Abstract
Although there is now convincing evidence that the infectivity of human immunodeficiency virus type 1 (HIV-1) is increased by incorporation of host intercellular adhesion molecule 1 (ICAM-1) in budding virions, the exact mechanism(s) through which ICAM-1 can so significantly affect HIV-1 biology remains obscure. To address this question, we focused our attention on the most proximal events in the virus life cycle. We made comparative analyses to estimate attachment and internalization of isogenic HIV-1 particles either lacking or bearing host-derived ICAM-1. Using attachment-and-entry assays and confocal fluorescence microscopy, we found that virus binding and uptake were both markedly enhanced by insertion of ICAM-1 within the virus envelope when PM1 lymphoid cells and primary human cells (i.e., peripheral blood lymphocytes and purified CD4+ T cells) were used as targets. Moreover, ICAM-1-bearing virions entered cells with faster uptake kinetics than viruses devoid of ICAM-1. Experiments conducted with fully competent viruses further confirmed the positive effect of virion-anchored host ICAM-1 on HIV-1 replication. Interestingly, subcellular-fractionation assays revealed that ICAM-1 incorporation modifies the HIV-1 entry route by increasing the level of viral material released in the cytosol, a process of internalization known to be mediated mainly by pH-independent membrane fusion and to result in productive infection. A virion-based fusion assay confirmed that the acquisition of ICAM-1 increases the efficiency of productive HIV-1 entry in primary CD4+ T lymphocytes. These observations provide new insights into how interactions other than those with gp120 and CD4-coreceptor complex can modulate the process of productive HIV-1 infection in CD4+ T lymphocytes, a cell target highly relevant to HIV-1 pathogenesis.
The initial binding of a virus to the surfaces of target cells represents a determinant factor in achieving efficient mammalian cell invasion. The first intimate contact between the virion and the cell surface must be rapid and strong to promote stable adhesion and to concentrate the virus at the cell surface. It is now well established that infection of CD4+ T lymphocytes by human immunodeficiency virus type 1 (HIV-1) mainly requires interaction between the viral glycoproteins gp120 and gp41 and a cellular complex made of CD4 and an appropriate coreceptor molecule (e.g., CCR5 or CXCR4). However, the importance of these cell surface structures in the process of HIV-1 attachment to host cells has been brought into question by recent observations. For example, the association between gp120 and CD4 is not sufficient per se to capture the virus (30), since the affinity of trimeric gp120 for CD4 is too low to permit firm adhesion of HIV-1 to the cell surface (15, 41). Moreover, a number of cell types can bind and/or be infected by the virus in the absence of CD4 expression. In such instances, HIV-1 may attach to these cells by using cell surface constituents other than CD4. It has been shown that sugar groups on the external envelope glycoprotein gp120 interact with sugar groups or lectin-like domains present on cell surface receptors, such as heparan sulfate (3, 26, 32, 39), galactosylceramide (12, 17), and dendritic cell-specific intercellular adhesion molecule 3 binding C-type lectin (DC-SIGN) (7). It was recently demonstrated that interactions between gp120 and CD4-CXCR4 do not influence the attachment of a T-cell line-adapted strain of HIV-1 (i.e., NL4-3) to different T lymphoid cell lines and primary cells (30).
The mechanism of HIV-1 entry into susceptible cells is a cooperative process requiring numerous events. First, interaction between gp120 and CD4 leads to conformational changes in gp120 which permit the subsequent ligation with the appropriate coreceptor. Second, a rearrangement of the transmembrane gp41 glycoprotein results in exposure of a fusion domain that is necessary for fusion of viral and cellular membranes. Electron microscopy analyses revealed that HIV-1 can also enter via a clathrin-mediated endocytic pathway in CD4+ T lymphocytes following fusion of the virus envelope with the vesicular membrane through a pH-independent mechanism (11, 33, 34). Although both entry processes seem to occur, data indicate that fusion taking place at the cell surface membrane is the most efficient route leading to a productive HIV-1 infection, since endocytosed viruses are directed toward a degradation pathway (25). Viral and/or cellular factors determining the route of virus entry (i.e., fusion between viral and cellular membranes versus vesicular uptake) remain almost undefined.
Despite the fact that productive infection of CD4+ T lymphocytes relies heavily on binding of virion-associated gp120 to cellular CD4, the process of attachment to these cells clearly implicates other cell surface constituents. Moreover, although CD4+ T lymphocytes are considered the major productive cellular reservoir of HIV-1, few studies have investigated the nature of cell surface molecules other than CD4 which might participate in HIV-1 attachment to such target cells. Several lines of evidence convincingly demonstrate that the association between virus-anchored proteins of host origin and their natural ligands on the target cell surface plays a key role in the initial phase of the HIV-1 life cycle (13, 14, 30, 31). Considering that multiple CD4 and coreceptor molecules are needed to permit virus entry (9), the additional virus-cell interactions might trigger membrane destabilization leading to redistribution of CD4 and chemokine coreceptors in the cellular membrane, an event that might facilitate creation of the fusion pore.
The cellular membrane contains a diversity of lipid microdomains asymmetrically distributed in the lipid bilayer. Lipid rafts are considered microdomains enriched in glycosphingolipids, glycophosphatidylinositol-anchored proteins, signaling molecules, and cholesterol. These microdomains are involved in numerous biological functions, such as endocytosis, membrane trafficking, cell morphogenesis, and signal transduction (6, 19, 28, 43). Moreover, these structures are also involved in the internalization of many pathogens, such as Mycobacterium bovis (16), Chlamydia trachomatis (29), simian virus 40 (35), and filoviruses (10). Interestingly, it has been reported that lipid raft integrity is essential for HIV-1 entry (20, 23, 24, 36, 48). Since cholesterol is crucial for the maintenance of lipid rafts, it was argued that depletion of this molecule from the plasma membrane would impair the process of HIV-1 infection. Fusion requires colocalization of receptor and coreceptor molecules that have been described as accumulating in microvilli on the surface of the T-cell membrane (44, 45). Studies have demonstrated that the molecules are located differently on the plasma membrane. For example, CD4 has been found in lipid rafts, whereas CXCR4 was located outside these microdomains. Hence, binding of HIV-1 must trigger lateral movement of membrane proteins to bring together CD4 and CXCR4. Accordingly, it was demonstrated that binding of HIV-1 to cell surface CD4 present in raft domains disturbs the plasma membrane and promotes the movement of virus-engaged CD4 molecules near to regions outside lipid rafts that are rich in CXCR4 (20). It was also observed that treatment of peripheral blood lymphocytes with a cholesterol-depleting drug reduced the colocalization of CD4 and CXCR4 with F-actin in microvilli, resulting in an impairment of HIV-1 fusion with the plasma membrane (48). It can thus be proposed that the first steps in the HIV-1 replicative cycle (i.e., attachment and fusion) will be favored by virus-associated host molecules that possess a strong affinity for their physiological counterligands, especially if such interactions mediate signaling events resulting in cytoskeleton-dependent recruitment of coreceptors to the attachment interface.
It is now well accepted that HIV-1 incorporates a vast array of host membrane molecules during its budding process, including intercellular adhesion molecule 1 (ICAM-1) (4, 13, 46). The engagement of ICAM-1 with its counterligand, LFA-1, on the cell surface enhances virus infectivity (13). This phenomenon is in agreement with the role played by LFA-1 in the immune response. Indeed, LFA-1 mediates the adhesion and migration of leukocytes during immune and inflammatory processes following its interaction with ICAM-1. In resting cells, integrins exist predominantly in the closed conformation. However, after binding to ICAM-1, the conformation state of LFA-1 changes to an open conformation that further enhances the affinity of LFA-1 for ICAM-1 (40). Clustering of LFA-1 following cell activation is also an important process in increasing ligand binding. Strikingly, LFA-1 acts not only as an adhesion receptor but also as a signaling receptor, since the engagement of this integrin with dimeric ICAM-1 initiates both polymerization and reorganization of F-actin, events that are known to be essential for many biological processes, including membrane fluidity (37). The primary objective of this study was to determine whether additional interactions between host-derived ICAM-1 within mature HIV-1 particles and cell surface LFA-1 affect the route of virus entry into CD4-expressing T lymphoid cells and primary human cells, such as CD4+ T lymphocytes.
(This work was performed by M.R.T. in partial fulfillment of requirements for the Ph.D. degree from the Microbiology-Immunology Program, Faculty of Medicine, Laval University.)
MATERIALS AND METHODS
Cells.
PM1 cells were obtained through the AIDS Research and Reference Reagent Program (Division of AIDS, National Institute of Allergy and Infectious Diseases, Rockville, Md.), and 293T cells were provided by W. C. Greene (The J. Gladstone Institutes, San Francisco, Calif.). The 293T cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (FCS), while the PM1 cells were cultured in RPMI 1640 medium supplemented with 10% FCS. Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll-Hypaque gradient centrifugation, whereas CD4+ T cells were purified from freshly isolated PBMCs by immunomagnetic negative selection (Miltenyi Biotec, Auburn, Calif.) as instructed by the manufacturer. The purity of CD4+ T cells was determined by cytofluometry analysis and was always >97%. Isolated PBMCs and purified CD4+ T cells were cultured for 2 days in RPMI medium supplemented with 10% FCS in the presence of phytohemagglutinin (1 μg/ml) and recombinant human interleukin-2 (rhIL-2) (50 U/ml).
Antibodies and reagents.
The anti-LFA-1 monoclonal antibodies MEM30 and MEM83 were kindly provided by V. Horejsi (Institute of Molecular Genetics, Prague, Czech Republic). Hybridomas that produce 183-H12-5C and 31-90-25, two antibodies recognizing different epitopes of the HIV-1 major viral core protein p24, were supplied by the AIDS Repository Reagent Program and the American Type Culture Collection (Manassas, Va.), respectively. Antibodies obtained from these cells were purified with mAbTrap protein G affinity columns (Amersham Biosciences, Inc., Uppsala, Sweden) according to the manufacturer's instructions. R-phycoerythrin-conjugated goat anti-mouse immunoglobulin G was purchased from Jackson ImmunoResearch Laboratories (West Grove, Pa.).
Plasmids and production of viral stocks.
pNL4-3 is a full-length infectious molecular clone of HIV-1, while pNL4-3 Luc+E−R+ produces Env-deficient reporter HIV-1 particles. These vectors were provided by the AIDS Repository Reagent Program. The pHCMV-G molecular construct codes for the broad-host-range vesicular stomatitis virus envelope glycoprotein G (VSV-G) under the control of the human cytomegalovirus promoter. pCD1.8 is a eukaryotic expression vector containing the entire human ICAM-1 (a generous gift from T. Springer, Center for Blood Research, Boston, Mass.). In some experiments, 293T cells were cotransfected with pNL4-3 Luc+E−R+ and pHCMV-G to produce VSV-G pseudotypes. β-Lactamase-tagged virions were produced by cotransfection of 293T cells with pNL4-3 and pCMV-BlaM-Vpr, a vector encoding a β-lactamase- Vpr fusion protein (provided by W. C. Greene). Viruses differing only in the absence or the presence of host-encoded ICAM-1 proteins on their surfaces were produced by the calcium phosphate coprecipitation method in 293T cells as described previously (13). Virus preparations were normalized for virion content by using an in-house enzymatic assay specific for the major viral p24 protein as described previously. In this test, 183-H12-5C and 31-90-25 are used in combination to quantify p24 levels (4).
Virus binding assay.
PM1 cells, PBMCs, or purified CD4+ T lymphocytes (1 × 106 or 3 × 106) were resuspended in 1 ml of culture medium containing isogenic HIV-1 particles either devoid of (NL4-3) or bearing (NL4-3/ICAM-1) host-derived ICAM-1 (100 or 300 ng of p24 per 106 cells) and were incubated at 37°C for different times. Next, the cells were extensively washed with ice-cold phosphate-buffered saline (PBS) to remove unbound viruses, transferred to fresh tubes, and finally lysed in 150 μl of ice-cold lysis buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, 0.5% Triton X-100). The virus content was assessed by monitoring the level of p24.
Virus entry assay.
Similar experimental conditions were used to estimate the process of virus entry, except that an additional step with trypsin was included to eliminate uninternalized virus. For virus entry kinetic studies, cells were incubated at 37°C with viruses for 5, 15, and 45 min for PM1 cells and 30, 90, and 180 min for PBMCs and purified CD4+ T lymphocytes. To monitor the role played by LFA-1 in the process of virus entry, cells were pretreated for 1 h at 37°C with an anti-LFA-1 antibody (MEM30) before the addition of virus. Following infection, the cells were washed and trypsinized for 5 min at 37°C to remove uninternalized viruses. Next, the cells were first washed once with RPMI supplemented with 10% FCS and then three times with PBS before lysis. The number of viruses entering cells was estimated by the p24 assay.
Infectivity analysis.
PBMCs and PM1 and CD4+ T cells (105) were incubated for 1 h at 37°C with isogenic NL4-3 or NL4-3/ICAM-1 virions (10 ng of p24). In some experiments, the cells were pretreated with an anti-CD4 antibody (i.e., SIM.2 at 10 μg/ml) or SDF-1 (500 ng/ml) for 30 min at 37°C to block either the CD4 primary receptor or the CXCR4 coreceptor before infection. The cells were then trypsinized for 5 min at 37°C to remove uninternalized viruses, washed with RPMI supplemented with 10% FCS, and extensively washed with PBS. Subsequently, the cells were resuspended in 200 μl of complete culture medium supplemented with rhIL-2 for PBMCs and purified CD4+ T cells and transferred into 96-well flat-bottom tissue culture plates. After 48 h of incubation, 150 μl of cell supernatant was harvested and frozen at −20°C until it was assayed by p24 enzyme-linked immunosorbent assay.
Cell fractionation assay.
PBMCs and PM1 and CD4+ T cells (5 × 106) were resuspended in 2.5 ml of culture medium containing isogenic NL4-3 or NL4-3/ICAM-1 virions (500 ng of p24 per 106 cells) or VSV-G pseudotypes (250 ng of p24) in six-well tissue culture plates. The cells were incubated at 37°C for either 90 min (PM1 cells) or 4 h (PBMCs and CD4+ T lymphocytes). The cells were washed with ice-cold PBS and trypsinized for 5 min at 37°C to eliminate uninternalized viruses. The cells were then washed with RPMI supplemented with 10% FCS, followed by three washes with ice-cold PBS. Cellular membranes were disrupted by resuspending the cells in 1 ml of ice-cold hypotonic buffer (10 mM Tris-HCl [pH 7.5], 10 mM KCl, and 1 mM EDTA) for 1 min before Dounce homogenization (three strokes with 7-ml B pestles). Nuclei, cell debris, and undamaged cells were pelleted by centrifugation (1,800 rpm for 5 min at 4°C in a Sorvall RT600B). Supernatants that contained the cytosol and vesicles (including endosomes) were centrifuged at 12,000 rpm for 90 min at 4°C in a Heraeus centrifuge. The supernatant representing the cytosolic fraction was adjusted to 0.5% Triton X-100, while the pellet (i.e., the vesicular fraction) was resuspended in 1 ml of lysis buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, and 0.5% Triton X-100). The levels of p24 present in both fractions were measured by enzyme-linked immunosorbent assay.
Fusion assay.
Primary CD4+ T lymphocytes (2 × 106) were pretreated with MEM83 and exposed for 2 h at 37°C to BlaM-Vpr-tagged virions (500 ng of p24). The virus-cell mixture was next extensively washed with PBS before incubation with the CCF2/AM dye (Aurora Bioscience, San Diego, Calif.) as described by the manufacturer. Briefly, 2 μl of CCF2/AM (1 mM) were mixed with 8 μl of a solution made of 0.1% acetic acid containing 100 mg of Pluronic-F127R/ml in 1 ml of CO2-independent medium (Gibco) to constitute the loading solution. The cells were incubated for 1 h at room temperature and then washed two times with CO2-independent medium. The BlaM reaction was maintained for 24 h at room temperature in 200 μl of CO2-independent medium supplemented with 10% FCS and 2.5 mM probenecid (Sigma), an inhibitor of anion transport. Finally, the cells were washed two times with PBS and fixed in 1 ml of a 2% solution of paraformaldehyde. The emission spectra of CCF2/AM (520 nm) and its cleaved product (447 nm) upon excitation at 409 nm were monitored with an 8000C spectrofluorometer (SLM AMINCO; SLM Instruments Inc., Urbana, Ill.).
Confocal microscopy.
PM1 cells and purified CD4+ T lymphocytes (3 × 105) were pretreated with the anti-LFA-1 MEM30 antibody for 30 min on ice or left untreated and then incubated for 90 min at 37°C with isogenic NL4-3 or NL4-3/ICAM-1 virions (100 ng of p24). The cells were washed three times with PBS, fixed in 2% paraformaldehyde for 20 min, and permeabilized for 4 min at 4°C with 0.3% Triton X-100 in PBS. The cells were next incubated with pooled human sera from HIV-1-positive patients for 45 min at 37°C to stain the viruses, followed by incubation with goat anti-human immunoglobulin secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, Oreg.). After several washes, slides were mounted in 90% glycerol in PBS. Bound and internalized viruses were visualized by confocal laser scanning microscopy (Olympus Fluoview FV300), and the digital images were processed with Adobe Photoshop. All the images were taken under similar experimental conditions (i.e., exposure time), and the processing was also the same for all the images shown in this study.
Statistical analysis.
The results presented are expressed as means ± standard deviations of triplicate samples. Statistical significance between groups was first accomplished by analysis of variance. Calculations were made with Microsoft Excel software. P values of <0.05 were considered statistically significant.
RESULTS
Virus attachment and internalization are both increased by the insertion of ICAM-1 into the HIV-1 envelope.
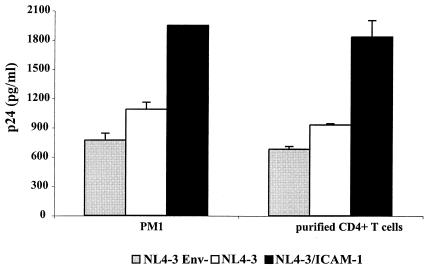
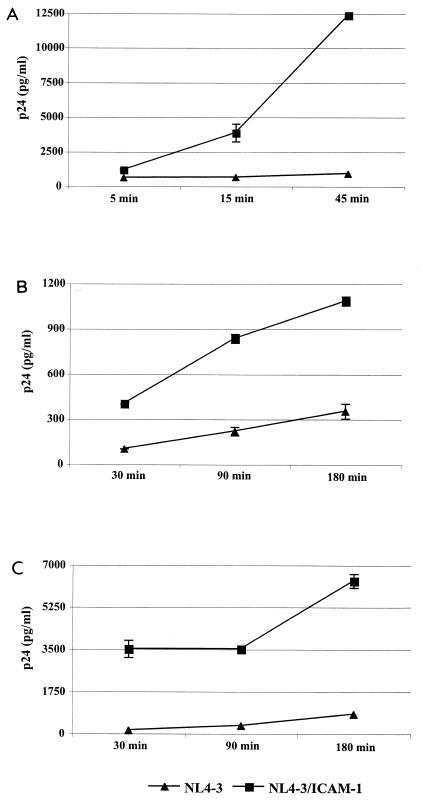
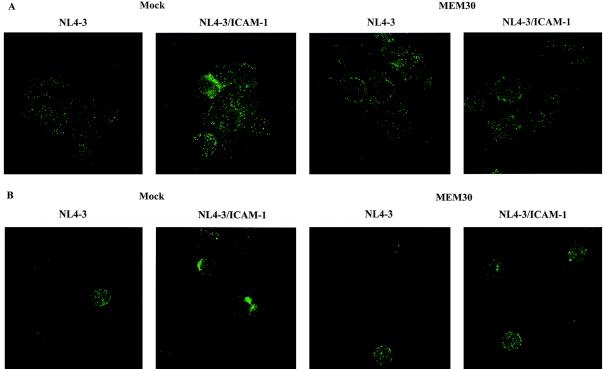
To decipher the contribution of virus-associated host ICAM-1 to HIV-1 adsorption and entry, isogenic virions either lacking or bearing ICAM-1 were incubated at 37°C with the T lymphoid PM1 cell line and purified CD4+ T lymphocytes for 45 and 90 min, respectively. The nonspecific attachment or internalization was measured by using envelope-deficient viruses. The amounts of virus that bound to PM1 and primary CD4+ T cells were enhanced by ICAM-1 incorporation (Fig. 1). These data indicate that the presence of a protein of host origin, such as ICAM-1, on the virion surface enhances the very first steps in the life cycle of HIV-1. We observed that the modulatory effect of ICAM-1 on virus attachment and entry was more dramatic at 37°C than when the virus-cell mixture was kept at 4°C (data not shown). Experiments aimed at testing the kinetics of virus binding and internalization events revealed that ICAM-1-bearing viruses attach and enter more rapidly than isogenic viruses lacking host ICAM-1 (Fig. 2). Differences at the earliest time point tested (i.e., 30 min) are more pronounced when primary human CD4+ T lymphocytes are used as targets. To further confirm the positive up-regulatory effect of ICAM-1 on the most proximal events in HIV-1 biology, we next examined the cellular uptake of HIV-1 by immunofluorescence analysis. PM1 cells and purified CD4+ T lymphocytes were again incubated at 37°C for 45 and 90 min, respectively, with equal amounts of p24 for each virus preparation and analyzed by confocal fluorescence microscopy using purified polyclonal anti-HIV-1 antibodies from pooled human sera. The involvement of cell surface LFA-1 in the attachment and entry of ICAM-1-bearing viruses was assessed by the use of a blocking anti-LFA-1 antibody (i.e., MEM30). A representative experiment depicted in Fig. 3 shows that virus-specific dots are more abundant in both cell types following inoculation with ICAM-1-bearing virions than after inoculation with viruses devoid of host ICAM-1. The augmentation of virus-specific dots for ICAM-1-bearing HIV-1 virions is abolished when interactions between ICAM-1 and LFA-1 are inhibited by MEM30. Interestingly, ICAM-1-bearing viruses are concentrated on specific areas of the target cell when infection is performed with purified CD4+ T lymphocytes at 37°C (Fig. 3B), suggesting that the process of virus entry requires a specific region on the cellular membrane.
FIG. 1.
Insertion of ICAM-1 into mature progeny virus enhances HIV-1 adsorption and entry. PM1 cells and purified CD4+ T lymphocytes (106) were incubated at 37°C for 45 and 90 min, respectively, with similar amounts of the indicated virus stocks (100 ng of p24). The cells were then extensively washed, lysed, and tested for p24. Experiments were performed in triplicate, and standard deviations are indicated. The data shown are representative of two separate experiments.
FIG. 2.
Kinetics of early steps in the HIV-1 life cycle is accelerated upon ICAM-1 incorporation. PM1 cells (A), PBMCs (B), and purified CD4+ T lymphocytes (C) (3 × 106) were incubated at 37°C for the indicated times with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (300 ng of p24). The cells were then extensively washed, lysed, and tested for p24. Experiments were performed in triplicate, and standard deviations are indicated. The data shown are representative of three separate experiments.
FIG. 3.
Confocal microscopy analyses confirm that the most proximal events in the virus replicative cycle are more efficient after insertion of host ICAM-1 into HIV-1. PM1 cells (A) and purified CD4+ T lymphocytes (B) were exposed at 37°C for 45 and 90 min, respectively, to the virus preparations studied. Next, the cells were extensively washed, fixed, and labeled as described in Materials and Methods. (Left) Cells were left untreated (Mock) before being infected with HIV-1. (Right) Cells were pretreated with a blocking anti-LFA-1 antibody (MEM30) before being incubated with HIV-1.
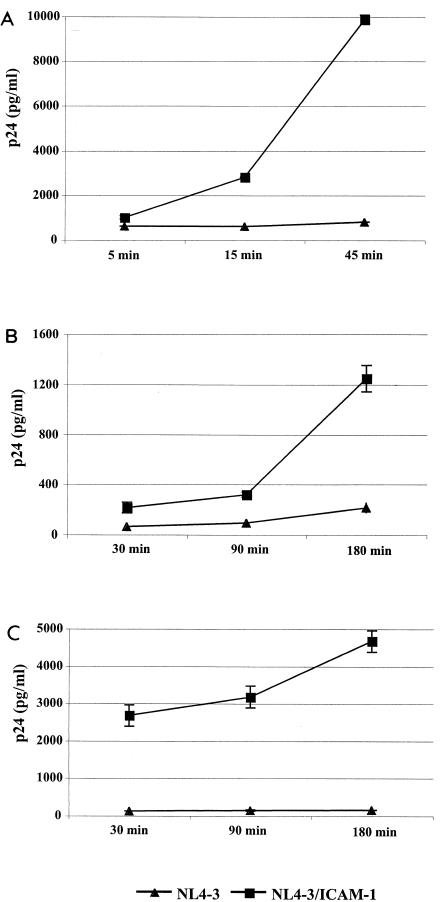
Next, we monitored the level of intracellular viral material by removing noninternalized virus particles attached to the cell surface. In agreement with our previous findings, virus entry kinetic studies revealed that ICAM-1-bearing virions entered the studied cells more rapidly and efficiently than their ICAM-1-negative counterparts (Fig. 4). Again, the most striking difference between the two isogenic virus preparations was observed in primary CD4+ T cells (Fig. 4C). The increase noted in the process of HIV-1 internalization is due to the specific association between virus-associated ICAM-1 and cell surface LFA-1, since it is abolished by the blocking anti-LFA-1 MEM30 antibody (Fig. 5). We also tested the importance of lipid raft integrity in HIV-1 internalization in both PM1 T cells and purified CD4+ T lymphocytes. To this end, cells were treated with methyl-β-cyclodextrin (MβCD) in an attempt to induce cholesterol efflux and disturb lipid rafts. Intact lipid rafts were found to be necessary to allow efficient entry of HIV-1 lacking or bearing host-derived ICAM-1 (data not shown).
FIG. 4.
Virus-anchored host ICAM-1 confers faster kinetics of HIV-1 internalization. PM1 cells (A), PBMCs (B), and purified CD4+ T lymphocytes (C) (3 × 106) were incubated at 37°C for the indicated times with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (300 ng of p24). The cells were next extensively washed with PBS and treated with trypsin to remove uninternalized virions before lysis to quantify the amount of virus that had entered the cells. Experiments were performed in triplicate, and standard deviations are indicated. The data shown are representative of three separate experiments.
FIG. 5.
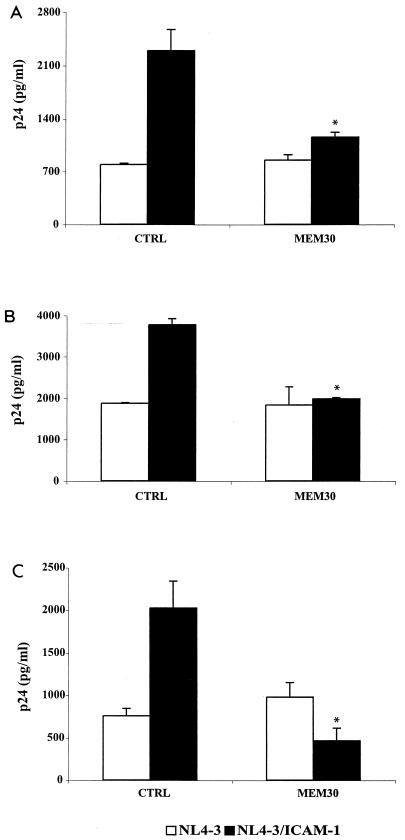
ICAM-1- LFA-1 interactions are responsible for more rapid attachment and fusion of ICAM-1-bearing virions. PM1 cells (A), PBMCs (B), and purified CD4+ T lymphocytes (C) were initially pretreated for 60 min at 37°C with the blocking anti-LFA-1 antibody MEM30. The cells were next incubated at 37°C for 30 min with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (100 ng of p24). The cells were extensively washed with PBS and treated with trypsin to remove uninternalized virions before lysis to quantify the amount of virus that had entered the cells. Experiments were performed in triplicate, and standard deviations are indicated. The data shown are representative of three separate experiments. The asterisks indicate a significant difference from cells inoculated with NL4-3/ICAM-1 that were not treated with MEM30 (CTRL) (P < 0.02).
Productive HIV-1 infection is augmented in established and primary CD4+ T cells upon insertion of host ICAM-1 into the viral envelope.
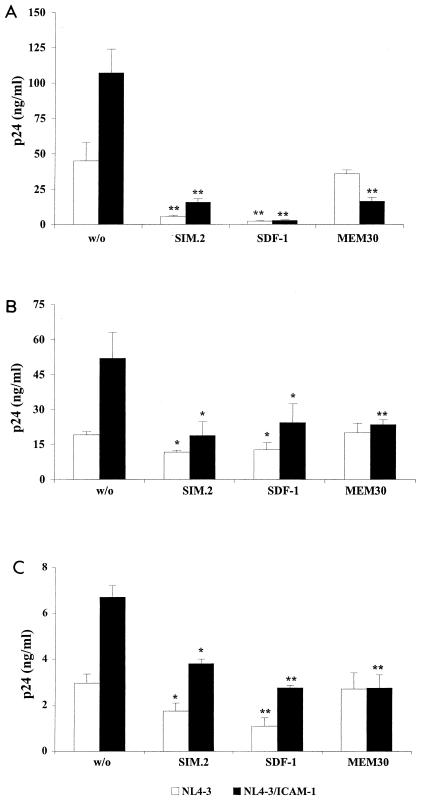
Previous works have clearly established that virus-anchored ICAM-1 positively affects HIV-1 infectivity in T lymphocytes (4, 13, 38). These observations were made using recombinant reporter viruses, i.e., incomplete virus in which the nef gene was replaced with a reporter gene (either chloramphenicol acetyltransferase or luciferase). To more closely parallel natural infection, fully infectious viruses either bearing or not bearing ICAM-1 were used to infect PM1 cells, PBMCs, and purified CD4+ T lymphocytes, and HIV-1 replication was monitored by measuring p24. As illustrated in Fig. 6, virus production was significantly more important for cells inoculated with ICAM-1-bearing virions than for cells infected with a similar amount of virus devoid of ICAM-1. The contribution of ICAM-1- LFA-1 interactions to this phenomenon was proven by demonstrating that pretreatment of target cells with MEM30 restored virus production to comparable levels (i.e., viruses lacking ICAM-1) independently of the source of the virus used for infection. Experiments performed with an anti-CD4 antibody (i.e., SIM.2), which inhibits the association between gp120 and CD4, and a blocking agent of the chemokine coreceptor CXCR4 (i.e., SDF-1) suggest that interactions of CD4 and CXCR4 with the HIV-1 envelope spike glycoprotein are equally important for the process of infection with viruses lacking or bearing ICAM-1 on their surfaces. Thus, both receptor and coreceptor are required for optimal virus entry and productive infection in the presence or absence of the host ICAM-1 in the mature viral entity.
FIG. 6.
Replication of fully competent HIV-1 particles is augmented by ICAM-1 incorporation through interactions between ICAM-1 and LFA-1. PM1 cells (A), PBMCs (B), and purified CD4+ T lymphocytes (C) were left untreated (w/o) or were treated with MEM30, SIM.2, or SDF-1. The cells were then incubated at 37°C for 60 min with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (10 ng of p24). The cells were trypsinized to remove uninternalized virus and were resuspended in complete culture medium. After 48 h, cell supernatants were collected and virus production was estimated by assessing p24 production. Experiments were performed in triplicate, and standard deviations are indicated. The data shown are representative of three separate experiments. The asterisks indicate a significant difference from cells infected with the listed virus preparations and left untreated (*, P < 0.05; **, P < 0.025).
The presence of host ICAM-1 in mature T-tropic HIV-1 particles affects the route of virus entry and promotes fusion at the cellular membrane.
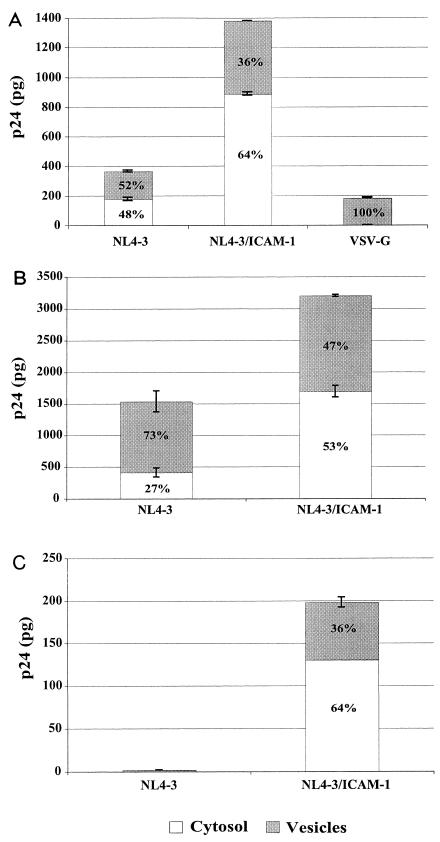
It has been demonstrated that HIV-1 enters susceptible cells either via intracellular vesicles, which leads to degradation by lysosomal enzymes, or via cytosolic delivery, which results in productive infection (25). In this work, a significant fraction of intracellular p24 was found to be present in intracellular vesicles. In another study, the vesicular uptake of p24 represented 62% of the total intracellular p24 when HIV-1 virions were incubated with Jurkat T lymphoid cells (42), whereas another study using trophoblastic cells showed that >85% of the intracellular p24 was found in the vesicular fraction (49). With the aim of testing whether the entry pathway could be modulated in T cells by the insertion of ICAM-1 within HIV-1, virus-infected cells were fractionated into separate subcellular cytosolic and vesicular fractions. Cells inoculated with HIV-1 particles pseudotyped with VSV-G were used as controls, because it targets virus entry to an endocytic route (1). The data shown in Fig. 7A demonstrate that following exposure of PM1 cells for 90 min to NL4-3 particles devoid of ICAM-1, ∼48% of total intracellular p24 appeared in the cytosolic fraction, in contrast to 64% when infection is allowed to proceed with isogenic ICAM-1-bearing virions. Cytosolic delivery of NL4-3/ICAM-1 progeny virus was further augmented when PM1 cells were pretreated with an anti-LFA-1 antibody that activates LFA-1 (i.e., MEM83) (data not shown). To eliminate the possibility that a fraction of the observed cytosolic p24 could result from disruption of endosomes while isolating the vesicular fraction, experiments were performed with VSV-G pseudotypes. The validity of our technique is confirmed by the observation that 100% of total intracellular p24 is located in the vesicular fraction once PM1 cells are incubated for 90 min with HIV-1 particles pseudotyped with VSV-G. Measurements of p24 were made in PBMCs and CD4+ T cells following a 4-h exposure because cytosolic p24 levels were not detectable at the earlier time points tested (i.e., 30 and 90 min) (data not shown). Cytosolic p24 levels were higher when PBMCs were exposed to NL4-3/ICAM-1 progeny virus, representing 53% of total intracellular p24 compared to 27% with HIV-1 particles devoid of host ICAM-1 (Fig. 7B). Levels of p24 were also higher in the cytosolic fraction (i.e., 64 versus 36%) when primary human CD4+ T lymphocytes were exposed to ICAM-1-bearing virions (Fig. 7C). Unfortunately, comparative analyses could not be done because p24 levels in both fractions were below the detection limit of the p24 assay even following exposure of purified CD4+ T lymphocytes for 4 h to isogenic viruses devoid of ICAM-1.
FIG. 7.
ICAM-1 incorporation favors cytosolic delivery of internalized virions. (A) PM1 cells (3 × 106) were incubated at 37°C for 90 min with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (300 ng of p24). PM1 cells were also exposed to HIV-1 particles pseudotyped with VSV-G envelope (100 ng of p24). (B and C) In some instances, PBMCs (B) and purified CD4+ T lymphocytes (C) (7 × 106) were incubated at 37°C for 240 min with similar amounts of isogenic NL4-3 particles either bearing or not bearing ICAM-1 (700 ng of p24). After virus exposure, the cells were washed, trypsinized, and resuspended in a swelling buffer. The cells were then disrupted by Dounce homogenization, and the cellular fractions (i.e., cytoslic and vesicular) were separated as described in Materials and Methods. The level of p24 in each fraction was monitored by a p24 assay. The percentages of cytosolic and vesicular p24 are indicated inside each bar. Experiments were performed in triplicate, and the data shown are representative of three separate experiments. The error bars indicate standard deviations.
In order to confirm that ICAM-1-bearing viruses were entering more efficiently into biologically relevant target cells (i.e., CD4+ T lymphocytes) than isogenic viruses lacking host ICAM-1, we performed a recently developed enzyme-based fusion assay (5, 27). This assay permits discrimination between the processes of HIV-1 entry by fusion or endocytosis. Primary CD4+ T cells were left uninfected or inoculated with HIV-1 that was either Env deficient, lacking ICAM-1, or bearing ICAM-1 and that was also loaded with an enzymatically active β-lactamase- Vpr fusion protein (i.e., BlaM-Vpr). Target cells were next exposed to the CCF2/AM dye, a BlaM substrate that is cleaved by the cytoplasmic BlaM-Vpr (for more details of the BlaM reaction, see reference 5). Emission of the cleaved substrate upon excitation at 409 nm was read at 447 nm with a spectrofluorometer, whereas the uncleaved substrate was read at 520 nm. The ratio of the intensities of the two wavelengths is the best way to compare results under each condition. It indicates the degree of BlaM-mediated cleavage with minimal interference from variations in cell size, probe concentration, and excitation and emission intensities (51). The ratios of the intensities at 447 and 520 nm shown in Table 1 indicate that virus uptake by fusion as monitored by changes in the signal-to-noise ratio was augmented by an average of 22% upon infection with NL4-3/ICAM-1 particles. Under the experimental conditions used, no change in the signal-to-noise ratio was seen when infection was allowed to proceed with either Env-deficient viruses or virions lacking host-encoded ICAM-1. Altogether, our data indicate that insertion of ICAM-1 into mature HIV-1 promotes the most proximal events in the virus life cycle (i.e., attachment and fusion) and favors delivery of the viral material into the cytoplasm.
TABLE 1.
Fusion of BlaM-Vpr-tagged virions into primary CD4+ T cells is enhanced by incorporation of host ICAM-1 into HIV-1
| Experiment no. | 447/520 ratioa
|
Change over uninfected cells (n-fold)b
|
|||||
|---|---|---|---|---|---|---|---|
| Mock | NL4-3 Env− | NL4-3 | NL4-3/ICAM-1 | NL4-3 Env− | NL4-3 | NL4-3/ICAM-1 | |
| 1 | 0.28 | 0.283 | 0.282 | 0.34 | 1 | 1 | 1.21 |
| 2 | 0.35 | 0.35 | 0.34 | 0.437 | 1 | 0.97 | 1.25 |
| 3 | 0.23 | 0.23 | 0.23 | 0.274 | 1 | 1.05 | 1.19 |
Ratio of intensities at 447 and 520 nm. Mock, mock infected.
The change (n-fold) is the ratio of the infected cell population to the mock-infected cells.
DISCUSSION
The initial attachment step between HIV-1 and the cell surface is critical for the virus replicative cycle. It is becoming clear that sequential interactions between virus-associated constituents and different cell surface molecules are required to promote adhesion and efficient entry into target cells. Although HIV-1 uses CD4 and CXCR4-CCR5 as the primary cellular receptor and coreceptors to infect human T cells, Olinger and coworkers have proposed that other virus-cell interactions are implicated in virus attachment to the surfaces of CD4-expressing T cells (30). It has been postulated that proteins of host origin that are acquired by HIV-1 could play a cardinal role in the early steps of the virus replicative cycle.
ICAM-1 has been reported to be inserted in mature HIV-1 particles and to increase virus infectivity severalfold in different experimental cell systems, such as in lymphoid T-cell lines, peripheral blood lymphocytes, and human lymphoid tissue cultured ex vivo (4, 13). The precise mechanism(s) through which the incorporation of this adhesion molecule can augment virus infectivity is still unclear. In this report, we made comparative analyses of isogenic viruses either lacking or bearing ICAM-1 and estimated the attachment and fusion process, as well as intracellular p24 in both vesicular and cytosolic fractions. Since lymphoid cells are natural targets of HIV-1, these studies were performed in an established T-cell line (i.e., PM1) and primary cells (i.e., unseparated PBMCs and purified CD4+ T lymphocytes). We found that the initial attachment step was accentuated by the presence of ICAM-1 in the virion envelope. Virus internalization was also up-regulated when target cells were exposed to ICAM-1-bearing viruses. Our findings suggest that the enhancing effect is optimal at a temperature that approaches physiological temperatures (i.e., 37°C). This observation is not a surprise, since thermodynamic processes, such as inside-out signaling, membrane fluidity, and actin cytoskeleton reorganization, regulate LFA-1 activity. Moreover, internalization of viruses either devoid of or bearing ICAM-1 was more rapid and efficient in PM1 cells than in primary cells. This observation is most likely related to the reported colocalization of CD4 and CXCR4 within the same lipid microdomains in PM1 cells (36), which is in sharp contrast to the situation prevailing in T lymphocytes, where the two cell surface molecules are segregated into separate membrane microdomains (20).
The use of integrins to gain entry into host cells is not restricted to HIV-1 but is a process shared by several viruses. In fact, echovirus (2), adenovirus (50), rotavirus (18), and human parechovirus (47) have all been shown to use integrins to bind to and/or penetrate target cells. Interestingly, all these viruses enter cells by endocytosis. Typically, enveloped viruses exploit three distinct pathways to penetrate target cells: (i) fusion between viral and cellular membranes, a process that uses HIV-1 to gain entry into CD4+ T lymphocytes; (ii) endocytosis via clathrin-coated pits; and (iii) endocytosis through caveolae or rafts. A possible change in HIV-1 entry that would be mediated by virus-associated ICAM-1 was tested by using fusion-incompetent viruses. Productive infection of human CD4+ T cells with ICAM-1-bearing viruses could not be achieved with fusion-deficient virus (data not shown), suggesting that fusion between viral and cellular membranes remains the principal route by which such viruses are internalized in CD4-expressing T lymphocytes. Moreover, both CD4 and CXCR4 are necessary to achieve a productive infection of CD4+ T cells, suggesting that viruses bearing ICAM-1 use the same receptor and coreceptor as viruses lacking this adhesion molecule to penetrate such target cells. Nevertheless, the mechanism through which each virus creates an optimal environment for the formation of the fusion pore might be different and favored when ICAM-1 is located on the virion surface.
It has been shown that lipid rafts are microdomains necessary for HIV-1 entry. In fact, depletion of cholesterol, as well as targeting CD4 to nonraft domains, abolishes HIV-1 infection in T-cell lines (8, 23, 24). While the distribution of CD4 and coreceptors in lipid rafts on purified CD4+ T cells has not yet been investigated, it was demonstrated that CXCR4, in contrast to CD4, is almost excluded from raft microdomains in mitogen- IL-2-treated PBMCs (20). Thus, the plasma membrane should allow a lateral movement of CXCR4 close to CD4 for HIV-1 infection to proceed. In order to investigate whether membrane fluidity and lipid raft integrity are still important for infection with ICAM-1-bearing virions, target cells were treated with MβCD. Penetration of viruses bearing and not bearing ICAM-1 was affected to the same level following MβCD treatment. These data suggest that both viruses require the movement of cellular proteins into membrane microdomains to achieve infection. Experiments are under way to define whether LFA-1 is localized near or inside lipid rafts upon binding of HIV-1 either lacking or bearing host ICAM-1. The possible presence of CD4 and CXCR4 in distinct membrane compartments will also be investigated following the attachment of these progeny viruses.
Subcellular-fractionation assays were performed with the tested virus preparations because HIV-1 internalization leading to productive infection has been shown to result from fusion events at the cell surface and not from endosomal uptake (25). It was proposed that vesicular uptake of HIV-1 does not involve appropriate envelope-receptor interactions and most likely results from nonspecific adhesion of HIV-1 particles to the cell surface. Interestingly, the process of ICAM-1 incorporation affected the distribution of intracellular p24 in the cytosolic and vesicular fractions. Indeed, after exposure to ICAM-1-bearing virions, cytosolic p24 represented a higher proportion of total intracellular p24 than following infection with HIV-1 particles devoid of ICAM-1. The increase in cytosolic p24 distribution, which is linked to a true infection process, is corroborated by our virus infectivity studies. Given that the cytosolic p24 value represents an indicator of HIV-1 entry events leading to successful infectious processes (25), our data suggest that ICAM-1 incorporation encourages viruses toward a route of entry ending in a more productive infection pathway. The use of virions loaded with β-lactamase- Vpr chimeric proteins confirmed that fusion and delivery into the cytoplasm are both accentuated upon the acquisition of ICAM-1 by HIV-1.
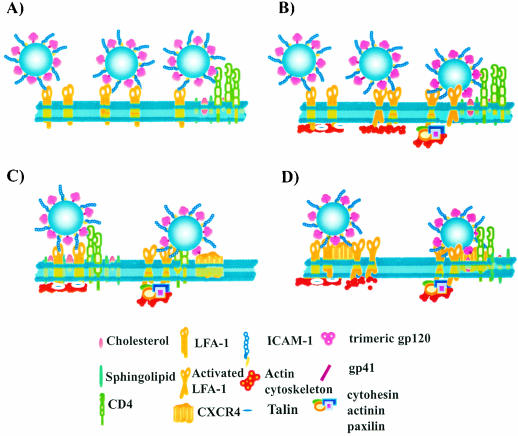
The main difficulty encountered by HIV-1 in achieving successful entry lies in the fact that the process is complex and cooperative, since it involves multiple Env proteins, as well as numerous CD4 glycoproteins and chemokine coreceptors that have to act in a concerted manner. Another obstacle that HIV-1 has to surmount in T lymphocytes is the fact that CD4 and CXCR4 are segregated into distinct cell surface microdomains (i.e., CD4 in lipid rafts and CXCR4 in nonraft regions). Therefore, the virus must destabilize the plasma membrane microenvironment to allow the formation of the fusion pore (20). Several hypotheses based on specific properties of the integrin can explain the higher proportion of the viral p24 input that reaches the cytosol once host ICAM-1 is found embedded in mature HIV-1. Since LFA-1- ICAM-1 interactions contribute to T-lymphocyte tethering and rolling in shear flow (21), it can be proposed that virus-embedded ICAM-1 promotes the rolling of virions onto the surfaces of target cells until a sufficient number of gp120-CD4 interactions are initiated (Fig. 8A). Alternatively, ligation of virus-anchored host ICAM-1 and cell surface LFA-1 can trigger LFA-1 activation, an event leading to mobilization of LFA-1 to the lipid raft and thus closer to CD4 (Fig. 8B). It can also be hypothesized that signal transduction events initiated by the association between LFA-1 and its ligand (i.e., ICAM-1) can mediate a remodeling of the cytoskeleton, resulting in destabilization of the plasma membrane and facilitation of secondary interactions with CXCR4 (Fig. 8C). This idea is supported by a recent study showing that the F-actin cytoskeleton is remodeled by ICAM-1-mediated signaling through LFA-1 (37). Finally, LFA-1 has a propensity to form complexes with other proteins and to allow their movement into lipid rafts upon cell activation (22). It is thus conceivable that LFA-1, which is excluded from lipid rafts in resting T cells (22), might be located near CXCR4 and that this complex could migrate to CD4-containing membrane rafts following the binding of ICAM-1-bearing HIV-1 particles to the cell surface (Fig. 8D). It should be emphasized that a possible effect on the lateral movement of cell surface molecules into the plasma membrane is a common denominator to explain how ICAM-1 incorporation can promote HIV-1 fusion and authentic cell infection. Studies are in progress to delineate the precise mechanism(s) of action of virus-anchored host ICAM-1 on the early events in HIV-1 biology in CD4+ T lymphocytes.
FIG. 8.
Proposed hypothetical models to explain how insertion of host ICAM-1 within HIV-1 results in an enhancement of virus attachment-fusion-internalization and cytosolic delivery. (A) Rolling of virus entity onto the cell surface due to the association between ICAM-1 and LFA-1 allows achievement of a sufficient number of interactions between gp120 and CD4. (B) Binding of virus-associated ICAM-1 to cell surface LFA-1 triggers activation of LFA-1, leading ultimately to a displacement of LFA-1 and thus of associated viruses toward lipid rafts that contain CD4. (C) Attachment of ICAM-1-bearing viruses to the surfaces of LFA-1-expressing cells can result in intracellular signal transduction events that facilitate interactions with the appropriate chemokine coreceptor. (D) A complex made of LFA-1 and CXCR4 can move into raft microdomains following binding of ICAM-1-bearing virions, leading to colocalization with CD4.
This study confirms previous observations indicating that even though CD4 and an appropriate coreceptor are needed for productive HIV-1 infection, there are other virus-cell interactions that can contribute to a successful infectious process. A better understanding of local factors important for HIV-1 entry and productive virus infection is needed, considering that several entry inhibitors are under clinical development.
Acknowledgments
We thank M. Dufour for performing flow cytometric analyses and Caroline Gilbert for technical assistance with the virus fusion assay.
This work was financially supported by an operating grant to M. J. Tremblay from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (grant no. HOP-14438). M.R.T. holds a CIHR Doctoral Award, and M.J.T. is the recipient of the Canada Research Chair in Human Immuno-Retrovirology (tier 1 level).
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergelson, J. M., N. St John, S. Kawaguchi, M. Chan, H. Stubdal, J. Modlin, and R. W. Finberg. 1993. Infection by echoviruses 1 and 8 depends on the alpha 2 subunit of human VLA-2. J. Virol. 67:6847-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco, J., J. Barretina, A. Gutierrez, M. Armand-Ugon, C. Cabrera, B. Clotet, and J. A. Este. 2002. Preferential attachment of HIV particles to activated and CD45RO+CD4+ T cells. AIDS Res. Hum. Retrovir. 18:27-38. [DOI] [PubMed] [Google Scholar]
- 4.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 6.Clague, M. J., and S. Urbe. 2001. The interface of receptor trafficking and signalling. J. Cell Sci. 114:3075-3081. [DOI] [PubMed] [Google Scholar]
- 7.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Real, G., S. Jimenez-Baranda, R. A. Lacalle, E. Mira, P. Lucas, C. Gomez-Mouton, A. C. Carrera, A. C. Martinez, and S. Manes. 2002. Blocking of HIV-1 infection by targeting CD4 to nonraft membrane domains. J. Exp. Med. 196:293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 10.Empig, C. J., and M. A. Goldsmith. 2002. Association of the caveola vesicular system with cellular entry by filoviruses. J. Virol. 76:5266-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 12.Fantini, J., D. G. Cook, N. Nathanson, S. L. Spitalnik, and F. Gonzalez-Scarano. 1993. Infection of colonic epithelial cell lines by type 1 human immunodeficiency virus is associated with cell surface expression of galactosylceramide, a potential alternative gp120 receptor. Proc. Natl. Acad. Sci. USA 90:2700-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortin, J. F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J. Virol. 71:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 17.Harouse, J. M., S. Bhat, S. L. Spitalnik, M. Laughlin, K. Stefano, D. H. Silberberg, and F. Gonzalez-Scarano. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320-323. [DOI] [PubMed] [Google Scholar]
- 18.Hewish, M. J., Y. Takada, and B. S. Coulson. 2000. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J. Virol. 74:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonen, E. 2001. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13:470-477. [DOI] [PubMed] [Google Scholar]
- 20.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence, M. B., E. L. Berg, E. C. Butcher, and T. A. Springer. 1995. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur. J. Immunol. 25:1025-1031. [DOI] [PubMed] [Google Scholar]
- 22.Leitinger, B., and N. Hogg. 2002. The involvement of lipid rafts in the regulation of integrin function. J. Cell Sci. 115:963-972. [DOI] [PubMed] [Google Scholar]
- 23.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 24.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols, B. J., and J. Lippincott-Schwartz. 2001. Endocytosis without clathrin coats. Trends Cell Biol. 11:406-412. [DOI] [PubMed] [Google Scholar]
- 29.Norkin, L. C., S. A. Wolfrom, and E. S. Stuart. 2001. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp. Cell Res. 266:229-238. [DOI] [PubMed] [Google Scholar]
- 30.Olinger, G. G., M. Saifuddin, M. L. Hart, and G. T. Spear. 2002. Cellular factors influence the binding of HIV type 1 to cells. AIDS Res. Hum. Retrovir. 18:259-267. [DOI] [PubMed] [Google Scholar]
- 31.Paquette, J. S., J. F. Fortin, L. Blanchard, and M. J. Tremblay. 1998. Level of ICAM-1 surface expression on virus producer cells influences both the amount of virion-bound host ICAM-1 and human immunodeficiency virus type 1 infectivity. J. Virol. 72:9329-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 33.Pauza, C. D. 1991. The endocytic pathway for human immunodeficiency virus infection. Adv. Exp. Med. Biol. 300:111-138. [DOI] [PubMed] [Google Scholar]
- 34.Pauza, C. D., and T. M. Price. 1988. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 36.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter, J. C., M. Bracke, A. Smith, D. Davies, and N. Hogg. 2002. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J. Immunol. 168:6330-6335. [DOI] [PubMed] [Google Scholar]
- 38.Rizzuto, C. D., and J. G. Sodroski. 1997. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J. Virol. 71:4847-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roderiquez, G., T. Oravecz, M. Yanagishita, D. C. Bou-Habib, H. Mostowski, and M. A. Norcross. 1995. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J. Virol. 69:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salas, A., M. Shimaoka, S. Chen, C. V. Carman, and T. A. Springer. 2002. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin LFA-1. J. Biol. Chem. [DOI] [PubMed]
- 41.Sattentau, Q. J., J. P. Moore, F. Vignaux, F. Traincard, and P. Poignard. 1993. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J. Virol. 67:7383-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 44.Singer, I. I., S. Scott, D. W. Kawka, J. Chin, B. L. Daugherty, J. A. DeMartino, J. DiSalvo, S. L. Gould, J. E. Lineberger, L. Malkowitz, M. D. Miller, L. Mitnaul, S. J. Siciliano, M. J. Staruch, H. R. Williams, H. J. Zweerink, and M. S. Springer. 2001. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J. Virol. 75:3779-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffens, C. M., and T. J. Hope. 2003. Localization of CD4 and CCR5 in living cells. J. Virol. 77:4985-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 47.Triantafilou, K., M. Triantafilou, Y. Takada, and N. Fernandez. 2000. Human parechovirus 1 utilizes integrins αvβ3 and αvβ1 as receptors. J. Virol. 74:5856-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viard, M., R. Blumenthal, and Y. Raviv. 2002. Improved separation of integral membrane proteins by continuous elution electrophoresis with simultaneous detergent exchange: application to the purification of the fusion protein of the human immunodeficiency virus type 1. Electrophoresis 23:1659-1666. [DOI] [PubMed] [Google Scholar]
- 49.Vidricaire, G., M. R. Tardif, and M. J. Tremblay. 2003. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J. Biol. Chem. 278:15832-15841. [DOI] [PubMed] [Google Scholar]
- 50.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 51.Zlokarnik, G., P. A. Negulescu, T. E. Knapp, L. Mere, N. Burres, L. Feng, M. Whitney, K. Roemer, and R. Y. Tsien. 1998. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science 279:84-88. [DOI] [PubMed] [Google Scholar]