, and as shown in Figure 6C, TPEN-treated cells showed a significant decrease in the phagocytosis of FITC-labeled bacteria. In contrast, treatment of cells with TPEN in the presence of Zn prevented this reduction in the phagocytosis by macrophages. Taken together, these findings indicate that pharmacological induction of Zn deficiency causes defects in alveolar macrophage GM-CSFRβ expression and phagocytosis that resemble the defects seen in the macrophages from HIV-1–transgenic rats.
Figure 6.
In vitro effect of Zn chelator N,N,N′,N′-tetrakis-(2-pyridyl-methyl) ethylenediamine (TPEN) on the expression of GM-CSFR and phagocytosis. Rat alveolar macrophage cell line NR8383 (American Type Culture Collection) was treated with 5 μM TPEN for 48 hours before staining for GM-CSFRβ. Membrane and intracellular expression was measured by flow cytometry. (A and B) Percent positive cells and MCF. Insets in A show representative histograms of percent positive cells for GM-CSFRβ. The shifts in the peaks after TPEN are indicated by arrows. Negative control cells (cells stained with isotype-matched control antibody) were routinely less than 1% for percentage of positive cells (data not shown) and were subtracted from the positive control cells. Open bars, no TPEN; solid bars, 5 μm TPEN. (C) Phagocytic index of alveolar macrophage cell line before and after treatment with TPEN or TPEN plus Zn. Insets show representative histograms of percent positive cells for phagocytosis. The shifts in the peaks after TPEN and TPEN plus Zn treatments are indicated by arrows. Each value in A–C represents the mean ± SEM of four separate experiments. *P < 0.05 compared to untreated group. **P < 0.05 compared to TPEN treatment.
In Vitro Supplementation of Alveolar Macrophages from HIV-1–Transgenic Rats with Zn Acetate–Restored Zn Levels and Phagocytosis
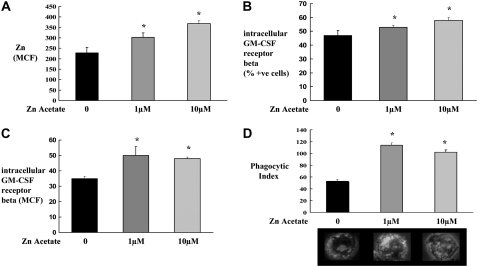
As we had determined in this HIV-1–transgenic rat model that dysfunctional alveolar macrophages are Zn deficient, we next tested whether or not Zn supplementation (Zn acetate at 1 or 10 μM for 48 h) could improve the functional status of alveolar macrophages from HIV-1–transgenic rats. As shown in Figure 7, Zn acetate supplementation increased intracellular Zn levels (Figure 7A), intracellular GM-CSFRβ expression (Figures 7B and 7C), and increased bacterial phagocytic function (Figure 7D) compared with untreated cells. The recovered Zn and phagocytic index levels were comparable to those for wild-type alveolar macrophages (data not shown). In contrast, comparable treatment with Zn acetate had no effect on any of these parameters in alveolar macrophages from wild-type rats (data not shown).
Figure 7.
Ex vivo Zn supplementation restored Zn levels, GM-CSFR β expression, and phagocytosis. Alveolar macrophages from HIV-1–transgenic rats (8 months old) were isolated as described in Materials and Methods. Cells were cultured with or without Zn (1 and 10 μM) for 48 hours. (A) MCF for intracellular Zn by flow cytometry. (B and C) Percent positive cells and MCF for intracellular GM-CSFRβ expression by flow cytometry. (D) Phagocytic index of alveolar macrophages. Also shown in (D) are the representative merged images of alveolar macrophages (phase contrast) with ingested FITC-labeled, inactivated S. aureus. Intracellular Zn (MCF) and phagocytic index of alveolar macrophages from wild-type rats were 325 ± 12 and 100 ± 8, respectively. Each value in A–D represents the mean ± SEM of duplicates of pooled samples from three rats in each group. *P < 0.05 compared to control group.
DISCUSSION
In this study, we determined that alveolar macrophages from HIV-1–transgenic rats have decreased intracellular expression of the GM-CSFRβ subunit and decreased bacterial phagocytic function. In parallel, HIV-1–transgene expression decreased the levels of Zn in the lungs, both in the alveolar epithelial lining fluid and within the alveolar macrophages. In vitro Zn depletion in alveolar macrophages from wild-type rats reproduced the HIV-1 phenotype, at least as reflected by decreased GM-CSFRβ subunit expression and decreased bacterial phagocytosis. In addition, Zn supplementation in vitro increased intracellular Zn levels and GM-CSFRβ subunit expression, and improved bacterial phagocytosis, in alveolar macrophages from HIV-1–transgenic rats. Taken together, these findings suggest a previously unrecognized immunomodulatory role of Zn in the pulmonary microenvironment during chronic HIV-1–related protein expression such as would be seen during chronic HIV-1 infection.
This study is unique in that we used a transgenic HIV-1 rat model in which multiple HIV-1–related proteins are expressed, but viral infection and replication do not occur, because both gag and pol have been deleted in this pro-virus construct. Even though there is no viral infection per se, these animals nevertheless develop an AIDS-like phenotype and, in our study, exhibit significant alveolar macrophage dysfunction. In terms of the progressive nature of the phenotype, this does not appear to require a progressive rise in HIV-1–related protein expression. However, the burden of the stress from these proteins appears to accumulate with age, just as other chronic diseases manifest progressive cellular dysfunction over time. These observations are important and consistent with the increased susceptibility to lung infections that cause so much morbidity and mortality in individuals infected with HIV-1 (31). Although there has been remarkable progress in our understanding of this disease, and effective therapies to limit the progression of HIV-1 infection are now in wide clinical use, the propensity of this virus to develop resistance to antiretroviral agents, and the eventual development of serious pulmonary infections and other complications, provide compelling reasons to identify novel adjunctive therapies that may limit the adverse effects of chronic HIV-1 infection. In the context of HIV-1 infection in humans, the overall viral burden (magnitude and duration of viral replication) is clearly the major issue, but this is accompanied by HIV-related protein burden as well. This HIV-1–transgenic rodent construct suggests that some of the AIDS phenotype is mediated by these HIV-related proteins, which is consistent with the fact that many affected tissues in individuals with AIDS are not directly infected by the virus. Clearly, HIV-1 infection and HIV-related protein expression are inextricably linked, and viral transmission and replication remain the principal therapeutic targets. However, this unique animal model and our experimental findings suggest that some morbidity of chronic HIV-1 infection could be modified by adjunctive therapies, such as dietary Zn supplementation.
In the lung, alveolar macrophages play an important role in both innate and adaptive immune responses. Within the alveolar microenvironment, GM-CSF is secreted by alveolar epithelial cells and is critically necessary for the maturation and function of alveolar macrophages. Macrophages respond to GM-CSF through specific membrane receptors that have a unique binding subunit (GM-CSFRα) and a signaling subunit (GM-CSFRβ) that is shared by the IL-3 and IL-5 receptors. We recently reported that chronic alcohol ingestion in rats decreased both GM-CSFRα and GM-CSFRβ expression in the alveolar macrophage, and, in parallel, dampened the expression of PU.1, the master transcription factor for GM-CSF signaling (16). Therefore, we speculated that HIV-1–related protein expression might cause similar effects, and examined the expression of GM-CSFRs in this recently available HIV-1–transgenic rat model. In contrast to chronic alcohol ingestion, chronic HIV-1–transgene expression had no demonstrable effect on GM-CSFRα expression in the alveolar macrophage. However, HIV-1–transgene expression significantly decreased GM-CSFRβ expression, and impaired bacterial phagocytosis. We did not observe any significant differences in the apparent viability or gross morphology of alveolar macrophages from wild-type versus HIV-1–transgenic rats (data not shown). It is well established that down-regulation of GM-CSFR expression and/or its master transcription factor, PU.1, adversely impacts the functional capacity of alveolar macrophages (32, 33). This GM-CSF signaling pathway also includes activation of Janus kinase family and subsequent activation of STAT. Our results regarding the effect of the Zn chelator, TPEN, on the expression of intracellular GM-CSFβ signaling unit are intriguing, because GM-CSFRβc chain–dependent activation of STAT5A was shown to contribute to defective macrophage function in individuals infected with HIV (34). Therefore, it is plausible that the effects of HIV-1–related proteins, and the consequent Zn deficiency, on GM-CSFRβ expression alone are sufficient to explain the functional defects in the alveolar macrophage. However, we clearly cannot exclude other possible mechanisms, and the recent availability of this transgenic rat model will provide a great opportunity for further mechanistic studies.
Regardless of the precise mechanisms, these findings are provocative, as alveolar macrophage dysfunction has been identified in individuals infected with HIV-1 (6). For example, reduced phagocytic activity of macrophages for S. aureus and P. carinii from individuals infected with HIV-1 has been reported (7). Importantly, the direct treatment of alveolar macrophages with the HIV-1 envelope protein, gp120, inhibited antifungal activity and reduced phagocytosis (35), consistent with our findings in this transgenic model. Furthermore, this observation suggests that some of the manifestations of AIDS can be attributed to the cellular toxicities of the HIV-1–related proteins rather than to viral replication alone.
The critical role for Zn deficiency in this model is fascinating, but, in retrospect, is perhaps not that surprising. Zn has been recognized as an important factor in the immune system for at least half a century (36). A central clinical feature of Zn deficiency is an increased susceptibility to infectious diseases, which led early researchers to speculate that Zn must be important for host immunity. Numerous studies have observed that even mild Zn deficiency can impair host immunity (37, 38), as is seen in individuals with chronic alcoholism and others that are immunosuppressed. Experimentally, Zn-deficient animals are more susceptible to a diverse range of infectious agents, including herpes virus, bacteria such as Mycobacterium tuberculosis, and Candida. In 1998, a clinical study showed that Zn supplementation reduced the incidence of acute lower respiratory infections in children in a double-blind, placebo-controlled study (39); however, the potential mechanisms were not examined. In patients with HIV infection, disease progression is accompanied by a decrease in serum Zn concentration (40), and these changes are at least partially reversible by Zn supplementation (30). In a murine model of AIDS, the amount of Zn in many tissues, including the spleen, was depressed; of note, these effects were exacerbated by alcohol consumption (41).
Interestingly, we found that, in this HIV-1–transgenic rat, the Zn concentrations in the alveolar space and in the alveolar macrophages decreases significantly with age. This is consistent with the observation that these animals are only mildly affected when they are young, but, with aging, develop a profound AIDS-like phenotype characterized by renal failure, muscle wasting, and respiratory problems. We did not assess whether or not this age-related phenotype is associated with progressively increased expression of HIV-1–related proteins, but this and other questions will be addressed as this unique model is studied in greater detail. The finding that Zn levels in the lungs progressively decline as the AIDS-like phenotype evolves is fascinating and raises the possibility that prolonged dietary Zn supplementation could slow the progression of HIV-1–related lung immune dysfunction.
This study does suggest that a direct connection between Zn deficiency and alveolar macrophage dysfunction during chronic HIV-1–transgene expression is plausible. Specifically, we treated a rat alveolar macrophage cell line with or without TPEN (a Zn chelator). Interestingly, TPEN-treated cells showed a similar dysfunctional phenotype to that seen in the HIV-1–transgenic rats, as reflected by decreased GM-CSFRβ expression and bacterial phagocytosis. Furthermore, supplementing intracellular Zn levels improved both GM-CSFRβ expression and bacterial phagocytosis by alveolar macrophages from HIV-1–transgenic rats. Taken together, these findings suggest that Zn bioavailability within the alveolar space modulates alveolar macrophage immune function in the setting of chronic HIV-1 expression. Future studies will need to address how HIV-1–related proteins limit Zn bioavailability in the alveolar space. Potential mechanisms would include abnormalities in Zn transporters that control intracellular levels. The fact that Zn supplementation improves alveolar macrophage Zn levels and immune function suggests that, whatever the defect may be, it is amenable to supplemental Zn and, therefore, clinical dietary treatments may prove to be effective.
In summary, these findings are clinically relevant, as they provide novel insights into the fundamental mechanisms by which HIV-1–related proteins impair host immune defense and increase the susceptibility to pulmonary infections. In addition, these findings raise the possibility that it may be beneficial to give Zn supplementation to individuals infected with HIV-1 to protect their lower airways from microbial infections. Because Zn is an essential micronutrient that is known to improve immune function, we can speculate that treating patients infected with HIV-1 with moderate amounts of Zn supplements as an adjunctive therapy could prevent, or at least limit, pulmonary infections, and perhaps nonpulmonary infections as well. Such therapy may be of enormous value in the developing countries, where illness and malnutrition can severely impair the immune system. Further studies are warranted to explore the roles for Zn bioavailability in the alveolar microenvironment of individuals infected with HIV-1, and whether Zn transporters or other cellular processes that regulate Zn levels are altered in this context. The unique HIV-1–transgenic rat model provides an exciting new tool to examine some of these questions in the laboratory and translate these findings to the clinical setting.
This work was supported by National Institutes of Health grants R21AA016271 and R01 AA011660 (D.G.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0344OC on February 28, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Thomas C, Limper A. Pneumocystis pneumonia. N Engl J Med 2004;350:2487–2498. [DOI] [PubMed] [Google Scholar]
- 2.Lin J, Nichol K. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome. Arch Intern Med 2001;161:441–446. [DOI] [PubMed] [Google Scholar]
- 3.Hirschtick R, Glassroth J, Jordan M, Wilcosky T, Wallace J, Kvale P, Markowitz N, Rosen M, Mangura B, Hopewell P. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med 1995;333:845–851. [DOI] [PubMed] [Google Scholar]
- 4.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest 2000;117:1017–1022. [DOI] [PubMed] [Google Scholar]
- 5.Thomas C, Limper A. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007;5:298–308. [DOI] [PubMed] [Google Scholar]
- 6.Stebbing J, Gazzard B, Douek D. Where does HIV live? N Engl J Med 2004;350:1872–1880. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese A, Vidotto V, Beltramo T, Torre D. Phagocytic activity in human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol 2005;12:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noursadeghi M, Katz D, Miller R. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis 2006;6:794–804. [DOI] [PubMed] [Google Scholar]
- 9.Klotman P, Notkins A. Transgenic models of human immunodeficiency virus type-1. Curr Top Microbiol Immunol 1996;206:197–222. [DOI] [PubMed] [Google Scholar]
- 10.van Maanen M, Sutton R. Rodent models for HIV-1 infection and disease. Curr HIV Res 2003;1:121–130. [DOI] [PubMed] [Google Scholar]
- 11.Bruggeman L, Dikman S, Meng C, Quaggin S, Coffman T, Klotman P. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 1997;100:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA 2001;98:9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell B. Granulocyte macrophage-colony stimulating factor augmentation therapy in sepsis: is there a role? Am J Respir Crit Care Med 2002;166:129–130. [DOI] [PubMed] [Google Scholar]
- 14.Joshi P, Applewhite L, Mitchell P, Fernainy K, Roman J, Eaton D, Guidot D. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol 2006;291:1150–1158. [DOI] [PubMed] [Google Scholar]
- 15.Pelaez A, Bechara R, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony–stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L106–L111. [DOI] [PubMed] [Google Scholar]
- 16.Joshi PC, Applewhite L, Ritzenthaler J, Roman J, Fernandez A, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony–stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol 2005;175:6837–6845. [DOI] [PubMed] [Google Scholar]
- 17.Prasad AS. Discovery of human Zn deficiency: impact on human health. Nutrition 2001;17:685–687. [DOI] [PubMed] [Google Scholar]
- 18.Zalewski PD. Zinc metabolism in the airway: basic mechanisms and drug targets. Curr Opin Pharmacol 2006;6:237–243. [DOI] [PubMed] [Google Scholar]
- 19.Truong-Tran AQ, Carter J, Ruffin R, Zalewski PD. New insights into the role of zinc in the respiratory epithelium. Immunol Cell Biol 2001;79:170–177. [DOI] [PubMed] [Google Scholar]
- 20.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med 2004;37:1182–1190. [DOI] [PubMed] [Google Scholar]
- 21.Izumi Y, Auberson YP, Zorumski CF. Zinc modulates bidirectional hippocampal plasticity by effects on NMDA receptors. J Neurosci 2006;26:7181–7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie GG, Oteiza PI. Zinc and the cycloskeleton in the neuronal modulation of transcription factor NFAT. J Cell Physiol 2007;210:246–256. [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS. The role of zinc in gastrointestinal and liver disease. Clin Gastroenterol 1983;12:713–741. [PubMed] [Google Scholar]
- 24.Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol Aspects Med 2005;26:391–404. [DOI] [PubMed] [Google Scholar]
- 25.Scrimgeour AG, Stahl CH, McClung JP, Marchitelli LJ, Young AJ. Moderate Zn deficiency negatively affects biochemical properties of rat tibiae independently of body composition. J Nutr Biochem 2007;18:813–819. [DOI] [PubMed] [Google Scholar]
- 26.Meunier N, O'Connor JM, Maiani G, Cashman KD, Secker DL, Ferry M, Roussel AM, Coudray C. Importance of zinc in the elderly: the ZENITH study. Eur J Clin Nutr 2005;59:S1–S4. [DOI] [PubMed] [Google Scholar]
- 27.Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15–mediated human NK cell function through down-regulation of common γ-chain. J Immunol 2001;166:885–891. [DOI] [PubMed] [Google Scholar]
- 28.Busetto S, Trevisan E, Patriarca P, Menegazzi R. A single-step, sensitive flow cytofluorometric assay for the simultaneous assessment of membrane-bound and ingested Candida albicans in phagocytosing neutrophils. Cytometry 2004;58:201–206. [DOI] [PubMed] [Google Scholar]
- 29.Jones CY, Tang AM, Forrester JE, Huang J, Hendricks KM, Knox TA, Spiegelman D, Semba RD, Woods MN. Micronutrient levels and HIV disease status in HIV-infected patients on highly active antiretroviral therapy in the Nutrition for Healthy Living cohort. J Acquir Immune Defic Syndr 2006;43:475–482. [DOI] [PubMed] [Google Scholar]
- 30.Wellinghausen N, Kern WV, Jochle W, Kern P. Zinc serum level in human immunodeficiency virus–infected patients in relation to immunological status. Biol Trace Elem Res 2000;73:139–149. [DOI] [PubMed] [Google Scholar]
- 31.Afessa B, Green B. Clinical course, prognostic factors, and outcome prediction for HIV patients in the ICU: the PIP (Pulmonary complications, ICU support, and prognostic factors in hospitalized patients with HIV) study. Chest 2000;118:138–145. [DOI] [PubMed] [Google Scholar]
- 32.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage–mediated innate host defense. Annu Rev Physiol 2002;64:775–802. [DOI] [PubMed] [Google Scholar]
- 33.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MC, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony–stimulating factor. Am J Physiol Lung Cell Mol Physiol 2003;285:L1132–L1136. [DOI] [PubMed] [Google Scholar]
- 34.Warby TJ, Crowe SM, Jaworowski A. Human immunodeficiency virus type 1 infection inhibits granulocyte-macrophage colony stimulating factor–induced activation of STAT5A in human monocyte–derived macrophages. J Virol 2003;77:12630–12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pittis MG, Sternik G, Sen L, Diez RA, Planes N, Pirola D, Estevez ME. Impaired phagolysosomal fusion in peripheral blood monocytes from HIV-1 infected subjects. Scand J Immunol 1993;38:423–427. [DOI] [PubMed] [Google Scholar]
- 36.Wellinghausen N, Kirchner H, Rink L. The immunobiology of zinc. Immunol Today 1997;18:519–521. [DOI] [PubMed] [Google Scholar]
- 37.Fischer WC, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004;24:255–275. [DOI] [PubMed] [Google Scholar]
- 38.Wirth JJ, Fraker PJ, Kierszenbaum F. Zinc requirement for macrophage function: effect of Zn deficiency on uptake and killing of a protozoan parasite. Immunology 1989;68:114–119. [PMC free article] [PubMed] [Google Scholar]
- 39.Black RE. Therapeutic and preventive effects of zinc on serious childhood infectious diseases in developing countries. Am J Clin Nutr 1998;68:476S–479S. [DOI] [PubMed] [Google Scholar]
- 40.Baum MK, Shor-Posner G, Campa A. Zinc status in human immunodeficiency virus infection. J Nutr 2000;130:1421S–1423S. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Liang B, Watson RR. The effect of alcohol consumption on nutritional status during murine AIDS. Alcohol 1994;11:273–278. [DOI] [PubMed] [Google Scholar]