Abstract
WAVE2 regulates T cell receptor (TCR)–stimulated actin cytoskeletal dynamics leading to both integrin clustering and affinity maturation. Although WAVE2 mediates integrin affinity maturation by recruiting vinculin and talin to the immunological synapse in an Arp2/3-dependent manner, the mechanism by which it regulates integrin clustering is unclear. We show that the Abl tyrosine kinase associates with the WAVE2 complex and TCR ligation induces WAVE2-dependent membrane recruitment of Abl. Furthermore, we show that WAVE2 regulates TCR-mediated activation of the integrin regulatory guanosine triphosphatase Rap1 via the recruitment and activation of the CrkL–C3G exchange complex. Moreover, we demonstrate that although Abl does not regulate the recruitment of CrkL–C3G into the membrane, it does affect the tyrosine phosphorylation of C3G, which is required for its guanine nucleotide exchange factor activity toward Rap1. This signaling node regulates not only TCR-stimulated integrin clustering but also affinity maturation. These findings identify a previously unknown mechanism by which the WAVE2 complex regulates TCR signaling to Rap1 and integrin activation.
Introduction
Stimulation of the T cell receptor (TCR) by peptide–major histocompatibility complex on an antigen-presenting cell (APC) triggers the activation of biochemical signaling pathways that not only lead to changes in gene transcription but also cytoskeletal reorganization resulting in the formation of the immunological synapse (IS) (Billadeau et al., 2007). Through a process known as “inside-out” signaling, the activation of intracellular signaling proteins in response to TCR ligation ultimately results in the regulation of cell surface integrins (clustering and affinity maturation) required for the stable interaction between the T cell and APC (Kinashi, 2005). The resulting characteristic structure of the IS contains signaling proteins in the center of the supramolecular activation complex (SMAC), whereas integrins and integrin scaffolding proteins such as talin are found in the peripheral SMAC (Anton van der Merwe et al., 2000).
Integrins are heterodimeric cell surface receptors that are responsible for cell adhesion during several biological processes or, in the case of T cells, conjugation to an APC or target cell. Among the different integrins expressed by T cells, LFA-1 (αLβ2) has been shown to be critical for conjugate formation and binds to its ligand ICAM-1 found on APCs (Dustin and Springer, 1989). In addition, VLA-4 (α4β1) also functions in T cell activation by binding to VCAM-1 and the extracellular matrix protein fibronectin (Mobley et al., 1994), functioning as a costimulatory molecule (Shimizu et al., 1990), and also localizes to the peripheral SMAC of the IS (Mittelbrunn et al., 2004) where it might interact with CD14 on the APC (Humphries and Humphries, 2007). The regulation of integrins can occur as a result of clustering of individual subunits on the cell surface, thereby increasing avidity, or by changes in the conformation of the integrin itself, thereby increasing affinity. Although TCR engagement leads to changes in both integrin affinity and avidity, the molecular mechanisms that control these individual processes remain largely unknown.
Recent studies have suggested that Rap1, a member of the Ras family of small GTPases, is a critical regulator of integrin activation in response to stimulation of both the TCR and chemokine receptors. In fact, both expression of a constitutively active form of Rap1 using a transgenic mouse model (Sebzda et al., 2002) and the creation of a Rap1A knockout mouse (Duchniewicz et al., 2006) have demonstrated the importance of this protein in regulating immune cell adhesion. Several effectors of Rap1 (RAPL, PKD, and RIAM) have been described and each appears to be required for appropriate integrin clustering to occur in response to receptor stimulation, whereas integrin affinity, when examined, was largely unaffected (Katagiri et al., 2003; Medeiros et al., 2005; Menasche et al., 2007). Despite the fact that Rap1 is a key regulator of integrin activation, neither the signaling pathways used by the TCR to activate Rap1 nor the specific guanine nucleotide exchange factors (GEFs) that activate Rap1 have been completely elucidated.
The Abl family of tyrosine kinases has been implicated in regulating cell shape and motility through the regulation of F-actin dynamics in several cell types (Hernandez et al., 2004). In fact, PDGF stimulation of fibroblasts results in increased Abl kinase activity, as well as Abl-dependent F-actin–mediated membrane ruffling (Plattner et al., 1999). Abl kinases can potentially regulate F-actin cytoskeletal changes through an association with the Abi (Abl-interactor) proteins, which interact directly with Arp2/3 (actin-related protein 2/3) regulatory WAVE proteins (Innocenti et al., 2004). More recently, it has been shown that T cells deficient for both Abl kinases (Abl and Arg) show profound defects in development, proliferation, and cytokine production (Gu et al., 2007). Although this study and others have demonstrated the importance of these proteins in regulating cellular function, the mechanisms by which Abl controls actin-dependent processes in both immune and nonimmune cells remain obscure.
It has been previously shown that TCR-stimulated changes in integrin avidity and affinity are dependent on the cytoskeletal regulatory protein WAVE2 and the Arp2/3 complex (Nolz et al., 2006; Gomez et al., 2007). It was subsequently found that the integrin scaffolding protein vinculin is also involved in TCR-mediated integrin activation and regulates integrin affinity maturation through an association with WAVE2–Arp2/3 complex and recruitment of talin to the β-chain of the integrin (Nolz et al., 2007). In contrast to WAVE2-depleted T cells, which fail to accumulate F-actin or integrins at the IS, vinculin-depleted T cells accumulate F-actin and integrins, thus suggesting that WAVE2 regulates both aspects of integrin regulation, whereas other proteins, such as Rap1 effectors and vinculin, are responsible for regulating either affinity or avidity in response to TCR engagement.
Because of the involvement of WAVE2 and Rap1 in regulating integrin avidity, we sought to determine whether WAVE2 contributed to TCR-mediated activation of Rap1. Our data demonstrate that the WAVE2 complex is required for TCR-mediated Rap1 activation. We show that the tyrosine kinase Abl is associated with the WAVE2 complex in T cells and that WAVE2 is involved in the TCR-stimulated plasma membrane recruitment of the tyrosine kinase Abl and the CrkL–C3G complex, leading to the activation of Rap1. Overall, these data demonstrate that the WAVE2 complex participates in the localization of key regulators of Rap1, leading to integrin clustering and affinity maturation during T cell activation.
Results
WAVE2 is involved in TCR- and PMA-mediated adhesion to fibronectin and ICAM-1 in normal human T cells
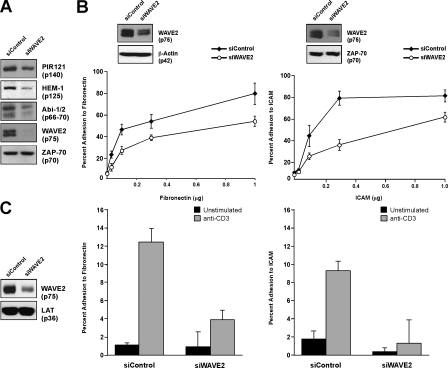
We have previously shown that WAVE2 is involved in TCR-stimulated changes in both integrin affinity and avidity in Jurkat T cells (Nolz et al., 2007). To determine if WAVE2 participated in adhesion in nontransformed primary human T cells, siRNA duplexes against WAVE2 were transfected into purified human CD4+ T cells, and the amount of suppression was determined by immunoblot. In agreement with previously published data, suppression of WAVE2 results in decreased protein levels of other WAVE complex members, including Abi-1 and 2, HEM-1, and PIR121 (Fig. 1 A). Because protein levels of the WAVE2 complex could be adequately decreased using this method, we next analyzed these cells for VLA-4–mediated adhesion to fibronectin in response to TCR stimulation. Stimulated T cells transfected with control siRNA showed limited basal binding to fibronectin but adhered readily to fibronectin in response to TCR stimulation, although not to wells containing only BSA (Fig. 1, B and C). However, cells in which WAVE2 had been suppressed were much less efficient at adhering to fibronectin at all concentrations tested (Fig. 1, B and C, left).
Figure 1.
WAVE2 is required for TCR-mediated adhesion to both fibronectin and ICAM-1 in primary human CD4+ T cells. (A) Purified human CD4+ T cells were transfected with either control siRNA (siControl) or siRNA against WAVE2 (siWAVE2). Whole cell extracts were then analyzed by immunoblot for expression of WAVE2, Abi-1/2, HEM-1, PIR121, and ZAP-70. (B and C) Cells were transfected as in A, stained with calcein AM, and added to a 96-well plate coated with increasing concentrations of fibronectin or ICAM-1-Fc (B) or 0.3 μg of each (C). Cells were then left unstimulated (C) or stimulated with anti-CD3 (B and C), and adhesion was determined as described in Materials and methods. Error bars represent SD.
Fibronectin is an extracellular matrix protein and adhesion to this molecule is mediated primarily through β1 integrins. In contrast, T cell–APC conjugation is primarily LFA-1 (αLβ2) mediated through the association with its ligand ICAM-1 found on the surface of the APC. Therefore, we next tested whether normal human T cells required WAVE2 for adhesion to ICAM-1 in response to TCR ligation. Similar to the results obtained with adhesion to fibronectin, WAVE2-suppressed T cells also demonstrated an overall reduction in adhesion to ICAM-1 in response to TCR stimulation (Fig. 1, B and C, right). Collectively, these data confirm the results obtained in Jurkat T cells, where WAVE2 is involved in TCR-mediated integrin activation leading to adhesion through both β1 and β2 integrins.
WAVE2 regulates TCR-mediated activation of Rap1
Rap1 is a small GTPase of the Ras family, and numerous studies have demonstrated the importance of this protein in regulating TCR-mediated integrin clustering and affinity maturation (Bos, 2005). We have previously shown that WAVE2 regulates the clustering of integrins and the recruitment of talin and vinculin to the IS leading to high-affinity integrin binding (Nolz et al., 2007). Thus, the WAVE2 complex may be involved in Rap1 activation leading to integrin clustering at the IS. We used shRNA against WAVE2 in the Jurkat T cell line and assessed Rap1 activation using a GST-tagged RalGDS GTPase binding domain (GBD). TCR-stimulated Jurkat T cells activated Rap1 rapidly, peaked at ∼1 min after stimulation, and returned to background levels by 5 min (Fig. 2 A). In contrast, WAVE2-suppressed cells were less effective at activating Rap1 in response to TCR stimulation when compared with the control cells (Fig. 2 A). Similarly, suppression of WAVE2 using siRNA in both human CD4+ and CD8+ T cells resulted in attenuated Rap1 activation downstream of the TCR (Fig. 2, B and C). The residual TCR-stimulated Rap1 activation seen in WAVE2-depleted cells may be caused by other TCR-regulated signaling pathways coupling to Rap1 activation or to incomplete suppression of WAVE2.
Figure 2.
WAVE2 is required for TCR-mediated Rap1 activation. (A) Jurkat T cells were transfected with either control vector or WAVE2 suppression vector and allowed to recover for 72 h. Cells were then stimulated with anti-CD3 for the indicated time, lysed, and GTP-bound Rap1 was detected using GST-RalGDS GBD and immunoblot. (B and C) Same as A except purified human CD4+ or CD8+ T cells were transfected with either siControl or siWAVE2 and stimulated with anti-CD3 for the indicated times. Black lines indicate that intervening lanes have been spliced out. (D and E) Jurkat T cells were transfected with the indicated vectors and stimulated with either anti-CD3 or 50 ng/ml PMA for 2 min. Subsequently, cytosolic and membrane fractions were prepared and probed as indicated. (F) Purified human CD4+ T cells were transfected as in B, stimulated with anti-CD3 over the indicated timecourse, and cell lysates were immunoblotted with a phospho-specific antibody against PAK (which cross-reacts with Mst1), total Mst1, and WAVE2. Numbers below top blots are arbitrary units based on densitometric analysis of the immunoblots.
Because WAVE2 regulates actin cytoskeletal remodeling, it is possible that actin reorganization may impact activation of Rap1. Cytochalasin D and latrunculin B are two actin-destabilizing toxins that prevent actin polymerization through different mechanisms. Treatment of purified human CD4+ T cells with either of these actin-destabilizing drugs resulted in impaired Rap1 activation in response to TCR stimulation (Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200801121/DC1). We had previously shown that WAVE2-dependent actin dynamics were required to recruit vinculin to the IS. We therefore examined whether vinculin suppression would affect TCR-stimulated Rap1 activation. In contrast to suppression of WAVE2 or pharmacological inhibition of actin dynamics, suppression of vinculin did not affect Rap1 activation after TCR ligation (Fig. S1 B). Thus, although actin dynamics (either WAVE2-dependent or -independent) are required for TCR-mediated Rap1 activation to occur, it is not mediated through vinculin.
Several downstream effectors of GTP-Rap1 have been identified that are required for the activation of inside-out signaling pathways leading to integrin activation. The nonconventional PKC isoform PKD (aka, PKCμ) binds to activated Rap1 through a noncanonical interaction within the pleckstrin homology domain of the protein, and both activated Rap1 and PKD localize to the cell membrane after receptor stimulation (Fig. 2, D and E). The interaction between PKD and activated Rap1 is also required for integrin clustering to occur in response to both TCR and PMA stimulation (Medeiros et al., 2005). We therefore determined whether WAVE2 suppression affected the recruitment of Rap1 or PKD to the cell membrane after cellular activation. Indeed, localization of Rap1 and PKD into the cell membrane is diminished after stimulation with either anti-CD3 or PMA in WAVE2-suppressed T cells (Fig. 2, D and E). In addition to PKD, GTP-Rap1 also associates with RapL, which will, in turn, form a complex with the kinase Mst1 (Katagiri et al., 2006), leading to its phosphorylation and activation, an event which is required for integrin clustering. Using an antibody that detects phosphorylation of Mst1 on threonine 183 (Katagiri et al., 2006), phosphorylated Mst1 could be detected in purified human CD4+ T cells after CD3 stimulation. However, WAVE2-suppressed CD4+ T cells fail to efficiently activate Mst1 in response to TCR stimulation (Fig. 2 F). Collectively, these data demonstrate that the WAVE2 complex is an essential component of signaling pathways initiated by the TCR, resulting in the plasma membrane localization and activation of Rap1 and its downstream effectors.
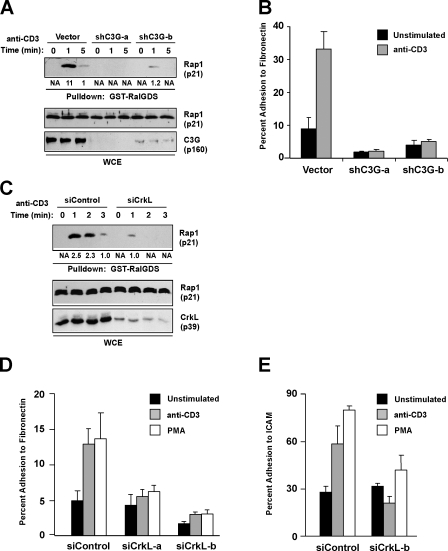
TCR-mediated activation of Rap1 is dependent on the adaptor CrkL and the GEF C3G
The GEF C3G can associate with the adaptor protein CrkL, and this CrkL–C3G complex has been implicated in regulating Rap1 activation in both BCR-ABL–transformed chronic myelogenous leukemia cells and other cell types (Sattler and Salgia, 1998; Arai et al., 1999; Ichiba et al., 1999; Sakakibara et al., 2002). Additionally, it has been proposed that this complex of proteins is involved in T cell adhesion because T cells lacking either c-Cbl or Cbl-b, two negative regulators of CrkL–C3G signaling, have increased adhesion (Shao et al., 2003; Zhang et al., 2003). However, it is unclear whether this complex regulates TCR-stimulated Rap1 activation. To determine whether C3G was required for Rap1 activation to occur in response to TCR stimulation, two different shRNAs against C3G were used in Jurkat T cells. Suppression of C3G dramatically impaired Rap1 activation (Fig. 3 A) and adhesion to fibronectin (Fig. 3 B) in response to TCR stimulation. Furthermore, suppression of CrkL using siRNA in human CD4+ T cells led to a defect in Rap1 activation (Fig. 3 C). Finally, suppression of CrkL in purified human CD4+ T cells impairs TCR-dependent adhesion to both fibronectin (Fig. 3 D) and ICAM-1 (Fig. 3 E). These data reveal that the CrkL–C3G complex is required for TCR-stimulated activation of Rap1 leading to integrin adhesion.
Figure 3.
C3G and CrkL are required for TCR-mediated activation of Rap1 and adhesion. (A) Jurkat T cells were transfected with the indicated suppression vectors, stimulated with anti-CD3, and Rap1 activation was determined. (B) Cells were transfected as in A, stained with calcein AM, and adhesion to 0.3 μg/well fibronectin was determined. (C) Purified human CD4+ T cells were transfected with the indicated siRNAs and Rap1 activation after anti-CD3 cross-linking was determined as described in Materials and methods. Numbers below top blots are arbitrary units based on densitometric analysis of the immunoblots. (D and E) Purified human CD4+ T cells were transfected with the indicated siRNAs and analyzed for the ability to adhere to 0.1 μg/well fibronectin or 0.3 μg/ml ICAM-1-Fc in response to either anti-CD3 or 50 ng/ml PMA. Error bars represent SD.
Effectors of Rap1 have been implicated in the regulation of integrin clustering and affinity maturation in response to both TCR and chemokine receptor stimulation in T cells (Katagiri et al., 2003; Medeiros et al., 2005). In agreement with this, localization of β1 integrins to the IS is impaired in both CrkL- and C3G-suppressed Jurkat T cells interacting with superantigen-pulsed Raji B cells (Fig. 4 A). Significantly, accumulation of F-actin at the IS was not affected in Jurkat T cells suppressed for either CrkL or C3G (Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200801121/DC1). In addition to affecting integrin clustering, CrkL and C3G are also required to generate the high-affinity state of β1 integrins, as demonstrated by an impaired ability to bind soluble VCAM-1-Fc in response to either TCR stimulation or treatment with PMA (Fig. 4 B). These data demonstrate that the CrkL–C3G complex regulates inside-out signaling pathways, leading to changes in both integrin affinity and avidity.
Figure 4.
CrkL and C3G regulate both integrin affinity and avidity. (A) Jurkat T cells were transfected with GFP-expressing control or suppression vectors targeting either CrkL or C3G. T cells (green) were allowed to form conjugates with superantigen (SEE)-pulsed Raji B cells (blue) and stained for β1 integrin (red) to determine localization of integrins to the IS. Data shown is the mean of three independent experiments. Bar, 5 μm. (B) Cells were transfected as in A and analyzed for their ability to bind soluble VCAM-1–Fc in response to either anti-CD3 or PMA stimulation. Stimulation with Mn2+ serves as a positive control for VCAM-1–Fc binding. Histograms represent VCAM-1–Fc binding in cell populations gated on different levels of GFP expression (negative, low, and high).
WAVE2 regulates the activation of the CrkL–C3G Rap1 exchange complex downstream of the TCR
Because CrkL and C3G are both required for TCR-mediated Rap1 activation and WAVE2-suppressed T cells are impaired in Rap1 activation, we next determined whether CrkL is recruited to the IS. For this experiment, we used latex beads coated with control mouse IgG or anti-CD3 because the high level of expression of CrkL in the Nalm 6 B cell line impaired visualization of CrkL recruitment to the IS. We did not observe any colocalization of F-actin with either WAVE2 or CrkL when Jurkat T cells were stimulated with mouse IgG-coated beads (unpublished data). However, we observed recruitment of F-actin and WAVE2, as well as F-actin and CrkL to the cell–bead contact site when stimulating with anti-CD3–coated beads (Fig. 5 A).
Figure 5.
WAVE2 is required for phosphorylation of CrkL and C3G. (A) Jurkat T cells were stimulated with OKT3-bound beads, and the localization of WAVE2 (green) or CrkL (green) and F-actin (red) was analyzed by confocal microscopy. Representative images are shown. Bar, 5 μm. (B) Jurkat T cells were transfected with the indicated vectors and stimulated with anti-CD3. Whole cell extracts were prepared and immunoblotted for pCrkL (Y207), total CrkL, and WAVE2. (C) Jurkat T cells were transfected with the indicated suppression/reexpression vectors, stimulated with anti-CD3, and whole cell extracts were immunoblotted as in B. (D) Jurkat T cells were transfected with the indicated expression vectors, stimulated with anti-CD3, and lysates immunoblotted with the indicated antibodies. (E) Jurkat T cells were transfected with control vector, CrkL-suppression vector, or suppression/reexpression vectors, which reexpressed either wild-type CrkL or point mutants disrupting the function of the SH2, SH3-N, or SH3-C domains. Rap1 activation was assessed as previously described. (F) Cells were transfected and stimulated as in B, and C3G was purified using a GST-CrkL SH3-N fusion protein and subsequently blotted using the indicated antibodies. (G) Purified human CD4+ T cells were transfected with the indicated siRNAs, stimulated with anti-CD3, and Rap1 activation and phosphorylation of CrkL was analyzed by immunoblot. Numbers below top blots are arbitrary units based on densitometric analysis of the immunoblots. Black lines indicate that intervening lanes have been spliced out.
Because WAVE2-suppressed T cells were found to be deficient in TCR-mediated Rap1 activation, we next wanted to determine whether the activation of CrkL and C3G was linked to the WAVE2 complex. CrkL is phosphorylated on Y207 by the Abl tyrosine kinase (de Jong et al., 1997; Zipfel et al., 2004). We therefore assessed whether WAVE2 suppression affected the phosphorylation of CrkL on Y207. Indeed, in response to TCR stimulation, CrkL is phosphorylated on Y207, and this phosphorylation was diminished in WAVE2-suppressed T cells (Fig. 5 B), suggesting the possibility that CrkL was not getting effectively recruited in WAVE2-suppressed cells. Interestingly, the effect of WAVE2 on CrkL phosphorylation appears to be independent of its ability to bind the Arp2/3 complex because reexpression of a ΔVCA version of WAVE2, as well as the wild-type version, are both sufficient to restore CrkL phosphorylation in WAVE2-suppressed T cells (Fig. 5 C). Moreover, suppression of Arp2/3 in Jurkat T cells does not affect Y207 phosphorylation of CrkL in response to TCR stimulation (JCN and DDB; unpublished data). In agreement with this finding, treatment of T cells with the actin depolymerizing agents cytochalasin D or latrunculin B, which abrogates Rap1 activation after TCR ligation, has no affect on TCR-stimulated tyrosine phosphorylation of CrkL (Fig. S1 C). These data suggest that although WAVE2 is required for CrkL phosphorylation in response to TCR engagement, it does so in an F-actin–independent manner.
It has been proposed that phosphorylation of CrkL on Y207 inhibits its localization through an autoinhibitory mechanism, where the SH2 domain of the protein binds with high affinity to a phosphorylated tyrosine between the amino- and carboxy-terminal SH3 domains (Feller et al., 1994; Rosen et al., 1995), thereby preventing the association with other binding partners and decreasing CrkL-mediated signaling. In fact, expression of an Y207F CrkL point mutant resulted in enhanced basal adhesion to fibronectin as compared with control and wild-type CrkL-expressing Jurkat T cells (Fig. S2, B and C). We therefore next determined which domains of CrkL were required for its phosphorylation and regulation of Rap1 after TCR ligation. Although wild-type CrkL underwent tyrosine phosphorylation, mutation of the critical residue within the SH2 domain (R39A) prevented TCR-stimulated phosphorylation of CrkL, whereas mutation of either SH3 domain had no effect (Fig. 5 D). In addition, we found that the CrkL SH2 domain and N-terminal SH3 domain (which binds to C3G) are involved in regulating Rap1 activation downstream of the TCR (Fig. 5 E). Collectively, these results suggest that the WAVE2 complex is involved in the recruitment of CrkL (via its SH2 domain) for optimal TCR-stimulated Rap1 activation (Fig. 6 C).
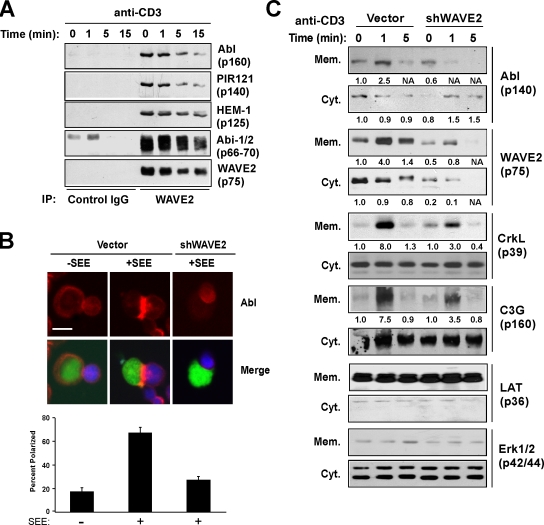
Figure 6.
The Abl tyrosine kinase interacts with and is localized to the membrane by the WAVE2 complex. (A) Purified human T cells were stimulated with anti-CD3. WAVE2 was immunoprecipitated and bound proteins were immunoblotted as indicated. (B) Control or WAVE2-suppressed Jurkat T cells (green) were allowed to form conjugates with antigen-pulsed Nalm 6 B cells (blue) and then stained for Abl (red). The percentage of T–B cell conjugates showing Abl accumulation at the IS were quantified as described in the Materials and methods. Error bars represent SD. Bar, 5 μm. (C) Jurkat T cells were transfected with either control or WAVE2 suppression vector and stimulated with anti-CD3. Subsequently, cytosolic and membrane fractions were collected as described in Materials and methods and immunoblotted with the indicated antibodies. Numbers below blots are arbitrary units based on densitometric analysis of the immunoblots.
The Rap1 GEF C3G can associate with the N-terminal SH3 domain of CrkL (Feller et al., 1995), and phosphorylation of Y504 within C3G regulates its GEF activity (Ichiba et al., 1999). To determine if C3G phosphorylation was affected by WAVE2 suppression, C3G protein was purified from cellular lysate using a GST N-terminal SH3 domain of CrkL (CrkL SH3-N; Fig. S2 D). Using this method, tyrosine phosphorylated C3G could be detected in TCR-stimulated cells, but this phosphorylation was diminished in WAVE2-suppressed Jurkat T cells (Fig. 5 F). In addition, C3G, although required for Rap1 activation, is not required for phosphorylation of CrkL on Y207 (Fig. 5 G). These data are consistent with the observation that although the N-terminal SH3 domain of CrkL is not required for TCR-stimulated phosphorylation of CrkL, it is required for Rap1 activation.
The WAVE2 complex regulates Abl tyrosine kinase membrane recruitment
Abl tyrosine kinases have been implicated in regulating both cytoskeletal dynamics and cellular adhesion (Woodring et al., 2003). In addition, WAVE2 is constitutively linked to the adaptor protein Abi-1 or Abi-2, which were identified as Abl kinase-interacting proteins (Dai and Pendergast, 1995; Shi et al., 1995). We therefore asked whether Abl is associated with the WAVE2 complex in T cells. Indeed, using two different antibodies against WAVE2, we found that Abl can be coimmunoprecipitated with the WAVE2 complex from human CD4+ T cells (Fig. S3 A, available at http://www.jcb.org/cgi/content/full/jcb.200801121/DC1). In addition, the SH3 domains of both Abi-1 and Abi-2 interact with Abl independently of the rest of the WAVE2 complex, whereas the SH3 domain of IRSp53, which binds the proline-rich region of WAVE2 directly, precipitates the entire WAVE2 complex, including Abl (Fig. S3 A). Additionally, Abl coimmunoprecipitates with WAVE2 from stimulated human CD4+ T cells throughout TCR stimulation (Fig. 6 A). Using an in vitro kinase assay, cytoplasmic Abl kinase activity does not appear to change in response to TCR stimulation (Fig. S4, A and B), but there is an increase in Abl kinase activity from the membrane fraction of TCR-stimulated cells (Fig. S4, C and D). Collectively, these data suggest that Abl is associated with the WAVE2 complex and that TCR stimulation results in Abl membrane recruitment where it can then phosphorylate its target substrates.
The defect of CrkL phosphorylation on Y207 in WAVE2-suppressed T cells suggested to us that Abl might not be properly recruited to its substrates without the WAVE2 complex. To test this, we first examined Abl cellular localization in T cell–Nalm 6 B cell conjugates formed with control and WAVE2-suppressed cells. Although Abl is efficiently recruited to the IS in control transfected T cells stimulated with SEE, Abl recruitment is diminished in T cells suppressed for WAVE2 (Fig. 6 B). To validate these results biochemically, membrane and cytosolic fractions from both control and WAVE2-suppressed Jurkat T cells were analyzed after TCR stimulation. Indeed, stimulation with anti-CD3 resulted in an increase in the amount of both WAVE2 and Abl in the membrane fraction of control cells but not in WAVE2-suppressed cells (Fig. 6 C). In fact, there appears to be more Abl in the cytoplasm of TCR-stimulated WAVE2-suppressed cells (Fig. 6 C). Interestingly, reprobing of these membranes indicates that WAVE2 suppression not only affects Abl membrane recruitment but also affects the recruitment of CrkL and C3G into the membrane fraction. Collectively, these data indicate that the WAVE2 complex is involved in the localization of Abl and the CrkL–C3G complex into the plasma membrane after TCR ligation.
Abl tyrosine kinase regulates TCR-stimulated Rap1 activation
The finding that Abl localization is defective in WAVE2-suppressed cells indicated that Abl kinase activity might be involved in regulating signaling pathways leading to the activation of Rap1. To test this hypothesis, we initially treated human CD4+ T cells with increasing concentrations of the Abl tyrosine kinase inhibitor Imatinib (Gleevec, STI-571) and assessed TCR-stimulated Rap1 activation and adhesion. Indeed, concentrations of Imatinib that sufficiently blocked phosphorylation of CrkL (5 and 10 μM) also resulted in impaired TCR-stimulated Rap1 activation and adhesion (Fig. S5, A–C). Because of the possibility that Imatinib could have off-target effects and/or inhibit the Abl-related kinase Arg, we decided to specifically target Abl using RNAi to directly examine the role of Abl in TCR-stimulated Rap1 activation and adhesion. Consistent with the Imatinib experiment, siRNA-mediated depletion of Abl from primary CD4+ T cells resulted in diminished TCR-stimulated Rap1 activation (Fig. 7 A) and Mst1 phosphorylation (Fig. 7 B) but had no effect on upstream signaling components such as phosphorylation of PLCγ1 (Fig. 7 C) or stability of the WAVE2 complex (Fig. S3 B). However, similar to the phenotype of CrkL-, C3G-, and WAVE2-suppressed T cells, suppression of Abl in either Jurkat T cells (Fig. 7 D) or primary CD4+ T cells (Fig. 7 E) resulted in impaired TCR-mediated adhesion to fibronectin and in the ability to bind soluble VCAM-1-Fc in response to both TCR and PMA stimulation (Fig. 7 F). Overall, these data demonstrate that the Abl tyrosine kinase is involved in a TCR signaling pathway, leading to Rap1 activation and integrin affinity maturation.
Figure 7.
Abl regulates TCR-mediated activation of Rap1 and integrin affinity maturation. (A) Purified human CD4+ T cells were transfected with the indicated siRNAs, and Rap1 activation was assessed after anti-CD3 cross-linking. Numbers below top blot are arbitrary units based on densitometric analysis of the immunoblots. (B) Same as A, except whole cell extracts were collected and analyzed by immunoblot for pMst1, Mst1, and Abl. (C) Jurkat T cells were transfected with suppression vectors against Abl (Abl-1 and Abl-3), stimulated with anti-CD3, and cell extracts were analyzed for pPLCγ1 (Y783), PLCγ1, pCrkL (Y207), CrkL, and Abl. (D and E) Jurkat T cells (D) or primary human CD4+ T cells (E) were suppressed for Abl and basal, and anti-CD3 and PMA-stimulated adhesion to fibronectin was analyzed as described in Fig. 1. Error bars represent SD. (F) Jurkat T cells were transfected with the indicated GFP-expressing suppression vector cells and analyzed for their ability to bind soluble VCAM-1-Fc as in Fig. 4 B.
Effect of Abl on CrkL–C3G membrane recruitment and activation
Because our data suggests that Abl regulates TCR-stimulated Rap1 activation and WAVE2 is involved in the recruitment of Abl into the membrane after TCR ligation, we reasoned that Abl might affect the membrane localization or activation of the CrkL–C3G complex. Consistent with the biochemical data shown in Fig. 6 C, we find that CrkL localization to the cell–bead contact site is affected in WAVE2-suppressed T cells (Fig. 8 A). In contrast, localization of CrkL to the cell–bead contact site, as well as the membrane recruitment of CrkL and C3G was only modestly affected in Abl-suppressed T cells (Fig. 8, A and B). Interestingly, suppression of Abl did not affect the recruitment of WAVE2 into the membrane fraction after TCR-stimulation (Fig. 8 B). Collectively, these data suggest that the recruitment of CrkL and C3G into the membrane is largely dependent on the WAVE2 complex.
Figure 8.
Affect of Abl suppression on CrkL–C3G membrane localization and activation. (A) Jurkat T cells transfected with control, WAVE2, or Abl EGFP suppression vectors were stimulated with IgG (column 1) or OKT3-coated beads (columns 2–4) and imaged for CrkL (aqua) and F-actin (red) recruitment. The percentage of EGFP+ T cell–bead conjugates showing localization of CrkL is indicated and was performed as described in Materials and methods. Representative images are shown. Bar, 5 μm. (B) Jurkat T cells were transfected with the indicated suppression vectors, stimulated by anti-CD3 cross-linking and cytosolic/membrane fractions, were prepared and immunoblotted with the indicated antibodies. Numbers below blots are arbitrary units based on densitometric analysis of the immunoblots. (C) Purified Abl and Fyn were incubated with the indicated GST fusion proteins in kinase buffer, and tyrosine phosphorylation was detected by anti-Tyr immunoblotting. Input levels of GST were detected by anti-GST immunoblotting. Numbers on the left are arbitrary units based on densitometric analysis of the immunoblots. (D) Jurkat T cells were transfected with the indicated suppression vectors, stimulated by anti-CD3 cross-linking, and C3G was precipitated as described in Fig. 5 E. Proteins were detected by immunoblotting with the indicated antibodies. Numbers below blots are arbitrary units based on densitometric analysis of the immunoblots.
Although our data indicate that Abl is involved in TCR-stimulated Rap1 activation, the recruitment of C3G into the membrane fraction of Abl-suppressed cells after TCR ligation was only moderately affected. It has been previously demonstrated that Src can phosphorylate Y504 within C3G to regulate its GEF activity (Ichiba et al., 1999). We therefore examined whether Abl might be involved in the phosphorylation of C3G. Using an in vitro kinase assay, we found that although purified Abl kinase can readily phosphorylate a GST fusion protein containing CrkL Y207, it had limited ability to phosphorylate a GST fusion protein containing the fragment of C3G containing Y504 (Fig. 8 C). This was not because of a problem with the fusion protein because the Src kinase Fyn readily phosphorylated the GST-C3G protein, as well as a positive control GST fusion protein containing Y100 of vinculin (Fig. 8 C). However, when examining the phosphorylation of C3G in cells, we found that suppression of Abl resulted in diminished TCR-stimulated tyrosine phosphorylation of C3G (Fig. 8 D). Collectively, these data suggest that Abl is involved in the tyrosine phosphorylation of C3G after TCR ligation in vivo.
WAVE2, Abl, CrkL, and C3G are involved in TCR-stimulated interleukin-2 (IL-2) production
To examine the physiological consequence of suppression of these mediators of TCR-stimulated integrin activation, we analyzed the ability of suppressed cells to produce IL-2 upon incubation with superantigen-pulsed Nalm 6 B cells. We reasoned that a decrease in the ability to form stable conjugates would affect TCR-stimulated activation of the IL-2 promoter and production of this cytokine. Consistent with this notion, we found that primary human CD4+ T cells suppressed for WAVE2, Abl, CrkL, or C3G showed diminished IL-2 secretion compared with siRNA control-transfected cells (Fig. 9).
Figure 9.
Suppression of WAVE2, Abl, CrkL, and C3G affects IL-2 production in T cells. Primary human CD4+ T cells were transfected with the indicated siRNA duplexes. After a 72-h incubation, cells were left unstimulated or stimulated with Nalm 6 B cells loaded with or without superantigen cocktail. 15 h after incubation, the amount or IL-2 in the supernatant was determined by ELISA as described in the Materials and methods. Error bars represent SD.
Discussion
Multiple studies have indicated that Rap1 regulates integrin clustering and affinity maturation in T cells. However, the signaling pathways initiated by the TCR that result in Rap1 activation remain relatively obscure. Our data demonstrate that the WAVE2 complex is an essential component of the TCR-stimulated signaling pathway, leading to activation of both Rap1 and its downstream effectors. Furthermore, our data suggest that WAVE2 regulates Rap1 activation through an Abl kinase-dependent mechanism that results in recruitment and activation of the CrkL–C3G exchange complex.
The data presented here are intriguing when combined with our earlier work showing that vinculin and talin are recruited to the IS via the VCA domain of WAVE2 and the Arp2/3 complex (Nolz et al., 2007). In terms of kinetics, our data suggests that Rap1 activation occurs during the early stages of cytoskeletal rearrangement when initial lamellipodial protrusion occurs. Because many effectors of Rap1 regulate both integrin avidity (e.g., Mst1) and affinity (e.g., RapL; Katagiri et al., 2003, 2006), WAVE2-dependent activation of Rap1 may help integrins localize to areas in the cell where de novo actin polymerization is occurring. Finally, as the IS begins to mature, the integrin scaffolding proteins vinculin and talin are then recruited to the WAVE2–Arp2/3 complex to generate the high-affinity state of the recently recruited integrins by binding both the β chain of the integrin and the actin cytoskeleton.
Surprisingly, suppression of WAVE2, Abl, CrkL, or C3G results in impaired TCR-mediated increases in both integrin affinity and avidity. To date, the described effectors of Rap1 appear to play a more important role in integrin localization rather than changing the conformation of the integrin required for increasing affinity. However, it has been suggested that Rap1 may act through RIAM to assist in talin binding to the integrin β chain, leading to its high-affinity state (Han et al., 2006). In addition, suppression of Rap1 in T cells results in impaired integrin affinity in response to chemokine stimulation (Parmo-Cabanas et al., 2007). Collectively, these data argue that Rap1 is important for both aspects of integrin regulation, even though certain Rap1 effectors probably regulate distinct steps of the process.
Although our data indicate that the activation of the CrkL–C3G complex requires WAVE2, the involvement of other adaptors is still probable. In fact, many proteins that associate with the SH2 domain of CrkL are phosphorylated in response to TCR stimulation, including Cbl (Donovan et al., 1994) and CasL (Kanda et al., 1997). In addition, chronic myelogenous leukemia cells, which express the BCR-ABL fusion protein, exhibit increases in CrkL, C3G, and CasL phosphorylation (Salgia et al., 1996). This is accompanied by both increased phosphorylation of several focal adhesion proteins and altered cellular adhesion. Therefore, it is likely that TCR stimulation causes Abl to activate the CrkL–C3G exchange complex, possibly though CasL, leading to Rap1 activation and adhesion.
WAVE2 has also been suggested to link to the BCR-ABL fusion protein through the adaptor Abi-1 (Li et al., 2007). That study indicates that recruitment of Abi and WAVE2 required direct interaction with BCR-ABL. This interaction resulted in F-actin accumulation, recruitment of several focal adhesion proteins including vinculin and talin, and overall increased adhesion to fibronectin mediated by β1 integrins. In fact, it has been shown that Abl phosphorylation of WAVE2 regulates its ability to polymerize actin via Arp2/3 (Leng et al., 2005). Consistent with this observation, it was recently shown that Abl is involved in the recruitment of WAVE2 and polymerization of F-actin at the IS (Huang et al., 2008). Our biochemical and microscopy data run contrary to this observation, instead suggesting that WAVE2 recruits Abl into the membrane in response to TCR ligation. Additionally, we find F-actin accumulation at the cell–bead contact site in Abl-suppressed cells. These inconsistencies could be caused by the differences in the experimental approach. Indeed, when examining T cell–B cell interactions, diminished adhesion in Abl-depleted cells might affect F-actin accumulation and, subsequently, WAVE2 localization.
Despite observing a significant affect on CrkL phosphorylation in Abl-suppressed cells and T cells treated with Imatinib, we fail to identify a defect in TCR-mediated tyrosine phosphorylation of PLCγ1 (Zipfel et al., 2004). This suggests that in this system, Abl does not function to coordinate proximal TCR signaling events, such as ZAP-70 phosphorylation and formation of the LAT signalosome, but, rather, Abl appears to work in concert with the WAVE2 complex to coordinate cytoskeletal dynamics and integrin activation. Yet, we cannot rule out the possibility that expression of the Abl-related protein Arg could be linking TCR-proximal signaling to PLC-γ1, while not being able to couple TCR signaling to CrkL phosphorylation, Rap1 activation, or integrin activation. Additionally, the level of suppression we attain with RNAi might be insufficient to affect TCR proximal signaling but does affect pathways leading to integrin activation. However, we do find that suppression of Abl or treatment with Imatinib does have an impact on IL-2 gene transcription. Future studies aimed at identifying mechanisms of WAVE2-independent Abl activity during T cell activation will help define the diverse functions of this tyrosine kinase.
In conclusion, the data presented here characterizes a previously unidentified function of the WAVE2 complex during T cell activation. Importantly, WAVE2 not only regulates integrin activation through Arp2/3-mediated actin polymerization and recruitment of integrins, talin, and vinculin but also mediates activation of Rap1 leading to integrin clustering and affinity maturation. In addition, this study suggests that the WAVE2 complex regulates Abl and CrkL–C3G recruitment into the plasma membrane, leading to Rap1 activation and integrin-mediated adhesion. It will be of interest to determine whether the ubiquitously expressed WAVE2 or its closely related homologues WAVE1 and WAVE3 use a similar pathway to regulate integrin activation in other cell types after receptor stimulation.
Materials and methods
Reagents and antibodies
Unless otherwise stated, all chemicals were obtained from Sigma-Aldrich. The antisera against WAVE2, Abi-1/2, PIR121, HEM-1, PLCγ1, LAT, and ZAP-70 have been previously described (Nolz et al., 2006). Antibodies for CrkL (clone 5–6) and phosphotyrosine (clone 4G10) were purchased from Millipore. Additionally, a rabbit polyclonal antibody was generated by Cocalico Biologicals, Inc. to a KLH-conjugated CrkL peptide (aa 280–303) and affinity purified using amino link (Thermo Fisher Scientific). The antibodies for phospho-CrkL (Y207), phospho-PLCγ1 (Y783), phospho-Pak1 Thr423 (Mst1 Thr183), total Mst1, and Erk were obtained from Cell Signaling Technology. The antibody for Abl (clone 8E9) was purchased from BD Biosciences, and the antibody for C3G (RapGEF1, Clone 3D10) was obtained from Novus Biologicals. The OKT3 mAb antibody was obtained from the Mayo Pharmacy and the anti-CD28 mAb was purchased from BD Biosciences. 5-μm latex beads were purchased from Invitrogen and labeled with IgG or anti-CD3 antibodies as previously described (Dombroski et al., 2005).
Cell culture and transfection
Jurkat T cells and Nalm 6 B cells were grown in RPMI-1640 supplemented with 5% FBS, 5% FCS, and 4 mM l-glutamine. Human CD4+ and CD8+ T cells were purified from cells retained in the leukoreduction systems chambers after plateletpheresis from blood donors at the Mayo Clinic Blood Bank using RosetteSep purification (StemCell Technologies Inc.). After purification, human CD4+ T cells were cultured in serum containing media along with 5 μg/ml PHA and IL-2 for 24 h. Cell were then washed and cultured in serum containing media with IL-2 for 72 h. Transient transfections in Jurkat were performed using 107 cells per sample along with 30–40 μg of plasmid DNA as previously described (Cao et al., 2002). Transfected Jurkat cells were used 48–72 h after transient transfection.
Plasmids and cloning
The pFRT and pCMS3 suppression vectors and the pCMS4 suppression/reexpression vector used for shRNA silencing and reexpression of resistant cDNAs have been described previously (Gomez et al., 2005). The following 19-nucleotide sequence was generated to target human WAVE2: GAGAAGAGAAAGCACAGGAA. The following sequence was used to target human CrkL: GAGGGCATTGACTGGTTAA. C3G was targeted using the following two sequences: a, GGGTTGTGTGAACTGAAAT; and b, GCCGGGATCCAGGAGAATG. Abl was targeted using the following two sequences: 1, GGGAGGGTGTACCATTACA; and 3, GGAAATCAGTGACATAGTG. Human CrkL was cloned from Jurkat cDNA using standard methods. Mutants of CrkL (SH2:R39A, SH3-N:W160Y,W161Y; SH3-C:W275Y,W276Y) were created using the Quick Change Site-Directed Mutagenesis kit (Stratagene) and cloned as a FLAG-tagged protein. The wild-type and ΔVCA versions of WAVE2 used in the suppression/reexpression vectors have been previously described (Nolz et al., 2007). Myc-tagged Rap1 and GFP-tagged PKD have been described previously (Medeiros et al., 2005). GST-fusion proteins of the SH3 domains of Abi-1 (438-End), Abi-2 (394-End), IRSp53 (370–439), and N-terminal CrkL (117–179) were generated in bacteria as previously described (Hamann et al., 2007).
siRNA transfection of purified human CD4+ T cells
All siRNAs were purchased from Thermo Fisher Scientific. Transfection of siRNA into purified human CD4+ T cells was performed essentially as previously described (Gomez et al., 2007). In brief, 5–6 × 106 cells were used per transfection using 300–500 pmol siRNA after T cell isolation and culture as described in Cell culture and transfection. Cells were then used 72 h after transfection. The following sequences were used to target proteins in primary human T cells: WAVE2, CACCCGAAAAGCCTTCAGA; CrkL-a, GAGGGCATTGACTGGTTAA; CrkL-b, GAGTTCTTTTGGATCATAA; Abl-a, GGGAGGGTGTACCATTACA; Abl-b, GGAAATCAGTGACATAGTG; and C3G, GGGTTGTGTGAACTGAAAT.
Cell stimulation and immunoblot analysis
For the stimulation timecourse studies, 10 × 106 Jurkat T cells or 25 × 106 human CD4+ T cells were stained on ice with 5 μg/ml anti-CD3 (OKT3, mAb) and then ligated using goat anti–mouse over the indicated time course at 37°C. After each time point, the cells were immediately washed in ice-cold PBS and lysed in NP-40 lysis buffer (20 mM Hepes, pH 7.9, 100 mM NaCl, 5 mM EDTA, 0.5 mM CaCl, 1% NP-40, 1 mM PMSF, 10 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM Na3VO4, and 5 μM MG-132) for 10 min on ice. Lysates were clarified by centrifugation at 18,000 g for 5 min at 4°C and then transferred to antibody-coated beads. The protein complexes were then washed twice with NP-40 lysis buffer, eluted in 60 μl SDS sample buffer, resolved by SDS-PAGE, and transferred to Immobilon-P membranes (Millipore). In cases where whole cell extracts were prepared, 50–100 μg of protein was resolved by SDS-PAGE. For immunoblots, antibodies were detected using goat anti–mouse IgG or goat anti–rabbit IgG coupled to HRP (Santa Cruz Biotechnology, Inc.) and SuperSignal Enhanced Chemiluminescence (Thermo Fisher Scientific). Bands were quantified by densitometry using Photoshop software (Adobe) and arbitrarily normalized to 1. All assays were performed a minimum of three times.
Rap1 activation assays
Cell stimulation was performed as described in Cell stimulation and immunoblot analysis, except 5 × 106 Jurkat or 10 × 106 human CD4+ T cells were used per sample. After washing with cold PBS, cells were lysed in 1 ml GTPase activation buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 10% glycerol, 1 mM PMSF, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM Na3VO4) vortexed for 5 s and immediately clarified for 10 min by centrifugation at 18,000 g. 0.5 ml of cell lysates were then transferred to glutathione agarose previously bound to GST-RalGDS GBD. Lysates were allowed to rotate for 10 min and washed once before elution. Cell extracts were obtained from unused cell lysate after clarification. After elution, samples were separated by SDS-PAGE on a 12.5% polyacrylamide gel, transferred, and immunoblotting was performed for Rap1.
Immunofluorescence microscopy
T cell–B cell and T cell–bead conjugates were formed essentially as described previously (Cannon et al., 2001; Dombroski et al., 2005; Nolz et al., 2007). In brief, Nalm 6 B cells were stained with CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin; Invitrogen) and pulsed with or without 2 μg/ml SEE (Toxin Technology, Inc.). B cells were centrifuged together with the same number of T cells, incubated at 37°C for 15 min, plated onto poly-l-lysine–coated coverslips, and fixed with 4% PFA/PBS for 10 min at room temperature. For T cell–bead conjugates, 106 cells/ml were incubated with IgG- or anti-CD3–coated beads (106/ml) in 0.5 ml of total volume, centrifuged at 450 rpm for 5 min, incubated at 37°C for 10 min, plated onto poly-l-lysine–coated coverslips, and fixed with 4% PFA/PBS for 10 min at room temperature. Fixed cells were quenched with 50 mM NH4Cl and permeabilized in 0.2% Triton X-100. Blocking and antibody incubations were performed in blocking buffer (PBS, 0.05% saponin, and 0.25% fish skin gelatin). Slides were labeled with purified anti-CrkL, anti-β1 integrin antibody (clone P5D2; Millipore), or anti-Abl (clone 8E9; BD Biosciences), followed by goat anti–mouse TRITC, goat anti–rabbit FITC (Invitrogen), or goat anti–rabbit Cy5 (Invitrogen). In some cases, conjugates were stained for polymerized F-actin using rhodamine-conjugated phalloidin (Invitrogen). Coverslips were mounted in Mowiol 4–88 (HOECHST; Celanese) containing 10% 1,4-diazobicyclo [2.2.2] octane. Fixed conjugates were examined at room temperature on a microscope (Axiovert 200; Carl Zeiss, Inc.) with a digital camera (AxioCam HRC; Carl Zeiss, Inc.) using a 40×/0.75 NA objective or a scanning confocal microscope (LSM 510; Carl Zeiss, Inc.) using a 63×/1.2 NA water-immersion objective. Images were captured with either the AxioVision or LSM software packages (Carl Zeiss, Inc.). Figure construction of images was performed in Photoshop and Canvas (ACD Systems). 100 conjugates were scored per condition, and cells were scored as positive if the T cell had bound a single bead or Nalm 6 B cell and demonstrated accumulation of integrin, F-actin, or protein at the contact area as previously described (Nolz et al., 2006, 2007). Data are presented as the percentage of cells polarized of the scored conjugates ±SE for a minimum of three independent experiments.
Adhesion assays
Adhesion assays were performed in flat-bottom 96-well plates. Fibronectin or ICAM-1Fc (R&D Systems) was diluted in sterile PBS, and wells were coated overnight at 4°C. Wells were then washed once with PBS, blocked with 2.5% BSA in PBS for 2 h at 37°C, and washed once more in PBS. Either Jurkat T cells or purified human CD4+ T cells were stained with 5 μM calcein AM (Invitrogen) for 30 min in serum-free media and washed twice with PBS. For anti-CD3 stimulation in purified human CD4+ T cells, cells were stained on ice with 5 μg/ml OKT3 for 15 min before addition to the coated wells. Cells were added to wells (105 for Jurkat and 2.5 × 105 for purified CD4+) containing media alone (unstimulated), 5 μg/ml OKT3 (anti-CD3 for Jurkat), 1:50 dilution of goat anti–mouse (anti-CD3 for purified human T cells prestained with OKT3), or PMA (10 ng/ml for Jurkat and 50 ng/ml for purified human T cells) in 100 μl of media total. The 96-well plate containing cells was then placed on ice for 30 min, followed by a 15-min incubation at 37°C. After incubation, the cellular input was measured using a fluorescent plate reader (MDS Analytical Technologies) using an excitation of 495 nm and emission of 517 nm. Plates were then subsequently washed with PBS and adhesion was continually monitored using the aforementioned excitations and emissions. Typically, data for adhesion to ICAM-1Fc was optimal at two to three washes with PBS and adhesion to fibronectin was optimal at five to six washes with PBS. All adhesion experiments were performed in triplicate and the SD is shown.
Soluble VCAM-1-Fc binding
Procedure was performed as previously described (Chan et al., 2001; Woodside et al., 2006). In brief, 106 Jurkat T cells were washed with modified Tyrode's buffer (12 mM NaHCO3, 20 mM Hepes, pH 7.4, 1 mg/ml glucose, 150 mM NaCl, 2.5 mM KCl, 1 mg/ml BSA, 1 mM Ca2+, 1 mM Mg2+) and incubated in 50 μl of Tyrode's with 1 μg of recombinant chimeric human 7-domain VCAM-1-Fc (R&D Systems). The cells were either left untreated or stimulated with OKT3, 50 ng/ml PMA, or 1 mM Mn2+ for 10 min at 37°C and then diluted in 3 ml of Tyrode's buffer and immediately fixed with 0.5 ml of 4% PFA for 20 min. The cells were then washed with Tyrode's buffer and stained with biotinylated goat anti–human Fc and streptavidin-allophycocyanin (eBioscience). Flow cytometry with a FACSCalibur system (BD Biosciences) was used to determine the degree of VCAM-1-Fc binding.
Cytosolic/membrane fractionation
Plasma membrane–enriched fractions were obtained essentially as previously described (Gradilone et al., 2003). In brief, after indicated stimulation, cells were washed with cold PBS and sonicated in 0.3 M sucrose in PBS containing protease and phosphatase inhibitors. Membrane fractions were obtained by centrifugation at 56,000 rpm for 60 min on a discontinuous 1.2-M sucrose gradient. Equal amounts of proteins were analyzed by immunoblot as described in Cell stimulation and immunoblot analysis.
In vitro kinase assay
Purified GST-fused proteins were incubated with recombinant c-Abl (Cell Signaling Technology), recombinant Fyn (BIOMOL International, L.P.), or full-length c-Abl (immunoprecipitated from Jurkat cells) as indicated, in a final volume of 20 μl containing 20 μM of nonlabeled ATP (Sigma-Aldrich) and kinase reaction buffer (25 μM Tris, pH 7.5, 5 μM β-glycerophosphate, 2 μM DTT, 5 μM Na3VO3, 5 μM MgCl2, and 0.2 mg/ml BSA). Reactions were performed at 30°C for 30 min and quenched by addition of SDS sampler buffer. Protein phosphorylation was analyzed by immunoblot with phosphospecific antibodies as described in Cell stimulation and immunoblot analysis.
IL-2 secretion and ELISA
In a 12-well plate, 2 × 105 CD4+ T cells and 105 Nalm 6 B cells were incubated on a tilt-angle rocking platform for 15 h under culture conditions. Where indicated, superantigens (SAB and SAE; 300 ng/well) were added to the reaction. After stimulation, supernatants were collected, and IL-2 secretion was determined with a sandwich ELISA kit (assay sensitivity 4 pg/ml; eBioscience) according to the manufacturer's protocol. In brief, a 96-well plate was coated with polyclonal anti–human IL-2 Antibody. After blocking, supernatants were diluted 1:4 and added to the wells overnight at 4°C. IL-2 was detected by incubation wells with biotinylated anti-human IL-2 antibody followed by HRP-Avidin. Color was developed using trimethylbenzidine substrate, and reaction was terminated with H2SO4. OD was measured at 450 nm and corrected at 570 nm on a plate reader (SpectraMax M2; MDS Analytical Technologies). IL-2 concentration was determined extrapolating OD values from the samples to a standard curve made using recombinant human IL-2 provided by the manufacturer.
Online supplemental material
Fig. S1 shows that inhibition of F-actin polymerization affects Rap1 activation but not CrkL phosphorylation. Fig. S2 shows that suppression of CrkL or C3G does not affect F-actin accumulation at the IS. Fig. S3 shows that Abl coimmunoprecipitates with, but is not an obligate component of, the WAVE2 complex. Fig. S4 shows that Abl kinase activity is recruited into the membrane fraction after TCR ligation. Fig. S5 shows that Imatinib affects TCR-stimulated Rap1 activation and adhesion to fibronectin. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200801121/DC1.
Supplementary Material
Acknowledgments
This work was supported by the Mayo Foundation and National Institutes of Health grants R01-AI065474 to D.D. Billadeau, R01-AI038474 and R01-AI031126 to Y. Shimizu, NIH-T32-CA009138 Cancer Biology Training Grant to J.S. Mitchell, and NIH-T32-AI07425 pre-doctoral Immunology Training Grant to J.C. Nolz. D.D. Billadeau is a Leukemia and Lymphoma Society Scholar.
The authors declare that they have no competing financial interests.
Abbreviations used in this paper: APC, antigen-presenting cell; GBD, GTPase binding domain; GEF, guanine nucleotide exchange factor; IL-2, interleukin-2; IS, immunological synapse; SMAC, supramolecular activation complex; TCR, T cell receptor.
References
- Anton van der Merwe, P., S.J. Davis, A.S. Shaw, and M.L. Dustin. 2000. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12:5–21. [DOI] [PubMed] [Google Scholar]
- Arai, A., Y. Nosaka, H. Kohsaka, N. Miyasaka, and O. Miura. 1999. CrkL activates integrin-mediated hematopoietic cell adhesion through the guanine nucleotide exchange factor C3G. Blood. 93:3713–3722. [PubMed] [Google Scholar]
- Billadeau, D.D., J.C. Nolz, and T.S. Gomez. 2007. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 7:131–143. [DOI] [PubMed] [Google Scholar]
- Bos, J.L. 2005. Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17:123–128. [DOI] [PubMed] [Google Scholar]
- Cannon, J.L., C.M. Labno, G. Bosco, A. Seth, M.H. McGavin, K.A. Siminovitch, M.K. Rosen, and J.K. Burkhardt. 2001. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity. 15:249–259. [DOI] [PubMed] [Google Scholar]
- Cao, Y., E.M. Janssen, A.W. Duncan, A. Altman, D.D. Billadeau, and R.T. Abraham. 2002. Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 21:4809–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J.R., S.J. Hyduk, and M.I. Cybulsky. 2001. Chemoattractants induce a rapid and transient upregulation of monocyte α4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J. Exp. Med. 193:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, Z., and A.M. Pendergast. 1995. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 9:2569–2582. [DOI] [PubMed] [Google Scholar]
- de Jong, R., J. ten Hoeve, N. Heisterkamp, and J. Groffen. 1997. Tyrosine 207 in CRKL is the BCR/ABL phosphorylation site. Oncogene. 14:507–513. [DOI] [PubMed] [Google Scholar]
- Dombroski, D., R.A. Houghtling, C.M. Labno, P. Precht, A. Takesono, N.J. Caplen, D.D. Billadeau, R.L. Wange, J.K. Burkhardt, and P.L. Schwartzberg. 2005. Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J. Immunol. 174:1385–1392. [DOI] [PubMed] [Google Scholar]
- Donovan, J.A., R.L. Wange, W.Y. Langdon, and L.E. Samelson. 1994. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J. Biol. Chem. 269:22921–22924. [PubMed] [Google Scholar]
- Duchniewicz, M., T. Zemojtel, M. Kolanczyk, S. Grossmann, J.S. Scheele, and F.J. Zwartkruis. 2006. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol. Cell. Biol. 26:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin, M.L., and T.A. Springer. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 341:619–624. [DOI] [PubMed] [Google Scholar]
- Feller, S.M., B. Knudsen, and H. Hanafusa. 1994. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 13:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller, S.M., B. Knudsen, and H. Hanafusa. 1995. Cellular proteins binding to the first Src homology 3 (SH3) domain of the proto-oncogene product c-Crk indicate Crk-specific signaling pathways. Oncogene. 10:1465–1473. [PubMed] [Google Scholar]
- Gomez, T.S., M.J. Hamann, S. McCarney, D.N. Savoy, C.M. Lubking, M.P. Heldebrant, C.M. Labno, D.J. McKean, M.A. McNiven, J.K. Burkhardt, and D.D. Billadeau. 2005. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat. Immunol. 6:261–270. [DOI] [PubMed] [Google Scholar]
- Gomez, T.S., K. Kumar, R.B. Medeiros, Y. Shimizu, P.J. Leibson, and D.D. Billadeau. 2007. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 26:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone, S.A., F. Garcia, R.C. Huebert, P.S. Tietz, M.C. Larocca, A. Kierbel, F.I. Carreras, N.F. Larusso, and R.A. Marinelli. 2003. Glucagon induces the plasma membrane insertion of functional aquaporin-8 water channels in isolated rat hepatocytes. Hepatology. 37:1435–1441. [DOI] [PubMed] [Google Scholar]
- Gu, J.J., N. Zhang, Y.W. He, A.J. Koleske, and A.M. Pendergast. 2007. Defective T cell development and function in the absence of abelson kinases. J. Immunol. 179:7334–7343. [DOI] [PubMed] [Google Scholar]
- Hamann, M.J., C.M. Lubking, D.N. Luchini, and D.D. Billadeau. 2007. Asef2 functions as a Cdc42 exchange factor and is stimulated by the release of an autoinhibitory module from a concealed C-terminal activation element. Mol. Cell. Biol. 27:1380–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., C.J. Lim, N. Watanabe, A. Soriani, B. Ratnikov, D.A. Calderwood, W. Puzon-McLaughlin, E.M. Lafuente, V.A. Boussiotis, S.J. Shattil, and M.H. Ginsberg. 2006. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr. Biol. 16:1796–1806. [DOI] [PubMed] [Google Scholar]
- Hernandez, S.E., M. Krishnaswami, A.L. Miller, and A.J. Koleske. 2004. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 14:36–44. [DOI] [PubMed] [Google Scholar]
- Huang, Y., E.O. Comiskey, R.S. Dupree, S. Li, A.J. Koleske, and J.K. Burkhardt. 2008. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood. 112:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries, J.D., and M.J. Humphries. 2007. CD14 is a ligand for the integrin alpha4beta1. FEBS Lett. 581:757–763. [DOI] [PubMed] [Google Scholar]
- Ichiba, T., Y. Hashimoto, M. Nakaya, Y. Kuraishi, S. Tanaka, T. Kurata, N. Mochizuki, and M. Matsuda. 1999. Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J. Biol. Chem. 274:14376–14381. [DOI] [PubMed] [Google Scholar]
- Innocenti, M., A. Zucconi, A. Disanza, E. Frittoli, L.B. Areces, A. Steffen, T.E. Stradal, P.P. Di Fiore, M.F. Carlier, and G. Scita. 2004. Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat. Cell Biol. 6:319–327. [DOI] [PubMed] [Google Scholar]
- Kanda, H., T. Mimura, N. Morino, K. Hamasaki, T. Nakamoto, H. Hirai, C. Morimoto, Y. Yazaki, and Y. Nojima. 1997. Ligation of the T cell antigen receptor induces tyrosine phosphorylation of p105CasL, a member of the p130Cas-related docking protein family, and its subsequent binding to the Src homology 2 domain of c-Crk. Eur. J. Immunol. 27:2113–2117. [DOI] [PubMed] [Google Scholar]
- Katagiri, K., A. Maeda, M. Shimonaka, and T. Kinashi. 2003. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4:741–748. [DOI] [PubMed] [Google Scholar]
- Katagiri, K., M. Imamura, and T. Kinashi. 2006. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 7:919–928. [DOI] [PubMed] [Google Scholar]
- Kinashi, T. 2005. Intracellular signalling controlling integrin activation in lymphocytes. Nat. Rev. Immunol. 5:546–559. [DOI] [PubMed] [Google Scholar]
- Leng, Y., J. Zhang, K. Badour, E. Arpaia, S. Freeman, P. Cheung, M. Siu, and K. Siminovitch. 2005. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proc. Natl. Acad. Sci. USA. 102:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., N. Clough, X. Sun, W. Yu, B.L. Abbott, C.J. Hogan, and Z. Dai. 2007. Bcr-Abl induces abnormal cytoskeleton remodeling, beta1 integrin clustering and increased cell adhesion to fibronectin through the Abl interactor 1 pathway. J. Cell Sci. 120:1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros, R.B., D.M. Dickey, H. Chung, A.C. Quale, L.R. Nagarajan, D.D. Billadeau, and Y. Shimizu. 2005. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 23:213–226. [DOI] [PubMed] [Google Scholar]
- Menasche, G., S. Kliche, E.J. Chen, T.E. Stradal, B. Schraven, and G. Koretzky. 2007. RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol. Cell. Biol. 27:4070–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn, M., A. Molina, M.M. Escribese, M. Yanez-Mo, E. Escudero, A. Ursa, R. Tejedor, F. Mampaso, and F. Sanchez-Madrid. 2004. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc. Natl. Acad. Sci. USA. 101:11058–11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley, J.L., E. Ennis, and Y. Shimizu. 1994. Differential activation-dependent regulation of integrin function in cultured human T-leukemic cell lines. Blood. 83:1039–1050. [PubMed] [Google Scholar]
- Nolz, J.C., T.S. Gomez, P. Zhu, S. Li, R.B. Medeiros, Y. Shimizu, J.K. Burkhardt, B.D. Freedman, and D.D. Billadeau. 2006. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr. Biol. 16:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz, J.C., R.B. Medeiros, J.S. Mitchell, P. Zhu, B.D. Freedman, Y. Shimizu, and D.D. Billadeau. 2007. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol. Cell. Biol. 27:5986–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmo-Cabanas, M., D. Garcia-Bernal, R. Garcia-Verdugo, L. Kremer, G. Marquez, and J. Teixido. 2007. Intracellular signaling required for CCL25-stimulated T cell adhesion mediated by the integrin alpha4beta1. J. Leukoc. Biol. 82:380–391. [DOI] [PubMed] [Google Scholar]
- Plattner, R., L. Kadlec, K.A. DeMali, A. Kazlauskas, and A.M. Pendergast. 1999. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 13:2400–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, M.K., T. Yamazaki, G.D. Gish, C.M. Kay, T. Pawson, and L.E. Kay. 1995. Direct demonstration of an intramolecular SH2-phosphotyrosine interaction in the Crk protein. Nature. 374:477–479. [DOI] [PubMed] [Google Scholar]
- Sakakibara, A., Y. Ohba, K. Kurokawa, M. Matsuda, and S. Hattori. 2002. Novel function of Chat in controlling cell adhesion via Cas-Crk-C3G-pathway-mediated Rap1 activation. J. Cell Sci. 115:4915–4924. [DOI] [PubMed] [Google Scholar]
- Salgia, R., E. Pisick, M. Sattler, J.L. Li, N. Uemura, W.K. Wong, S.A. Burky, H. Hirai, L.B. Chen, and J.D. Griffin. 1996. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J. Biol. Chem. 271:25198–25203. [DOI] [PubMed] [Google Scholar]
- Sattler, M., and R. Salgia. 1998. Role of the adapter protein CRKL in signal transduction of normal hematopoietic and BCR/ABL-transformed cells. Leukemia. 12:637–644. [DOI] [PubMed] [Google Scholar]
- Sebzda, E., M. Bracke, T. Tugal, N. Hogg, and D.A. Cantrell. 2002. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat. Immunol. 3:251–258. [DOI] [PubMed] [Google Scholar]
- Shao, Y., C. Elly, and Y.C. Liu. 2003. Negative regulation of Rap1 activation by the Cbl E3 ubiquitin ligase. EMBO Rep. 4:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., K. Alin, and S.P. Goff. 1995. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 9:2583–2597. [DOI] [PubMed] [Google Scholar]
- Shimizu, Y., G.A. van Seventer, K.J. Horgan, and S. Shaw. 1990. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J. Immunol. 145:59–67. [PubMed] [Google Scholar]
- Woodring, P.J., T. Hunter, and J.Y. Wang. 2003. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J. Cell Sci. 116:2613–2626. [DOI] [PubMed] [Google Scholar]
- Woodside, D.G., R.M. Kram, J.S. Mitchell, T. Belsom, M.J. Billard, B.W. McIntyre, and P. Vanderslice. 2006. Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1 binding to integrin alpha4beta1. J. Immunol. 176:5041–5049. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Y. Shao, D. Fang, J. Huang, M.S. Jeon, and Y.C. Liu. 2003. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. J. Biol. Chem. 278:23978–23983. [DOI] [PubMed] [Google Scholar]
- Zipfel, P.A., W. Zhang, M. Quiroz, and A.M. Pendergast. 2004. Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 14:1222–1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.