Abstract
Study Objectives:
Weight loss may reduce the severity of obstructive sleep apnea (OSA), but persistence of OSA following surgical weight loss has not been defined. We sought to clarify the impact of bariatric surgery on OSA. We hypothesized that, despite substantial weight loss and reductions in the apnea-hypopnea index (AHI), many will have persistent disease.
Methods:
Consecutive patients referred for preoperative sleep evaluation underwent polysomnography before and 1 year following bariatric surgery. We compared the effects of weight loss on body mass, OSA, and continuous positive airway pressure requirements. We defined OSA severity using the AHI (normal < 5 events per hour, mild 5 to 14 events per hour, moderate 15 to 29 events per hour, and severe 30 or more events per hour). We identified predictors of OSA severity following weight loss and assessed compliance with therapy.
Results:
Twenty-four patients (aged 47.9 ± 9.3 years; 75% women) were enrolled. At baseline, all subjects had OSA, the majority of which was severe. Weight loss reduced body mass index from 51.0 ± 10.4 kg/m2 to 32.1 ± 5.5 kg/m2 (p < 0.001) and the AHI from 47.9 ± 33.8 to 24.5 ± 18.1 events per hour (p < 0.001). At follow-up, only 1 patient (4%) experienced resolution of OSA. The majority (71%) had moderate or severe disease. The most important predictor of the follow-up AHI was the baseline AHI (R2 = 0.603). All patients with residual OSA required continuous positive airway pressure to ablate apneic events, but the required pressures decreased from 11.5 ± 3.6 cm H2O to 8.4 ± 2.1 cm H2O (p = 0.001). Only 6 patients were compliant with continuous positive airway pressure therapy at the follow-up visit.
Conclusions:
Surgical weight loss reduces the AHI, but many patients have residual OSA one year after bariatric surgery.
Citation:
Lettieri CJ; Eliasson AH; Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med 2008;4(4):333–338.
Keywords: Obstructive sleep apnea, apnea-hypopnea index, obesity, weight loss, bariatric surgery, prediction, continuous positive airway pressure
Obesity and untreated obstructive sleep apnea (OSA) have been linked to numerous health consequences, including diabetes, cardiovascular disease, and premature mortality.1–10 Although OSA may occur in a wide range of individuals, including those who are thin and physically fit, obesity is an important risk factor for this disorder.1,10–14 The prevalence of OSA among obese individuals is high and correlates with increasing body mass index (BMI). Among the severely obese, the prevalence of OSA ranges from 55% to 90%.11,12,15–17 OSA itself may promote weight gain through ineffective sleep, impaired glucose metabolism, and imbalances of leptin, ghrelin, and orexin levels.14 Obese individuals frequently have more severe disease, as manifested by a higher apnea-hypopnea index (AHI) and a lower nadir on nocturnal pulse oximetry (SpO2).11,15–17 In view of the close association of OSA and obesity, it might be assumed that bariatric surgery resulting in significant weight loss would also improve OSA. However, many patients report persistent somnolence and snoring despite substantial weight loss. Furthermore, many individuals who initially achieve significant weight reductions regain a portion of their weight.18,19 The persistent symptoms and subsequent weight gain may be associated with or result from residual sleep-disordered breathing.
Obesity is neither necessary nor sufficient for the development of OSA. A corollary to this statement is that weight loss following bariatric surgery may not be sufficient to resolve OSA. Unfortunately, the dramatic reductions in AHI reported with weight loss in some studies of bariatric surgery overshadow the fact that surgical weight loss may not cure sleep-disordered breathing. Few studies have reported outcomes in terms of frequency of disease resolution using postoperative polysomnography.
The AHI is a measure used to identify the presence of OSA and define its severity. Individuals with an AHI of less than 5 events per hour are not considered to have OSA. One commonly used scheme categorizes patients with an AHI from 5 to 14 as having mild OSA, from 15 to 29 as moderate OSA, and an AHI of 30 or greater as having severe OSA.20 Many obese patients evaluated by polysomnography prior to bariatric surgery have been shown to have very severe disease, with AHIs exceeding 100 events per hour.17,21,22 In these patients, even large reductions in the AHI may leave them with persistently severe OSA that necessitates ongoing treatment.
In this paper, we report descriptive data from our cohort and attempt to identify predictors of the presence and severity of OSA following surgical weight loss. In addition, we explore the effects of surgical weight loss on continuous positive airway pressure (CPAP) requirements and identify predictors of discontinuation of CPAP therapy.
METHODS
Subjects
Consecutive patients referred to our sleep medicine clinic for preoperative evaluation of excessive daytime somnolence (EDS) prior to bariatric surgery were included. Our sleep center is part of an academic, tertiary-care referral hospital. From January 2003 until January 2005, 145 people were evaluated for bariatric surgery in our bariatric surgery clinic, and 118 patients elected to undergo gastric banding procedures. Of these, 25 patients were referred to our sleep clinic for perioperative evaluation. Polysomnography was obtained in all subjects prior to undergoing bariatric surgery. One patient died perioperatively from a pulmonary embolus, and data from this patient are not included in this study. The remaining 24 patients were reevaluated in our sleep clinic approximately 1 year after surgery for a follow-up evaluation and polysomnography. The protocol was approved by our institution's scientific research review committee.
Measurements
Subjects were clinically evaluated by a board-certified sleep specialist prior to and 1 year following bariatric surgery. This time interval was chosen to optimize the resultant weight loss and allow adequate time for recovery from surgery. Both evaluations included a clinical assessment and an overnight attended polysomnogram. If OSA was diagnosed, the subject also underwent a formal CPAP titration and was prescribed CPAP for continued home use. Patients were educated on the potential benefits of treating OSA and the proper use of CPAP. All patients received appropriate follow-up care.
For each subject, we collected demographic, clinical, and polysomnographic data. Demographic data included age, sex, and BMI. BMI was calculated from measured height and weight in each subject and is expressed as kg/m2. A BMI less than 25 was considered normal and 30 or greater was defined as obese. Subjective complaints of EDS and snoring and results of the Epworth Sleepiness Scale (ESS) were recorded.23 The attended overnight polysomnogram in our sleep laboratory consisted of a standardized 12-channel montage (Sensormedics Alpha Somnostar system, Sensormedics, Yorba Linda, CA), and studies were scored in 30-second epochs following Rechtschaffen and Kales criteria for sleep staging.24,25 Polysomnographic data recorded for this analysis included the AHI, SpO2 nadir, percentage of time with an SpO2 below 80% and below 90%, and CPAP pressure needed to ablate obstructive respiratory events. OSA was diagnosed using the accepted criteria and standards of the American Academy of Sleep Medicine.20,26 This included an AHI of 5 or more events per hour associated with subjective EDS and/or an ESS > 10. OSA was considered mild if the AHI was 5 or more per hour but less than 15 per hour, moderate if 15 or more per hour but less than 30, and severe if 30 or more per hour.20,26 During the second clinical evaluation, when applicable, CPAP compliance was determined by both self-reported use and interrogation of the automated device-driven recorded information, or so-called “smart-card” download.
Endpoints
Our primary endpoint was severity of OSA following surgical weight loss. Secondary variables included absolute changes in AHI, weight, BMI, ESS, subjective complaints of snoring and EDS, percentage of time with nocturnal SaO2 below 90%, SaO2 nadir on polysomnography, and quantity of CPAP required to ablate respiratory events between the preoperative and postoperative polysomnograms.
Statistical Analysis
We compared continuous variables with the paired Student t-test and analyzed categorical variables with the Fisher exact test. All tests were 2 tailed, and p values of less then 0.05 were assumed to represent statistical significance. When applicable, data are presented as mean ± standard deviation. Multivariate linear regression was used to identify independent predictors of the AHI following surgical weight loss. All analyses were completed using Stata ver. 9.2 (StataCorp, College Station, TX).
RESULTS
The baseline characteristics of the study participants, including demographic information, Malampati grade, and presence of obesity-related medical complications are presented in Table 1. At enrollment, patients were, on average, 47.9 ± 9.3 years of age, and most (75%) were women. The BMI of enrolled patients averaged 51.0 ± 10.4 kg/m2 and ranged from 37 kg/m2 to 73 kg/m2. Women had slightly higher BMIs (53.1 vs 44.2 kg/m2, p = 0.06), were younger (46.6 vs 51.8 years of age, p = 0.24), and had lower Malampati grades (2.6 vs 3.3, p = 0.14) than the men in our sample.
Table 1.
Demographics of Study Participants by Sex
| Men (n = 6) | Women (n = 18) | |
|---|---|---|
| Age (mean ± SD) | 51.8 ± 8.4 | 46.6 ± 9.4 |
| Malampati grade | 3.3 ± 0.5 | 2.6 ± 0.8 |
| Race, no. (%) | ||
| White | 5 (83) | 7 (39) |
| Black | 1 (17) | 7 (39) |
| Other | 0 | 4 (22) |
| Obesity-related comorbidities, no. (%) | ||
| Hypertension | 6 (100) | 11 (61) |
| Diabetes | 3 (50) | 6 (33) |
| Hyperlipidemia | 3 (50) | 3 (17) |
| GERD | 2 (33) | 5 (28) |
| Coronary artery disease | 2 (33) | 0 |
| Depression | 2 (33) | 2 (11) |
| Fibromyalgia | 0 | 1 (6) |
Data are presented as mean ± SD or number (%). GERD refers to gastroesophageal reflux disease.
Data from the preoperative and postoperative evaluations are presented in Table 2. During the initial preoperative evaluation, all patients reported subjective complaints of EDS, and the average ESS was 16.1 ± 4.5. Most (n = 22, 92%) reported habitual snoring. All patients met accepted criteria for OSA per the American Academy of Sleep Medicine and the AHI averaged 47.9 ± 33.8 events per hour (range 5–135).20,26 Most patients (n = 17, 71%) had severe OSA. Nocturnal desaturation below 90% was common (n = 20, range of nadir 52%–96%). All patients required CPAP to ablate apneic events.
Table 2.
Effects of Bariatric Surgery on Sleep and Obstructive Sleep Apnea
| Preoperative (n = 24) |
Postoperative (n = 24) |
p Value | |
|---|---|---|---|
| BMI, kg/m2 | 51.0 ± 10.4 | 32.1 ± 5.5 | < 0.001 |
| Weight, kg | 146.8 ± 28.9 | 92.8 ± 18.4 | < 0.001 |
| EDS, % | 100 | 47.1 | 0.02 |
| ESS, score | 15.0 ± 4.9 | 10.6 ± 4.0 | < 0.001 |
| Snore, % | 91.7 | 95.8 | 0.33 |
| TST, min | 298.4 ± 54.7 | 319.8 ± 50.6 | 0.51 |
| Sleep efficiency, % | 92.8 ± 27.5 | 83.6 ± 10.8 | 0.66 |
| Sleep latency, min | 31.0 ± 25.6 | 34.4 ± 33.2 | 0.85 |
| Sleep stage, % | |||
| 1 | 18.6 ± 8.6 | 5.8 ± 4.4 | 0.004 |
| 2 | 44.4 ± 13.8 | 58.6 ± 12.9 | 0.07 |
| 1 & 2 | 31.5 ± 15.5 | 32.2 ± 27.1 | 0.93 |
| 3 & 4 | 11.0 ± 10.7 | 8.5 ± 8.6 | 0.67 |
| REM | 8.0 ± 6.1 | 11.5 ± 2.6 | 0.24 |
| PLMI | 8.7 ± 7.0 | 6.3 ± 6.6 | 0.39 |
| SpO2, % of TST | |||
| < 80% | 3.6 ± 1.0 | 1.6 ± 0.6 | 0.005 |
| < 90% | 14.6 ± 2.7 | 11.9 ± 2.4 | < 0.001 |
| SpO2 nadir, % | 76.5 ± 12.1 | 84.5 ± 5.8 | 0.004 |
| AI, events/h | 42.8 ± 17.3 | 23.3 ± 8.1 | 0.003 |
| AHI, events/h | 47.9 ± 33.8 | 24.5 ± 18.1 | < 0.001 |
| CPAP requirement, cm H2Oa | 11.5 ± 3.7 | 8.4 ± 2.1 | 0.004 |
Data are as mean ± SD or number (%). BMI refers to body mass index; ESS, Epworth Sleepiness Scale; EDS, excessive daytime sleepiness; TST, total sleep time; REM, rapid eye movement sleep; AI, apnea index; AHI, apnea-hypopnea index.
Continuous positive airway pressure (CPAP) required to abate obstructive events.
Patients completed the second evaluation approximately 1 year following bariatric surgery. Mean duration between the surgery and final evaluation was 418 (range: 328–677) days. At the time of the follow-up evaluation, the average BMI had been reduced from 51.0 ± 10.4 kg/m2 to 32.1 ± 5.5 kg/m2 (p < 0.001), an average reduction of 18.9 ± 8.3 kg/m2. The average AHI decreased 23.4 events per hour (p < 0.001), but substantial variability was noted (SD = 22.8, range: −2 to 97). Postoperative polysomnography revealed reductions in the AHI in 22 subjects. Two individuals experienced an increase in their AHI despite a mean reduction in BMI of 18.4 kg/m2. Using AHI cutoffs for mild, moderate, and severe disease, the severity of OSA improved in only 50% of subjects. Of the 12 patients whose AHI improved sufficiently to reclassify their OSA severity, only 3 (12.5%) improved by more than 1 category of severity. Twenty-three patients had persistent OSA at follow-up. Only 1 patient had an AHI less than 5 and no longer met the criteria for a diagnosis of OSA. Most patients (n = 20, 83%) continued to have transient nocturnal hypoxia below 90%. Although only 7 patients (29%) subjectively complained of snoring postoperatively, all but 1 (96%) snored during the follow-up polysomnogram (p = 0.33). Significant improvements were noted on the ESS. However, nearly half reported a persistence of daytime somnolence, and more than half (n = 13, 54%) continued to have ESS scores greater than 10.
We noted significant sex difference between the initial and the follow-up AHIs. Men had higher baseline AHIs (93.7 ± 28.1 vs 32. 6 ± 18.0, p < 0.001) and experienced much larger absolute reductions than women (49.5 ± 26.7 vs 14.7 ± 13.5 events per hour, p < 0.001). Men tended to have higher relative reductions in their AHIs than did women (52% vs. 38%, p = 0.27). Despite the more dramatic reductions in the AHI, the men in our study continued to have more severe disease than the women on repeat polysomnography, as measured by the AHI (44.2 ± 23.5 vs 17.9 ± 9.9, p < 0.001). We observed a tendency for women's classification of OSA severity to improve more commonly than men's classification (61.1% vs 16.7%, p = 0.16).
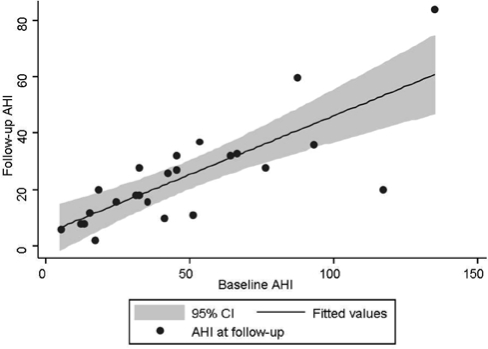
We used multivariate linear regression to identify predictors of the AHI following surgical weight loss. Candidate variables included the AHI and BMI at baseline, the absolute change in BMI or AHI, age, sex, and Malampati grade. After controlling for the baseline AHI (β coefficient = 0.777, p < 0.001), no other markers predicted the AHI following weight loss. The majority of the variance in the follow-up AHI was explained by the baseline AHI (R2 = 0.603), suggesting that the most important predictor of the AHI following weight loss is the baseline AHI (Figure 1). When we limited our regression models to those who achieved a BMI of less than 30 at follow-up, we again found that the only predictive marker of the follow-up AHI was the baseline AHI.
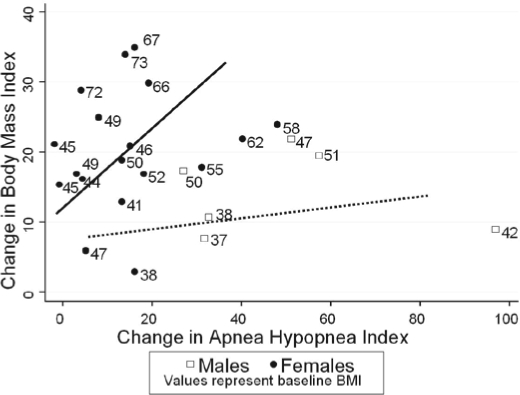
Figure 2.
Individual changes in body mass index versus apneahypopnea index. BMI refers to body mass index.
Figure 2.
Scatter plot of apnea-hypopnea index before and after weight loss. The thick line represents the line of best fit for the follow-up apnea-hypopnea index (AHI). The shaded gray area represents the 95% confidence interval (CI) around the best-fit line.
We also investigated the effects of surgical weight loss on CPAP requirements and compliance. Twenty of the 23 patients who had persistent OSA at follow-up underwent CPAP titration following weight loss. The mean pressure required to ablate apneic events was reduced by 3.1 ± 3.7 cm H2O (p = 0.001). Despite the high prevalence of residual OSA, only 6 individuals (26%) were compliant with CPAP therapy at the time of their follow-up. Those who had discontinued CPAP at the time of the follow-up visit included all 6 individuals with mild OSA (ESS 10.7 ± 3.7), 7 of 10 with moderate OSA (ESS 10.7 ± 4.4), and 4 of 7 with severe OSA (ESS 11.0 ± 4.1). Individuals who discontinued CPAP had similar measures of daytime somnolence (ESS = 10.8 ± 4.1 vs 11.5 ± 3.8, p = 0.53) and measures of OSA severity (AHI = 20 ± 20.5 vs 29 ± 5.8, p = 0.49) than did those who were compliant with CPAP. Despite the fact that all patients with OSA were observed to snore during the follow-up PSG, most patients (70%) reported that they were no longer snoring at the time of the follow-up visit. Individuals who denied snoring at the follow-up visit were more likely to have discontinued CPAP therapy than were individuals who reported persistent snoring (odds ratio = 10.0, 95% confidence interval = 1.2–81.8).
DISCUSSION
Surgical weight loss resulted in reductions in the AHI in nearly all patients. However, the majority of individuals in our study experienced persistent OSA. Patients and healthcare practitioners should recognize that reliance on weight loss as a “cure” for OSA may lead to an inappropriate cessation of CPAP therapy. Failing to recognize or treat persistent OSA may significantly impact health and quality of life, leading to ensuing weight gain and increased cardiovascular risks.
Despite numerous claims in the lay press that bariatric surgery can cure OSA, several studies have shown that OSA may persist following weight loss. These investigations showed substantial reductions in AHI with concomitant improvements in ESS.17,21 However, a very small minority of patients experienced resolution of obstructive events after sustained weight loss,27 and many patients continued to require CPAP therapy.22 Recurrence or worsening of sleep apnea has been observed following an initial weight reduction even without a concomitant weight increase.28 Reports that purport to show resolution of OSA in a majority of subjects do not describe how resolution was defined or if polysomnography was obtained after the weight loss.29
In our study, OSA was present in all individuals referred to our sleep clinic prior to bariatric surgery. Our cohort was similar to those in prior reports in that the severity of disease was high prior to weight loss. As expected, weight loss following bariatric surgery almost universally lowered the AHI and resulted in subjective improvements in somnolence. However, despite dramatic reductions in our patients' AHIs the overall prevalence and severity of OSA remained high. In spite of their ongoing disease, few patients continued to receive therapy for OSA. We observed that patients who felt that their snoring had resolved were at much higher risk of inappropriately discontinuing CPAP therapy. Unfortunately, resolution of subjective snoring did not predict reductions in the severity of OSA. To patients, snoring may have represented an important signal that they were suffering from a sleep-related disease. Perceived resolution of this signal may have led many of the patients in our study to assume that their OSA had resolved and, therefore, no longer warranted treatment. Patients and physicians need to recognize that subjective resolution of snoring does not equate to improvements or cure of OSA following weight loss. Finally, we show that the most important predictor of OSA severity following weight loss is the preoperative severity of disease, as measured by the AHI.
Our study has several limitations. Although we included all patients referred for preoperative sleep evaluation, our cohort represented only 20% of those undergoing bariatric procedures at our institution. Our study is therefore subject to selection bias, and these findings may not be applicable to the general population and may not reflect the true prevalence and severity of OSA among obese individuals considering bariatric surgery. However, we believe that our findings are real-world observations that are meaningful to clinicians, especially in view of numerous reports that have found a near universal presence of sleep apnea among morbidly obese individuals.11,12,15–17 In addition, the fact that OSA was observed in every referred patient suggests that the threshold for referral in our bariatric surgery clinic may be too high. The prevalence of OSA among patients pursuing bariatric surgery is becoming increasingly recognized. Symptoms-based referral practices are increasingly being replaced by universal sleep evaluations for candidates for bariatric surgery because clinical screening parameters may be falsely negative in this high-risk population. Another limitation is that we did not have anthropometric variables such as neck circumference, percentage of body fat, or waist-to-hip ratio on all subjects. These markers have been suggested as important risk factors for OSA. Inclusion of these markers may have enabled us to more accurately identify predictors of OSA severity following surgical weight loss. Our limited sample size precluded complex multivariate modeling to identify independent predictors of the follow-up AHI. However, the findings from our study do suggest that the most important predictor of the follow-up AHI is the baseline AHI. A final limitation concerns our definition of how we defined a meaningful response following surgical weight loss. For this paper, we evaluated whether bariatric surgery recategorized the severity of an individual's OSA using accepted, well-defined cutpoints of the AHI.20,26 Some authors have reported data concerning the effects of surgical weight loss on OSA by defining a clinically significant response as a 50% reduction in the AHI and an absolute AHI of less than 15 or less than 20. By applying these criteria to our study, only 3 or 4 patients, respectively, would have had a significant improvement in their OSA following surgical weight loss. Use of these criteria supports our concern that many, if not most, individuals will have persistent OSA despite significant weight loss following surgery.
Despite an elevated AHI, most of the patients in our study independently discontinued treatment for OSA. Individuals who discontinued CPAP had slightly lower measures of daytime sleepiness and were more likely to report a resolution of snoring despite polysomnographic evidence of persistent OSA. The results of a recent study by Buchner et al suggests that these patients may benefit from CPAP therapy despite improvement of their symptoms.30 The authors of this large prospective study report that treatment of individuals with mild to moderate OSA with CPAP reduced their absolute risk for experiencing a cardiovascular event within the subsequent decade (absolute relative risk = 28.5%, number needed to treat to prevent 1 event/10 years = 3.5). Treatment with CPAP remained an independent predictor of cardiovascular outcomes after adjusting for age, sex, BMI, and cardiovascular risk factors and disease (hazards ratio = 0.34, 95% confidence interval = 0.20–0.58). The authors conclude that CPAP therapy should be considered for primary and secondary prevention of cardiovascular events even in patients with mild OSA.
Future questions that remain to be answered include identifying which patients with OSA are likely to benefit from undergoing bariatric surgery. Surgical weight loss alone frequently does not cure OSA, although it does tend to reduce the severity of disease as measured by the AHI and may lower CPAP pressures required to ablate apneic events as was found in this study. Lower CPAP pressures may improve compliance with OSA treatment. However, no long-term outcome data exist to clearly demarcate how much of a reduction in the AHI or CPAP pressures is required to result in meaningful reductions in symptoms and comorbidities related to OSA. Until the impact of surgical weight loss is better defined, patients should be counseled that, although bariatric surgery may result in significant weight loss and improvement in other obesity-related comorbidities such as hypertension and diabetes, the patients are likely to continue to require treatment for OSA.
Despite significant weight loss, OSA persists in the majority of patients following bariatric procedures. Due to negative impacts on health and quality of life associated with OSA, it is recommended that patients continue CPAP therapy and undergo reevaluation with polysomnography to assess for residual disease following surgical weight loss regardless of potential subjective improvements in sleep-related symptoms.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The opinions expressed herein are not to be construed as official or as reflecting the policies of either the Department of the Army or the Department of Defense.
REFERENCES
- 1.Health Implications of Obesity. NIH Consensus Development Conference Statement. Ann Intern Med. 1985;103:1073–7. [PubMed] [Google Scholar]
- 2.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 4.Drenick EJ, Bale GS, Seltzer F, et al. Excessive mortality and causes of death in morbidly obese men. JAMA. 1980;243:443–5. [PubMed] [Google Scholar]
- 5.Garfinkel L. Overweight and cancer. Ann Intern Med. 1985;103:1034–6. doi: 10.7326/0003-4819-103-6-1034. [DOI] [PubMed] [Google Scholar]
- 6.Yaggi H, Mohsenin V. Sleep-disordered breathing and stroke. Clin Chest Med. 2003;24:223–37. doi: 10.1016/s0272-5231(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 7.Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. State of the art. Am J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Whitney CW, Redline S, et al. Sleep disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 9.Lavie P, Herer P, Peled R, et al. Mortality in sleep apnea patients: a multivariate analysis of risk factors. Sleep. 1997;20:377–80. doi: 10.1093/sleep/18.3.149. [DOI] [PubMed] [Google Scholar]
- 10.Peker Y, Hedner J, Norum J, et al. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 11.Rajala R, Partinen M, Sane T, et al. Obstructive sleep apnea syndrome in morbidly obese patients. J Intern Med. 1991;230:125–19. doi: 10.1111/j.1365-2796.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–6. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 13.Lettieri C, Eliasson A, Andrada T, et al. Obstructive sleep apnea syndrome: are we missing an at risk population. J Clin Sleep Med. 2006;1:285–9. [PubMed] [Google Scholar]
- 14.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31:S303–9. doi: 10.2337/dc08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83. doi: 10.1381/096089203322509228. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 17.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea in patients with clinically significant obesity. Obes Surg. 2003;13:58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 18.Shah M, Simha V, Garg A. Long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–31. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 19.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research; the report of an American Academy of Sleep Medicine task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 22.Guardiano SA, Scott JA, Ware JC, Schechner SA. The long-term results of gastric bypass on indexes of sleep apnea. Chest. 2003;124:1615–9. doi: 10.1378/chest.124.4.1615. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A, editors. Los Angeles, CA: University of California Los Angeles, Brain Information Service/Brain Research Institute; 1968. A manual of standardized techniques and scoring system for sleep stages of human sleep. [Google Scholar]
- 25.Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 26.American Sleep Disorders Association. Practice parameters for the indications for polysomnography and related procedures: Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20:406–22. [PubMed] [Google Scholar]
- 27.Sampol G, Muñoz X, Sagalés MT, et al. Long-term efficacy of dietary weight loss in sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:1156–9. doi: 10.1183/09031936.98.12051156. [DOI] [PubMed] [Google Scholar]
- 28.Pillar G, Peled R, Lavie P. Recurrence of sleep apnea without concomitant weight reduction increase 7.5 years after weight reduction surgery. Chest. 1994;106:1702–4. doi: 10.1378/chest.106.6.1702. [DOI] [PubMed] [Google Scholar]
- 29.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 30.Buchner NJ, Sanner BM, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med. 2007;76:1274–80. doi: 10.1164/rccm.200611-1588OC. [DOI] [PubMed] [Google Scholar]