Abstract
Objectives:
Research on the effects of sleep-disordered breathing (SDB) on sleep structure has traditionally been based on composite sleep-stage summaries. The primary objective of this investigation was to demonstrate the utility of log-linear and multistate analysis of the sleep hypnogram in evaluating differences in nocturnal sleep structure in subjects with and without SDB.
Methods:
A community-based sample of middle-aged and older adults with and without SDB matched on age, sex, race, and body mass index was identified from the Sleep Heart Health Study. Sleep was assessed with home polysomnography and categorized into rapid eye movement (REM) and non-REM (NREM) sleep. Log-linear and multistate survival analysis models were used to quantify the frequency and hazard rates of transitioning, respectively, between wakefulness, NREM sleep, and REM sleep.
Results:
Whereas composite sleep-stage summaries were similar between the two groups, subjects with SDB had higher frequencies and hazard rates for transitioning between the three states. Specifically, log-linear models showed that subjects with SDB had more wake-to-NREM sleep and NREM sleep-to-wake transitions, compared with subjects without SDB. Multistate survival models revealed that subjects with SDB transitioned more quickly from wake-to-NREM sleep and NREM sleep-to-wake than did subjects without SDB.
Conclusions:
The description of sleep continuity with log-linear and multistate analysis of the sleep hypnogram suggests that such methods can identify differences in sleep structure that are not evident with conventional sleep-stage summaries. Detailed characterization of nocturnal sleep evolution with event history methods provides additional means for testing hypotheses on how specific conditions impact sleep continuity and whether sleep disruption is associated with adverse health outcomes.
Citation:
Swihart BJ; Caffo B; Bandeen-Roche K; Punjabi NM. Characterizing sleep structure using the hypnogram. J Clin Sleep Med 2008;4(4):349–355.
Keywords: Sleep disruption, sleep-disordered breathing, sleep structure and event history modeling
Quantifying sleep fragmentation is central in assessment of sleep quality. Traditionally, measures such as the arousal frequency and sleep-stage percentages have been used to appraise sleep quality in research and clinical practice. Although conventional metrics of sleep structure have provided useful insight into the biology of sleep, these parameters explain only part of the variance in outcomes such as daytime sleepiness associated with conditions that fragment sleep.1–3 Furthermore, many of the conventional measures provide an overall summary of the entire night and unable to capture the temporal evolution of overnight events, the frequency of sleep-stage transitions, and the time between these transitions. Given the remarkable progress in our understanding of the neurobiology of the sleep-wake switch4 and the underlying neural circuitry responsible for transitioning between rapid eye movement (REM) and non-REM (NREM) sleep,5 adequately characterizing sleep-stage transitions is a priority to better define the influence of specific factors (e.g., age and sex) on normal sleep structure and organization. In addition, a careful portrayal of sleep-stage transitions is essential in clarifying the putative mechanisms through which conditions such as sleep-disordered breathing (SDB) mediate adverse health outcomes.
Several techniques have been used to derive measures of sleep quality that complement the repertoire of traditional metrics. Power spectral analysis of the sleep electroencephalogram (EEG),6 sleep spectrograms based on cardiopulmonary coupling,7 and visual identification of cyclical alternating patterns8 in sleep EEG have revealed clinically meaningful changes in the sleep structure in health and disease. Although these techniques provide unique insight into sleep continuity, their use requires specialized expertise along with an appreciation of the associated limitations. With improvements in digital technology, many of aforementioned techniques are automated and being increasingly incorporated in commercially available software.9 A relatively underutilized, but universally available, method for assessing sleep continuity is the hypnogram. The graphic representation of sleep-stage sequence across the night provides a visual depiction of the normal ultradian cycling of sleep. While the hypnogram provides a qualitative description of sleep structure, quantitative measures based on the hypnogram are not as commonly used in research or clinical practice as are other measures such as the frequency of arousals. Visual scoring of arousals is labor intensive, time consuming, and fraught with low to modest interscorer and intrascorer reliability. Even when coupled with the distribution of sleep-stage amounts, the frequency of arousals is unable to characterize the full extent of information embedded within the hypnogram. It is certainly plausible that a clinical disorder increases the frequency of sleep-stage transitions but has no material impact on the total amount of time spent in each stage or perhaps even the number of arousals. Tabulating the number of sleep-stage shifts can be helpful10,11 but is insufficient because it describes only one dimension of the hypnogram (i.e., number of shifts) while neglecting another (i.e., the time spent in a sleep stage before transitioning). Methods to describe temporal histories as depicted in the hypnogram are common in epidemiologic studies but have had limited application in sleep medicine. Although event-history models have been previously used in the context of examining determinants of sleep latency, such methods have not been employed in assessing the sleep-stage transitions and quantifying the impact of SDB on sleep structure.12–16 Thus, the primary objective of the current investigation was to determine whether event-history models are able to quantify sleep fragmentation using the overnight hypnogram. Specifically, log-linear models and multistate survival analysis methods were used to model the number and rate of sleep-stage transitions, respectively, in a community sample of middle-aged and older adults with and without SDB.
METHODS
Study Sample and Covariate Data
The current investigation used data from the Sleep Heart Health Study (SHHS), a multicenter study on SDB, hypertension, and cardiovascular disease.17 Subjects for the SHHS were recruited from ongoing cohort studies on cardiovascular and respiratory disease. Details regarding the design and methodology for recruiting and characterizing study subjects have been previously described.18 Approval for the study protocol was acquired from the institutional review board of each participating institution and informed consent was obtained from all subjects. The baseline visit included interviewer-administered questionnaires to assess prevalent medical comorbidities (e.g., hypertension and cardiovascular disease), smoking history, caffeine and alcohol consumption, race, sex, and age. Systolic and diastolic blood pressure, height, weight, and neck circumference were also obtained on the night of the polysomnogram.
To assess the independent effects of SDB on sleep structure, a matched subset of the SHHS cohort with and without SDB was selected for the current study. Subjects with moderate to severe SDB were identified as those with a respiratory disturbance index that exceeded the 90th percentile of the entire cohort (RDI ≥ 22.3 events/h). Subjects without SDB were identified as those with an RDI below the 25th percentile of the entire cohort (RDI < 1.33 events/h). Extremes of SDB severity were used to increase the likelihood of finding differences in conventional measures of sleep structure. Confounding due to demographic factors was minimized by matching subjects with and without SDB on age, sex, race, and body mass index (BMI). The limits imposed on age and BMI were such that no matching pair differed by more than 10 years (1 standard deviation of SHHS cohort) of age and 5 kg/m2 (1 standard deviation) in BMI. Other exclusion criteria included prevalent cardiovascular disease, hypertension, chronic obstructive pulmonary disease, asthma, coronary heart disease, history of stroke, and current smoking. Despite having a baseline cohort of 6441 subjects, only 60 subject pairs with and without SDB (n = 120) met the strict inclusion criteria outlined above and could be individually matched to each other.
Polysomnography
An overnight sleep study in the subject's home was conducted using the Compumedics P-series recording system (Compumedics, Australia). The recording montage included the following physiologic recordings: EEG (C3-A2 and C4-A1), right and left electroocculograms, single-lead electrocardiogram, chin electromyogram, measurement of abdominal and thoracic effort by impedance plethysmography, oxyhemoglobin saturation by pulse oximetry, airflow (oral-nasal thermistor), body position (by mercury gauge), and ambient light. All sleep recordings were sent to a centralized reading center for visual analysis. Details of polysomnographic equipment, hook-up procedures, failure rates, scoring criteria, and quality assurance have been previously described.18
Sleep-stage scoring was performed by trained technicians according to the published guidelines.19 Apneas were identified if airflow was absent or nearly absent for at least 10 seconds. Hypopneas were identified if discernible reductions in airflow or thoracoabdominal movement (at least 30% below baseline values) occurred for at least 10 seconds. The RDI was defined as the number of apneas or hypopneas, each associated with a 4% decrease in oxygen saturation, per hour of sleep. Arousals were identified as abrupt shifts in the EEG frequency for at least 3-seconds. In REM sleep, scoring of arousals also required a concurrent increase in activity of the chin electromyogram.20 The arousal index was defined as the average number of arousals per hour of sleep. Conventional parameters of sleep structure included sleep latency, total sleep time, sleep efficiency (total sleep time/time in bed), and percentages of NREM and REM sleep. Subjects with visually identified poor-quality EEG were not eligible for the current analysis. Other exclusionary criteria included poor-quality oximetry or respiratory signals and inability to visually score sleep.
Statistical Analysis
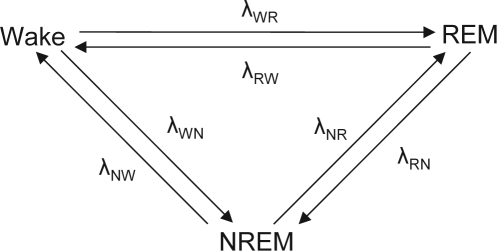
To characterize nocturnal event histories in the sleep hypnogram, two distinct methods were employed: multistate survival analysis and log-linear models. Multistate survival models describe a finite number of states together with all possible transitions that can occur between those states.21,22 The movement of subjects between states is governed by a set of transition rates (or hazard rates) that can be modeled using proportional hazards regression.23 In the context of modeling overnight stage transitions, sleep was represented using three states: wake, NREM sleep, and REM sleep (Figure 1). The multistate variant24 of the proportional hazards regression model that depicts the dynamics of sleep-stage transitions for subject i can be written as follows:
Figure 1.
A schematic of the six possible transitions between wake, REM [rapid eye movement], and non-REM [NREM]). λpq is the hazard rate of making the transition from stage p to stage q.
Here, the strata index s indicates the type of sleep-stage transition (e.g. NREM-to-REM, Figure 1), λo(s)(t) is the distinct baseline hazard rate for each type of sleep-stage transition, xi is an indicator variable for disease status (SDB versus no-SDB), and βs is the regression coefficient for strata specific log(transition rate) comparing those with SDB compared to those without SDB.25 Due to the fact that a subject can cycle through all three states several times during the night, six different types of transitions are distinguished, and each of these transitions can occur more than once. To estimate rates of transitioning in the multistate model, the data must be structured in a person-period format taking into account all possible competing transitions.26 For example, a NREM sleep duration that transitions into REM sleep would be expanded to two data records: NREM-to-REM transition (observed record) and NREM-to-wake transition (censored record). The designation of the former as “observed” and the latter as “censored” indicates the occurrence of the NREM-to-REM transition during a period of risk for either transition (see appendix). A stratified extension of proportional hazard models was fitted with the PHREG procedure in SAS (SAS Institute, Inc., Cary, NC). The robust sandwich variance estimator was used to account for intrasubject correlation, and ties were handled as proposed by Efron.27 The stratified proportional hazards model was used because it can incorporate several states (e.g., wake, NREM, and REM) between which transitions may take place at distinct hazard rates. The STRATA specification of the PHREG procedure allows model fitting when the hazard functions across groups can be assumed to be parallel for a particular transition type but not across the different types of transitions. Thus, the stratified proportional hazards model accommodates the requirement that the baseline hazard rates for the six different transitions shown in Figure 1 are not necessarily similar.
To model the frequency of transitions as a function of group status, Poisson log-linear models were employed.28 Poisson log-linear models, a specialized case of generalized linear models, are commonly used to model contingency tables. In the context of modeling the frequency of sleep-stage transitions, there are two distinct groups that can each repeatedly experience six possible transition types. The basic concept of the log-linear modeling involves fitting a model to the observed frequencies contained within the 2 × 6 contingency table. The model is parameterized for row and column effects as follows:
In the above equation, log(Fab) is the log of the expected cell frequency for cell ab in the contingency table; μ is an intercept (the referent cell's mean natural log of expected frequency); ϕaG andϕbS represent the main effects of group status and transition-type, respectively; and ϕabGS estimates the interaction between group and transition-type effects. Generalized estimating equations were used to account for the interdependence among the cells.29,30 As opposed to the multistate approach, which models events over time and accounts for censoring, the structure of the data for log-linear analysis is only concerned with the number of the transitions observed. The coefficients produced by this model and linear combinations thereof were appropriately transformed to render estimates of relative frequencies of particular sleep-stage transition types as a function of group status (SDB versus no SDB). The log-linear analysis was conducted using the GENMOD procedure in SAS. Both the multistate and log-linear models included the matching variables age, sex, race, and BMI. All p values are for 2-sided tests.
RESULTS
A matched sample of 60 subjects with and without SDB was identified from the SHHS cohort. As expected, the two groups were similar with respect to age, sex, and race (Table 1). However, a small but statistically significant difference was noted in the BMI between subjects with and without SDB. Restricting the matching limits in BMI to less than 5 kg/m2 or age to less than 10 years to improve the degree of matching led to a significant decrease in the overall sample size. Thus, BMI, age, and other matching covariates were included in all multivariable models. Subjects with SDB had a mean RDI of 34.0 events per hour (median: 30.6, interquartile range: 26.4–39.1), whereas those without SDB had a mean RDI of 0.63 events per hour (median: 0.67, interquartile range: 0.32–0.91). As expected, the overall arousal frequency was higher in SDB subjects, compared with healthy subjects (no SDB). Surprisingly, despite obviously large differences in disease severity (i.e., RDI), conventional measures of sleep structure such as total sleep time, percentage of total sleep time in each sleep stage, and sleep efficiency were similar between the two groups (Table 1).
Table 1.
Characteristics of Subjects With and Without Sleep-Disordered Breathing (SDB)
| Variable | SDB | No SDB | p valuea |
|---|---|---|---|
| Demographic | |||
| Age, y | 62.7 ± 10.8 | 62.3 ± 10.6 | 0.31 |
| Male, no. (%) | 38 (63.3) | 38 (63.3) | 1.00 |
| White, no. (%) | 52 (86.7) | 52 (86.7) | 1.00 |
| BMI, kg/m2 | 30.7 ± 5.2 | 29.2 ± 4.5 | < 0.0001 |
| Polysomnographic | |||
| RDI, events/h | 34.0 ± 12.1 | 0.63 ± 0.4 | < 0.0001 |
| Total sleep time, min | 353.3 ± 59.5 | 362.9 ± 56.3 | 0.38 |
| Sleep latency, minb | 20.5 ± 15.7 | 22.1 ± 18.5 | 0.69 |
| Sleep efficiency, % | 81.9 ± 10.3 | 83.0 ± 9.2 | 0.98 |
| Sleep stage, %c | |||
| 1 | 6.5 ± 4.5 | 5.7 ± 3.5 | 0.21 |
| 2 | 58.6 ± 10.5 | 57.3 ± 11.8 | 0.52 |
| SWS | 15.6 ± 11.6 | 16.0 ± 12.5 | 0.86 |
| REM | 19.2 ± 7.2 | 21.0 ± 5.6 | 0.09 |
| Arousal frequency, events/h | 28.0 ± 12.0 | 13.7 ± 5.7 | < 0.0001 |
Data are presented as mean ± SD unless otherwise indicated.
Group differences by sleep-disordered breathing (SDB) status were determined by the Wilcoxon signed-rank test for categorical variables and paired t test for continuous variables.
Denotes the latency to the first onset of sleep.
Stage 1, stage 2, slow-wave (SWS), and rapid eye movement (REM) sleep are expressed in percentage of total sleep time. SWS represents the combination of sleep stages 3 and 4.
The transition frequencies of wake-to-NREM sleep and NREM sleep-to-wake were significantly higher in subjects with SDB (Table 2). Log-linear models revealed that SDB conferred a 26% and 32% increase in propensity of wake-to-NREM sleep and NREM sleep-to-wake transitions, respectively. The higher relative frequency of these two transition types suggests that SDB can disrupt sleep continuity with oscillations between NREM sleep and wakefulness. The log-linear model also showed that SDB increases the number of transitions from wake-to-REM sleep. Multistate models examining the hazards of each sleep-stage transition type revealed that there was an increase in the rate of wake-to-NREM sleep and NREM sleep-to-wake transitions (Table 2), confirming the findings of the log-linear analysis. The adjusted hazard rate ratios of wake-to-NREM sleep and NREM sleep-to-wake transitions were 1.10 (95% confidence interval: 1.02, 1.20) and 1.50 (95% confidence interval: 1.30, 1.74), respectively. In addition, multistate models demonstrated an increase in the rate for REM sleep-to-wake, depicting those with SDB having a 2.26 (95% confidence interval: 1.51, 3.40) times greater likelihood of transitioning from REM sleep to wakefulness than those without SDB.
Table 2.
Results from Log-Linear and Multistate Models for Sleep-Stage Transitions in SDB for the Entire Night
| Sleep-stage transition | Log-linear analysis |
Multistate analysis HRb |
||||||
|---|---|---|---|---|---|---|---|---|
| Frequency of transitionss |
RRb |
|||||||
| SDB | No SDB | RR | 95% CI | p value | HR | 95% CI | p value | |
| Wake → NREM | 1725 | 1368 | 1.26 | 1.07, 1.48 | 0.005 | 1.10 | 1.02, 1.20 | 0.02 |
| NREM → Wake | 1579 | 1200 | 1.32 | 1.11, 1.56 | 0.001 | 1.50 | 1.30, 1.74 | <0.0001 |
| NREM → REM | 346 | 351 | 1.02 | 0.81, 1.29 | 0.85 | 1.04 | 0.68, 1.57 | 0.87 |
| REM → Wake | 358 | 324 | 1.17 | 0.91, 1.50 | 0.21 | 2.26 | 1.51, 3.40 | <0.0001 |
| REM → NREM | 160 | 134 | 1.20 | 0.90, 1.59 | 0.20 | 1.57 | 0.89, 2.77 | 0.12 |
| Wake → REM | 175 | 114 | 1.42 | 1.02, 1.96 | 0.04 | 0.86 | 0.49, 1.50 | 0.60 |
Frequency of transitions comparing subjects with sleep-disordered breathing (SDB) to those without SDB (No SDB).
Results are adjusted for age, sex, race, and body mass index. RR refers to relative ratio and HR refers to hazard ratio; CI, confidence interval; REM, rapid eye movement NREM, non-REM.
Recognizing the overnight heterogeneity in the distribution of NREM and REM sleep and in the frequency of sleep-stage transitions over the course of the night, analyses were performed dividing each subject's total sleep period into two segments. Accounting for differences in total sleep time, the first and second segments of sleep were determined as the first half and second half, respectively, of each subject's total sleep time, as opposed to using a global arbitrary cutpoint. Log-linear and multistate models were then reconstructed for each segment of the sleep period (Table 3). Analyses by segment of the night showed that those with SDB had higher rates of awakening from both NREM and REM sleep in both segments. Transition frequencies for wake-to-NREM sleep and NREM sleep-to-wake were significantly higher in SDB subjects regardless of segment of night, confirming the overall-night results. It was also found that subjects with SDB had a higher transition frequency for wake-to-REM sleep and REM sleep-to-wake in the first but not in the second half of the sleep period. Finally, multistate analyses showed that, compared with the first half of the sleep period, subjects with SDB also displayed a greater propensity for sleep fragmentation, with significantly higher rates of wake-to-NREM sleep and REM-to-NREM transitions in the second half of the sleep period.
Table 3.
Results from Log-Linear and Multistate Models for Sleep-Stage Transitions by Segments Of Night
| Sleep-stage transition | Log-linear analysis |
Multistate analysis HRb |
||||||
|---|---|---|---|---|---|---|---|---|
| Frequency of transitionsa |
RRb |
|||||||
| SDB | No SDB | RR | 95% CI | p value | HR | 95% CI | p value | |
| First segment of night | ||||||||
| Wake → NREM | 764 | 611 | 1.26 | 1.07, 1.48 | 0.005 | 1.09 | 0.99, 1.20 | 0.08 |
| NREM → Wake | 712 | 553 | 1.30 | 1.10, 1.54 | 0.002 | 1.46 | 1.20, 1.78 | 0.0002 |
| NREM → REM | 145 | 149 | 1.07 | 0.87, 1.32 | 0.53 | 1.16 | 0.71, 1.90 | 0.56 |
| REM → Wake | 125 | 96 | 1.38 | 1.05, 1.81 | 0.02 | 2.77 | 1.55, 4.93 | 0.001 |
| REM → NREM | 69 | 72 | 1.05 | 0.80, 1.37 | 0.73 | 1.05 | 0.56, 1.96 | 0.88 |
| Wake → REM | 63 | 32 | 1.87 | 1.29, 2.72 | 0.001 | 1.05 | 0.37, 3.00 | 0.92 |
| Second segment of night | ||||||||
| Wake → NREM | 961 | 757 | 1.26 | 1.02, 1.55 | 0.03 | 1.12 | 1.01, 1.24 | 0.03 |
| NREM → Wake | 867 | 647 | 1.33 | 1.06, 1.67 | 0.01 | 1.53 | 1.27, 1.86 | <0.0001 |
| NREM → REM | 201 | 202 | 1.01 | 0.76, 1.33 | 0.96 | 0.96 | 0.58, 1.62 | 0.89 |
| REM → Wake | 233 | 228 | 1.06 | 0.81, 1.37 | 0.68 | 2.08 | 1.29, 3.35 | 0.003 |
| REM → NREM | 91 | 62 | 1.19 | 0.85, 1.68 | 0.31 | 2.37 | 1.12, 5.02 | 0.02 |
| Wake → REM | 112 | 82 | 1.15 | 0.82, 1.6 | 0.43 | 0.79 | 0.41, 1.52 | 0.48 |
Frequency of transitions comparing subjects with sleep-disordered breathing (SDB) to those without SDB (No SDB).
Results are adjusted for age, sex, race, and body mass index. RR refers to relative ratio and HR refers to hazard ratio; CI, confidence interval; REM, rapid eye movement NREM, non-REM.
DISCUSSION
The primary objective of the current study was to investigate whether characterizing sleep through log-linear and multistate analyses would demonstrate differences in sleep structure between subjects with and without SDB. Using a matched sample of middle-aged and older adults recruited from the general community, the current investigation demonstrates that, in the absence of confounding medical conditions, conventional measures of sleep structure were similar between those with and without SDB despite being at the extremes of health and disease, respectively. In contrast, log-linear and multistate models showed notable differences in sleep structure between the two groups. Subjects with SDB were noted to have a greater number and higher rates of sleep-stage transitions than those without SDB, suggesting that the occurrence of apneas and hypopneas during sleep can alter the duration spent in distinct sleep stages throughout the night without altering the overall summaries of sleep-stage amounts or total sleep time. In addition, the present study also indicates that the propensity to transition from one stage to another is different between the first and second half of the sleep period.
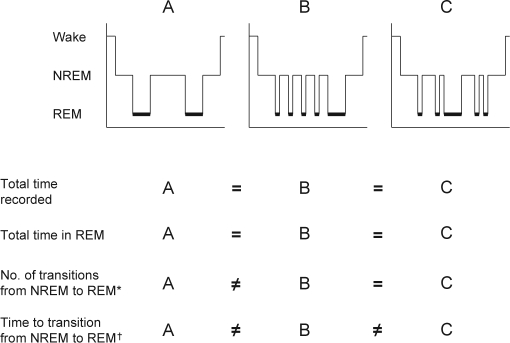
The finding that SDB disrupts sleep continuity is not unexpected. It is well established that apneas and hypopneas often terminate with a brief EEG arousal. Recurrent arousals lead to state instability with recurrent to-and-fro transitions between different sleep stages. Event-related arousals from sleep are not all equal because there is much interindividual and intraindividual variation in whether an apnea or hypopnea leads to a shift between sleep stages or a transition to wakefulness. The results of the current study illustrate that conventional measures of sleep structure tend to collapse a temporally evolving process and limit the ability to reach inferences regarding secular trends across groups. Nevertheless, composite summary measures provide useful and necessary information because knowing if the total time spent in a stage is similar across groups assists in distinguishing whether sleep is actually more fragmented or the greater frequency of transitions is simply a result of differences in total sleep time. In addition to characterizing transition frequencies and rates, event history methods, as employed herein, also afford modeling of directionality of transitions. For example, a state change from wake-to-NREM sleep is clearly distinct from the transition of NREM sleep-to-wake.Moreover, the methods of multistate survival analysis allow the dynamic notion of competing risks to be applied to the evolution of sleep, in which a transition from one to any of the other states is possible. Such distinction of state evolution of sleep is not possible with sleep-stage percentages or arousal counts because markedly differing sleep profiles can be congruent on these measures. Even counting the number of sleep-stage transitions is insufficient because it does not describe the time or rate of a particular type of sleep-stage transition. As shown in Figure 2, the percentages of NREM and REM sleep across different hypnograms can be similar, whereas overt differences can exist in the number and the rate of sleep-stage transitions. Log-linear and multistate models quantify these differences that are often visually apparent in the hypnogram.
Figure 2.
Differences in results obtained from survival and log-linear analysis of sleep-stage transitions illustrated using 3 hypothetical hypnograms. Total time recorded and time in rapid eye movement (REM) sleep are equivalent among the three hypnograms, demonstrating the limits of information gained by relying on composite summary measures alone to capture differences in sleep structure. Number of transitions from non-REM (NREM) to REM allow for a quantitative distinction to be made between profiles A and B and between A and C but not between B and C. Time to transition from NREM to REM provides a fuller description of profiles, enabling a quantifiable distinction among the three hypnograms.
*Differences detectable with log-linear modeling
†Differences detectable with multi-state survival analysis
There are several strengths of this study that merit discussion. The exclusion of medical comorbidities and matching on race, sex, BMI, and age minimized the concern for confounding and permitted a thorough assessment of the independent effects of SDB on sleep structure. Given that SDB is commonly associated with substantial medical comorbidity, identifying a sample of matched subjects free of such conditions is a major strength. Furthermore, the finding of similar conventional sleep-stage summaries in subjects with and without SDB in our sample highlights the importance of characterizing the temporal evolution of sleep with methods that capitalize on distinct dimensions (i.e., frequency and rate) of an event. Availability of sleep recordings in the home is an additional strength because home-based studies limit the potential of the “first-night” effect on sleep structure that is common with in-laboratory polysomnograms.31–33 Finally, the use of a well-characterized cohort recruited from the general-community cohort minimizes potential biases that are often inherent in clinic-based samples.
Despite these strengths, the current study has several limitations. First, a simplified approach for the assessment of transition frequency and rate was used that did not include distinct stages of NREM sleep. The decision to limit classification to wake, NREM sleep, and REM sleep was driven by the need to answer the question of whether event-history methods provided any additional insight into the macrostructure of sleep. In light of the robust findings from modeling the three states, further application of these methods to the assessment of sleep structure with distinct NREM sleep stages represents a logical extension. Second, because both of the methods employed in our analyses are based on visually scored sleep stages, poor reliability of scoring could impact the derived inferences. As observed by others, scoring of stage 1 sleep was least reliable in the SHHS. However, if stages are recoded as wake, NREM sleep, and REM sleep, as was needed for the log-linear or multistate analyses, interscorer comparisons in the SHHS yield kappa statistics in the range of 0.87 to 0.90.34 Third, stratification by sleep-stage transition type and further by segments of the sleep period diminishes the power to detect differences, especially if particular transition types occur infrequently (e.g., wake-to-REM sleep). However, the current analyses set the stage for future work with the entire SHHS cohort that will provide sufficient power to identify potential determinants of sleep-stage transitions. Fourth, a distinct feature of the proportional hazards model is that it leaves the underlying hazard for a specific type of transition unspecified. Although this is a major strength of the proportional hazards model, knowing the hazard rate in the reference group is sometimes desirable. Implementation of parametric models can provide these reference hazards and represent yet another extension of the current work.35 Finally, the methods proposed herein characterize the continuity of sleep using 30-second epochs and thus cannot fully depict events (e.g., arousals or periods of microsleep) that occur within the epoch. Nonetheless, event-history methods can be applied to sleep stages that are scored using a shorter epoch period (e.g., 4 seconds) to better describe sleep microstructure.
The major implication of this study is that the characterization of sleep structure in SDB and other sleep disorders is better served by encompassing new quantitative characterizations along with the classical measures, particularly to aptly test hypotheses regarding the function and health-related effects of normal and abnormal sleep. Within this broader scope, understanding various dimensions of sleep continuity may carry significance with regard to predicting the relationship between sleep and general health. In light of findings of the current study, the newly suggested approaches of examining sleep structure could provide a more thorough understanding of how comorbidities affect sleep, as well as how normal sleep function, in turn, fulfills a crucial role in health and disease.
APPENDIX
The following table illustrates the data expansion necessary for multistate survival analysis. On the left, a data record of 1 state change is shown. Subject i transitions from non-rapid eye movement (NREM)-to-rapid eye movement (REM) sleep. To convert this record to a person-period format, the record would be expanded to 2 records that reflect all possible transitions from NREM sleep. These include a NREM-to-REM transition (observed) and NREM-to-wake transition (censored).
| id | Transition type | Duration of state, min | id | Transition type | Duration of state, min | Observed (1) or Censored (0) | |
|---|---|---|---|---|---|---|---|
| i | NREM → REM | 24.3 | → | i | NREM → REM | 24.3 | 1 |
| i | NREM → Wake | 24.3 | 0 |
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Carskadon MA, Brown ED, Dement WC. Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging. 1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 2.Chervin RD, Aldrich MS. Characteristics of apneas and hypopneas during sleep and relation to excessive daytime sleepiness. Sleep. 1998;21:799–806. [PubMed] [Google Scholar]
- 3.Wesensten NJ, Balkin TJ, Belenky G. Does sleep fragmentation impact recuperation? A review and reanalysis. J Sleep Res. 1999;8:237–245. doi: 10.1046/j.1365-2869.1999.00161.x. [DOI] [PubMed] [Google Scholar]
- 4.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 6.Knott JR, Gibbs FA, Henry CE. Fourier transforms of the electroencephalogram during sleep. J Exp Psychol. 1942;31:465–477. [Google Scholar]
- 7.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–1161. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 8.Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP) Sleep Med Rev. 2000;4:101–123. doi: 10.1053/smrv.1999.0083. [DOI] [PubMed] [Google Scholar]
- 9.Penzel T, Conradt R. Computer based sleep recording and analysis. Sleep Med Rev. 2000;4:131–148. doi: 10.1053/smrv.1999.0087. [DOI] [PubMed] [Google Scholar]
- 10.Merica H, Blois R, Bovier P, Gaillard JM. New variables for defining sleep continuity. Physiol Behav. 1993;54:825–831. doi: 10.1016/0031-9384(93)90289-r. [DOI] [PubMed] [Google Scholar]
- 11.Haba-Rubio J, Ibanez V, Sforza E. An alternative measure of sleep fragmentation in clinical practice: the sleep fragmentation index. Sleep Med. 2004;5:577–581. doi: 10.1016/j.sleep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Fahrmeir L, Klinger A. A nonparametric multiplicative hazard model for event history analysis. Biometrika. 1998;85:581–592. [Google Scholar]
- 13.Punjabi NM, O'hearn DJ, Neubauer DN, et al. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159:1703–1709. doi: 10.1164/ajrccm.159.6.9808095. [DOI] [PubMed] [Google Scholar]
- 14.Yassouridis A, Steiger A, Klinger A, Fahrmeir L. Modelling and exploring human sleep with event history analysis. J Sleep Res. 1999;8:25–36. doi: 10.1046/j.1365-2869.1999.00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–314. [PubMed] [Google Scholar]
- 16.Norman RG, Scott MA, Ayappa I, Walsleben JA, Rapoport DM. Sleep continuity measured by survival curve analysis. Sleep. 2006;29:1625–1631. doi: 10.1093/sleep/29.12.1625. [DOI] [PubMed] [Google Scholar]
- 17.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 18.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington: US Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 20.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 21.Kay R. The analysis of transition times in multistate stochastic-processes using proportional hazard regression-models. Commun Stat Part A-Theory Methods. 1982;11:1743–1756. [Google Scholar]
- 22.Islam MA, Singh KP. Multistate survival models for partially censored data. Environmetrics. 1992;3:223–234. [Google Scholar]
- 23.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 24.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11:91–115. doi: 10.1191/0962280202SM276ra. [DOI] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling Survival Data. New York: Springer; 2001. The Cox Model; pp. 39–76. [Google Scholar]
- 26.Therneau TM, Grambsch PM. Modeling Survival Data. New York: Springer; 2001. Multiple Events per Subject; pp. 169–229. [Google Scholar]
- 27.Efron B. Efficiency of coxs likelihood function for censored data. J Am Stat Assoc. 1977;72:557–565. [Google Scholar]
- 28.Agresti A. An introduction to categorical data analysis. New York: Wiley; 1996. [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 30.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 31.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt HS, Kaelbling R. The differential laboratory adaptation of sleep parameters. Biol Psychiatry. 1971;3:33–45. [PubMed] [Google Scholar]
- 33.Browman CP, Cartwright RD. The first-night effect on sleep and dreams. Biol Psychiatry. 1980;15:809–812. [PubMed] [Google Scholar]
- 34.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 35.Hosmer DW, Jr, Lemeshow S. New York: John Wiley & Sons, Inc; 1999. Applied survival analysis: regression modeling of time to event data. [Google Scholar]