Abstract
Background:
Adaptive servoventilation (ASV) can be effective therapy for specific types of central apnea such as Cheyne-Stokes respiration (CSR). Patients treated chronically with opioids develop central apneas and ataxic breathing patterns (Biot's respiration), but therapy with CPAP is usually unsuccessful. There are no published studies of ASV in patients with sleep apnea complicated by chronic opioid therapy.
Methods:
Retrospective analysis of 22 consecutive patients referred for evaluation and treatment of sleep apnea who had been using opioid medications for at least 6 months, had an apnea-hypopnea index (AHI) >20/h, and had been tested with ASV. Baseline polysomnography was compared with CPAP and ASV. Outcome variables: AHI, central apnea index (CAI), obstructive apnea index (OAI), hypopnea index (HI), desaturation index, mean SpO2, lowest SpO2, time SpO2 <90%, and degree of Biot's respiration.
Results:
Mean (SD) AHI measured 66.6/h (37.3) at baseline, 70.1/h (32.6) on CPAP, and 54.2/h (33.0) on ASV. With ASV, the mean OAI was significantly decreased to 2.4/h (p < 0.0001), and the mean HI increased significantly to 35.7/h (p < 0.0001). The decrease of CAI from 26.4/h to 15.6/h was not significant (p = 0.127). Biot's breathing persisted, and oxygenation parameters were unimproved with ASV.
Conclusions:
Due to residual respiratory events and hypoxemia, ASV was considered insufficient therapy in these patients. Persistence of obstructive events could be due to suboptimal pressure settings (end expiratory and/or maximal inspiratory). Residual central events could be related to fundamental differences in the pathophysiology of CSR compared to opioid induced breathing disturbances.
Citation:
Farney RJ; Walker JM; Boyle KM; Cloward TV; Shilling KC. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med 2008;4(4):311-319.
Keywords: Opioid medications, sleep disordered breathing, obstructive sleep apnea, central sleep apnea, Biot's respiration, adaptive servoventilation, continuous positive airway pressure, polysomnography
Adaptive servoventilation (ASV) appears to be an effective therapy for Cheyne-Stokes respiration (CSR).1–3 ASV also appears to be effective for patients with complex sleep apnea syndrome (CompSAS), a term used to describe central apneas that develop in patients with obstructive sleep apnea when CPAP is applied.4,5 As previously reported,6–10 chronic opioid therapy is associated with a variety of respiratory disturbances during sleep, including central apnea. However, there have been no published studies regarding the efficacy of ASV in patients who have sleep disordered breathing related to chronic opioid therapy.
There are numerous factors which may induce periodic breathing (CSR),11–14 but the critical factor leading to central apnea is the gap between eucapnia and the hypocapnic threshold.11,15,16 Depending upon the position and slope of the individual's ventilatory response to CO2 and his or her set point for apnea, relatively modest changes in ventilation can reduce the PaCO2 below the apneic threshold, thereby inhibiting ventilation until the accumulation of CO2 is sufficient to stimulate respiratory neurons. That is, a narrow difference or gap between the prevailing CO2 during normal ventilation and the apneic threshold increases ones susceptibility to central apneas. It is possible to widen the gap either by manipulating the hypocapnic threshold itself17–19 or by increasing the PaCO2. Adaptive servoventilation machines track the patient's breathing pattern and, using an internal algorithm, adjust breath-by-breath inspiratory pressure support to maintain a slightly reduced minute ventilation to prevent periodic hyperventilation and episodic hypocapnia. Thus, the breathing pattern is stabilized and central apneas are prevented.1 It seems that ASV might function optimally when there is a more or less regular “predictable” respiratory pattern which can be used by the machine to track ventilation and then to drive the bilevel device.
In contrast, respiration associated with chronic exposure to opioids is usually irregular and is best characterized as ataxic breathing or Biot's respiration,6,8 which is readily distinguished by the completely erratic ventilatory pattern of rate and tidal volume. In addition to having an underlying ataxic breathing pattern, these patients also tend to respond paradoxically to CPAP with more central apneas (similar to CompSAS patients). Since ASV has never been evaluated in patients who have sleep disordered breathing complicated by chronic opioid therapy, the purpose of this study was to assess the efficacy of ASV in these patients.
METHODS
IRB Approval
The institutional review board of the hospital approved the study protocol and waived patient consent requirement.
Patient Selection
Subjects were referred to the Intermountain Sleep Disorders Center at LDS Hospital, Salt Lake City, Utah (elevation 1,500 m), for testing and therapy of suspected sleep apnea. The records were reviewed of all patients tested with ASV since February 2006, when ASV became available in our laboratory. Patients were selected if they had previously undergone baseline diagnostic polysomnography showing an apnea-hypopnea index (AHI) ≥ 20/h and had been treated with opioid medications on a daily basis ≥ 6 months. The majority of these patients underwent CPAP titration between the diagnostic study and the ASV study. Clinical information was obtained from sleep medicine consultation and/or comprehensive sleep medicine questionnaires routinely completed by all patients studied. Patients were excluded if they had CSR.
Protocol
Attended 17-channel polysomnography (Cadwell Laboratories, Inc., Kennewick, WA) was performed consisting of central (C3/A2 and C4/A1) and occipital (O1/A2 and O2/A1) electroencephalogram, right and left electrooculogram, and submentalis electromyogram. During baseline polysomnography, airflow was detected by air pressure transducers in the nares (PTAF II, Pro-Tech Services, Inc. Mukilteo, WA). During CPAP and ASV testing, the PTAF device was attached to the tubing immediately adjacent to the mask. Respiratory effort was determined by measurement of chest and abdomen motion with piezoelectric transducers. The arterial oxygen saturation by pulse oximetry (SpO2) was measured simultaneously by an internal Cadwell oximeter set in the 4-beat averaging mode and a backup external oximeter (Masimo Rad-9, Masimo Corporation, Irvine, CA) set in the 2-sec averaging mode. Standard protocols for our laboratory were used to titrate CPAP, and a variety of masks were employed according to patient preference. With ASV, full face masks were used, and any evidence of leak was monitored and corrected. Adaptive servoventilation testing was accomplished with a ResMed VPAP adapt SV (ResMed Ltd., NSW, Australia). Initially we used default settings as recommended by the manufacturer (end expiratory pressure 5.0 cm H2O, minimum inspiratory pressure support 3.0 cm H2O, maximum inspiratory pressure support 10.0 cm H2O, and backup rate of 15/min). Recently, we have titrated end-expiratory pressure (EEP) to a maximum of 10.0 cm H2O or as tolerated if respiratory events were not eliminated using default settings.
The decision to perform a particular polysomnographic protocol or to be tested with ASV was made by the physician responsible for the care of the patient. Some patients underwent a split-night study with and without titration of CPAP (N = 5). In these cases, baseline data was obtained from the diagnostic phase of the recording. Split-night studies using ASV were not performed. In cases where continuous positive pressure breathing failed to correct hypoxemia, oxygen was sometimes added, however only data obtained while breathing room air was compared for the purposes of statistical analysis in this study. Three patients required continuous oxygen therapy while awake and therefore were tested while receiving oxygen during both the baseline and ASV polysomnograms. One patient was studied while breathing room air during the baseline and CPAP study but was tested with ASV and oxygen.
Analysis
Sleep was scored according to standard criteria.20 Apneas were scored on the basis of absence of airflow ≥ 10 sec. Obstructive apneas were defined by the presence of respiratory effort; central apneas required the absence of respiratory effort and hypopneas by reduction in airflow ≥ 10 sec and ≥ 3% decrease in SpO2.21 In accordance with standard scoring, hypopneas were not differentiated as obstructive or central. Computation of the respiratory events using the airflow signal obtained from PTAF II was confounded during ASV because of the pressure signal generated by the programmed backup rate of the VPAP adapt SV machine. Therefore, respiratory events characterized by absent thoracic and abdominal motion ≥ 10 sec were scored as central apnea. AHI, as well as obstructive apnea, central apnea, and hypopnea indices were computed as the total of defined respiratory events divided by the total sleep time in hours. The percent time SpO2 was below 90%, lowest SpO2, mean SpO2, and desaturation index (defined as the number of SpO2 decreases ≥ 4% divided by the total sleep time in hours) were calculated for each condition during the room air portion of the study.
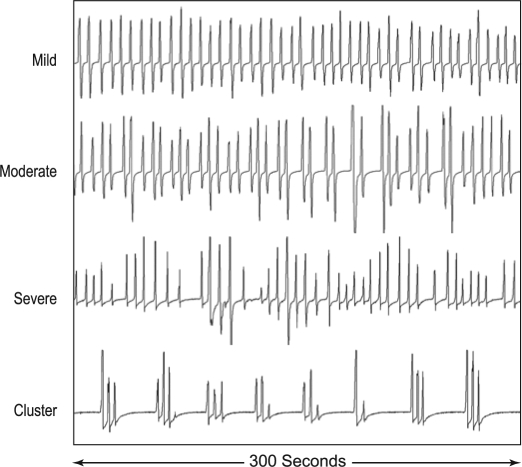
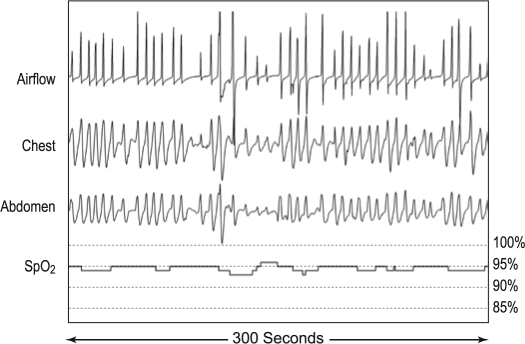
Respiratory patterns were evaluated for ataxic breathing or Biot's respiration by one author (RJF). Pattern recognition was best when records were viewed using 120- to 300-sec epochs. The irregular respiratory patterns normally seen in REM sleep were excluded from consideration. As with atrial fibrillation, quantification of variability of an irregular pattern can be very difficult. Based upon the degree of irregularity or presence of cluster breathing as illustrated in Figure 1, we classified the severity of Biot's breathing as follows: Normal = no or rare variability of rate and tidal volume; Mild = predominantly mild to occasionally moderate variability; Moderate = moderate ataxia clearly present throughout most of the record; and Severe = severe variability of rate and tidal volume and/or cluster breathing throughout the majority of the record. Note that both moderate and severe categories required at least unambiguous variability or cluster breathing throughout the majority of NREM sleep. Scoring of Biot's breathing while being tested with ASV cannot be blinded because of the air pressure (“airflow”) signals generated by the ASV device. In these cases, it is necessary to inspect the respiratory pattern using thoracic and abdominal motion signals.
Figure 1.
Variations of “Biot's Breathing”. Air flow patterns obtained from PTAF signals showing varying degrees of ataxic or irregular breathing (mild, moderate and severe) plus an example of “cluster breathing” obtained from patients in this series who were chronically receiving opioid medications. Note the marked variability of VT and frequency in the moderate and severe categories.
Statistical Analysis
Means and standard deviations were calculated for all respiratory variables and standard sleep parameters. In addition, variables under the ASV conditions were compared to baseline. A D'Agostino-Pearson test for normality was conducted for each variable within conditions. If the assumptions of normality were met, then a t-test for repeated measures was used for comparisons. Otherwise, a Wilcoxon test was used. A Bonferroni correction was applied to control for probability of a type I error. The primary outcome of the AHI was considered significantly different if p < 0.05 (2-tailed test). Significant differences were concluded for the components of AHI (obstructive apnea index, central apnea index, and hypopnea index) if p < 0.017 (0.05/3). For similar reasons, significant differences were concluded for mean SpO2, SpO2 <90%, and low SpO2 if p < 0.017.
RESULTS
Demographics
The patient demographics are shown in Table 1. There were 9 males and 13 females. In general, these patients were middle aged (mean 50.1 years old), obese (mean BMI 32.9 kg/m2), and sleepy (Epworth Sleepiness Scale score mean 12.5). There was a considerable range in both age (22–76 years) and weight (BMI 21.9–47.6 kg/m2). Opioid therapy had been prescribed only for non-malignant pain (e.g., back pain, fibromyalgia, and neuropathy). There were frequent comorbidities (depression in 5 males and 11 females; hypertension in 3 males and 8 females).
Table 1.
Patient Demographics
| MALES (n= 9) |
FEMALES (n = 13) |
ALL (n = 22) |
|
|---|---|---|---|
| Age (SD) y | 49.2 ± 9.3 | 50.8 ± 14.8 | 50.1 ± 12.6 |
| Weight (SD) kg | 102.4 ± 19.8 | 90.1 ± 19.1 | 95.2 ± 19.9 |
| Height (SD) cm | 179.9 ± 6.9 | 163.0 ± 7.8 | 169.9 ± 11.2 |
| BMI kg/m2 | 31.8 ± 6.5 | 33.7 ± 5.9 | 32.9 ± 6.1 |
| ESS (normal <10) | 13.4 ± 6.5 | 11.9 ± 5.4 | 12.5 ± 5.7 |
BMI = body mass index; ESS = Epworth Sleepiness Scale (normal < 10).
Sleep Parameters
The major sleep parameters are shown in Table 2. There was no significant difference in the sleep parameters. The sleep profile was rarely improved with ASV (the case presentation below being an exception). Sleep scoring was difficult in certain cases due to the presence of α profusion. REM sleep was frequently completely absent, and in some cases a very unusual sleep histogram was observed, in which stage 2 NREM sleep predominated throughout with rare awakenings or arousals, low stage 1 NREM, and absent REM sleep. The arousal and periodic limb movement indices remained elevated across all 3 conditions.
Table 2.
Means and Standard Deviations for Sleep Measures Under Baseline, CPAP, and ASV Conditions
| BASELINE | CPAP | ASV | |
|---|---|---|---|
| Total sleep time (h) | 5.0 ± 1.7 | 5.4 ± 1.6 | 6.1 ± 1.6 |
| Sleep efficiency (%) | 79.3 ± 12.8 | 79.1 ± 16.6 | 79.9 ± 15.9 |
| Stage 1 NREM (%) | 8.4 ± 7.8 | 8.6 ± 12.0 | 7.8 ± 5.9 |
| Stage 2 NREM (%) | 73.5 ± 12.5 | 79.3 ± 15.9 | 73.6 ± 14.6 |
| Stage 3 NREM (%) | 6.0 ± 10.8 | 3.7 ± 5.5 | 7.5 ± 9.8 |
| Stage REM (%) | 12.0 ± 10.9 | 7.9 ± 10.6 | 11.0 ± 9.8 |
| Arousal index | 16.0 ± 14.3 | 19.2 ± 18.5 | 13.4 ± 6.7 |
| PLMS index | 11.4 ± 18.0 | 12.5 ± 18.7 | 35.1 ± 34.1 |
Total sleep time = total sleep time recorded during only room air phase of study (unless the whole study was performed using oxygen); sleep efficiency % = (total sleep time/total recording time)*100; stage 1-3 NREM and stage REM % = (sleep stage time/total sleep time)*100; arousal index = (number of arousals/total sleep time)*100; PLMS = periodic limb movement index = (number of periodic limb movements/total sleep time)*100. No significant differences (p < 0.01) for sleep variables.
Baseline Respiratory Characteristics
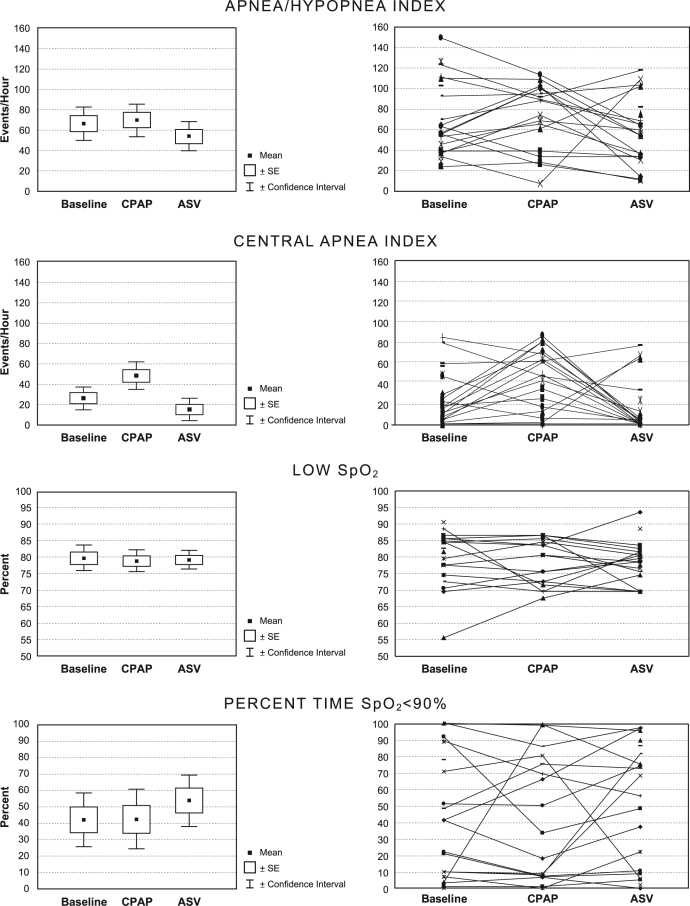
The primary respiratory outcome parameters are shown in Figure 2 and Table 3. Severe sleep apnea was present during the baseline. The mean ± SD AHI measured 66.6/h ± 37.3, central apnea index (CAI) 26.4/h ± 25.1, obstructive apnea index (OAI) 25.8/h ± 23.7, and hypopnea index (HI) 14.5/h ± 14.5. On an ordinal scale of none, mild, moderate, and severe, 18 of 22 patients (82%) of patients demonstrated moderate to severe Biot's respiration. Substantial hypoxemia was evident during the baseline condition. The mean SpO2 was 89.9%; however, the SpO2 measured < 90% for 42.2% of the sleep time. In 13 of 22 patients (59%), the SpO2 measured < 90% for > 10% of the sleep time. The mean low SpO2 for baseline was 79.5%, for CPAP was 78.7%, and for ASV was 79.0%.
Figure 2.
Comparison of various respiratory parameters obtained at baseline, CPAP, and ASV.
Table 3.
Means and Standard Deviations for Respiratory Measures Under Baseline, CPAP, and ASV Conditions
| BASELINE (n = 22) |
CPAP (n = 18) |
ASV (n = 22) |
|
|---|---|---|---|
| AHI | 66.6 ± 37.3 | 70.1 ± 32.6 | 54.2 ± 33.0 |
| OAI | 25.8 ± 23.7 | 10.7 ± 12.4 | 2.4 ± 4.3** |
| CAI | 26.4 ± 25.1 | 48.1 ± 27.1* | 15.6 ± 23.9 |
| HI | 14.5 ± 14.5 | 11.3 ± 9.1 | 35.7 ± 18.9** |
| Desaturation index | 31.2 ± 34.7 | 43.3 ± 32.5 | 20.8 ± 21.3 |
| Mean SpO2 | 89.9 ± 4.3 | 89.5 ± 4.3 | 89.2 ± 3.9 |
| SpO2 % <90% | 42.2 ± 36.5 | 42.5 ± 36.2 | 53.6 ± 35.3 |
| Low SpO2 | 79.5 ± 8.1 | 78.7 ± 6.7 | 79.0 ± 6.0 |
AHI = apnea-hypopnea index; OAI = obstructive apnea index; CAI = central apnea index; HI = hypopnea index; desaturation index = SpO2 decreases ≥4%; SpO2 % <90% = percentage of sleep time in which the SpO2 measured <90%.
Differs from baseline p < .01
Differs from baseline p < .001
Comparison of Respiratory Measures of CPAP and ASV to Baseline
Compared to baseline, the AHI remained elevated and was not significantly different in either CPAP or ASV conditions. The mean AHI measured 66.6/h at baseline compared to 70.1/h with CPAP (p = 0.558) and 54.2/h with ASV (p = 0.158). Despite the absence of significant changes in the overall AHI, the component indices changed markedly when comparing ASV with the baseline. The mean OAI was significantly reduced from 25.8/h to 2.4/h (p < 0.0001) whereas mean HI was significantly increased from 14.5/h to 35.7/h (p <0.0001). Compared to baseline, the mean CAI (26.4/h) was not significantly reduced with ASV (15.6/h) (p = 0.127) but was significantly increased during CPAP (48.1/h) (p < .01). The transition in type of respiratory disturbance from obstructive apnea to hypopnea reflects, in large part, scoring issues related to the backup respiratory rate feature of ASV as well as the therapeutic effect of positive airway pressure. Indicative of the persistence of respiratory events, the desaturation index remained elevated in all conditions without a significant reduction with ASV compared to the baseline (p = 0.131).
Figure 2 shows individual changes with CPAP and ASV compared to baseline. The AHI decreased below 20/h in only 4 patients on ASV. Of these, one had been tested breathing room air at baseline but was on oxygen when tested using ASV. In 2 others, the baseline AHI was only moderately abnormal and decreased from 21.6/h to 8.4/h and from 27.6/h to 9.1/h, respectively. In the fourth case, the AHI decreased from 53.1/h at baseline to 23.7/h with CPAP and to 12.4/h with ASV. Interestingly, the degree of ataxic breathing or Biot's respiration in this case was considered to be mild at baseline, and normal with CPAP and ASV.
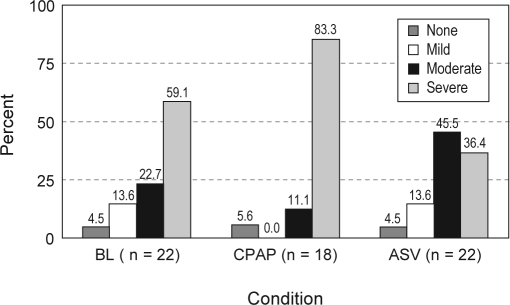
Neither CPAP nor ASV markedly reduced Biot's respiration which was rated moderate or severe in 94% of patients with CPAP and 82% of those with ASV (Figure 3). Statistical comparison of Biot's respiration severity showed no difference between a baseline mean rating of 3.3 and that of CPAP of 3.7 (p = 0.093) or baseline and VPAP of 3.1 (p = 0.280).
Figure 3.
Severity of ataxic breathing (Biot's respiration). See text for definitions. Markedly irregular breathing was seen in the majority of patients across all conditions.
The oxygenation parameters showed persistent hypoxemia, even in some cases with combination therapy using oxygen and ASV. Although the mean SpO2 measured 89.2% with ASV (3 cases using oxygen), the SpO2 measured <90% for ≥10% of the sleep time in 16 of 22 cases breathing room air, and in 2 of 3 cases while receiving oxygen with ASV. The lowest SpO2 increased above 90% in only one case.
Illustrative Case Analysis
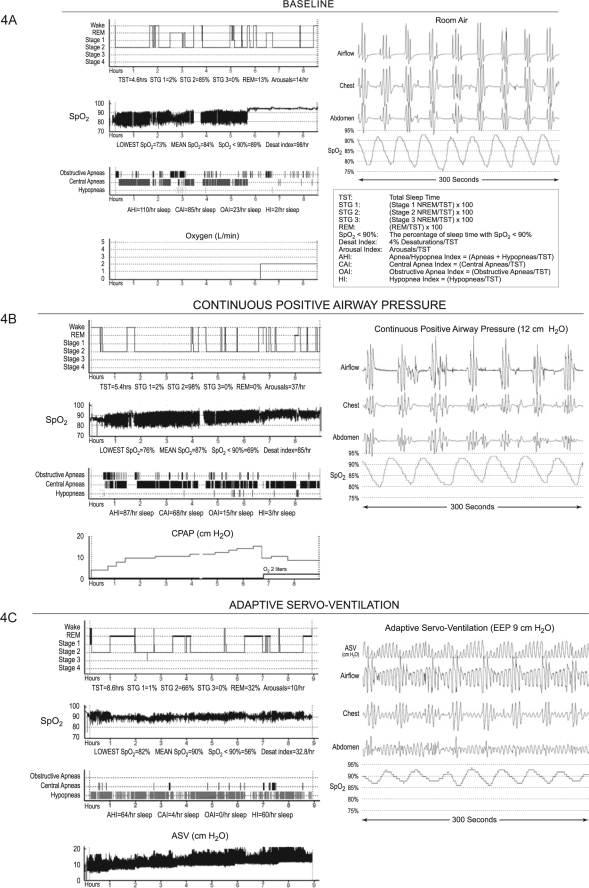
It is useful to review the data from one patient with very severe sleep apnea in whom EEP was titrated to the maximum of 10.0 cm H2O (Figure 4). Although there was clearly a response to therapy with modulation of the respiratory pattern, she continued to have frequent oxygen desaturations associated with respiratory events that were primarily scored as hypopneas.
Figure 4.
Case Illustration. See text for details. The scored graphic results of polysomnography at baseline (4A), CPAP (4B), and ASV (4C) are shown. The respiratory and sleep data refer only to the room air portions of the studies. With each study is shown a representative 120-sec sample of raw data.
The patient was a 52-year-old female (height 160 cm, weight 76.8 kg; BMI 30) who presented with chronic back pain, depression, anxiety, and poor sleep. She did not report symptoms of loud snoring or witnessed apneas, and she did not have hypersomnolence (Epworth Sleepiness Scale score 3). Medications included hydromorphone (intrathecal), hydrocodone 10 mg/acetaminophen 500 mg (4/day), eszopiclone 3.0 mg at bedtime and sertraline100 mg daily. Baseline polysomnography (Figure 4A) showed severe hypoxemia with predominant unambiguous central apneas with a cluster pattern (not CSR). Supplemental oxygen increased the SpO2 to 95%, and interestingly, the frequency of central apneas was reduced; however, the presence of markedly ataxic underlying breathing pattern emerged, consistent with Biot's respiration (Figure 5). With CPAP (Figure 4B), there were continuous central apneas, oxygen desaturations, and chaotic breathing. The SpO2 improved slightly only with addition of oxygen, but apneas remained in high frequency. With ASV (Figure 4C), there was a slight decrease in magnitude of oxygen desaturations with increasing end-expiratory pressure (EEP) from 5.0 to 10.0 cm H2O (the maximum possible with current technology). Frank apneas were almost eliminated; however, there were some obvious events scored as central in which there was no apparent thoraco-abdominal volume change, even with EEP of 9-10 cm H2O and maximal inspiratory pressure of 10 cm H2O (Figure 6). Persistent ataxic breathing could also be readily appreciated by viewing the thoraco-abdominal signals in 300-sec epochs. Due to the persistence of the respiratory events, desaturations, and arousals, therapy was considered to be insufficient. It was of interest, however, that her sleep architecture was improved.
Figure 5.
Ataxic breathing (Biot's respiration) during 120-sec epoch while breathing supplemental oxygen on the baseline study. Typical category 4 ataxic breathing pattern.
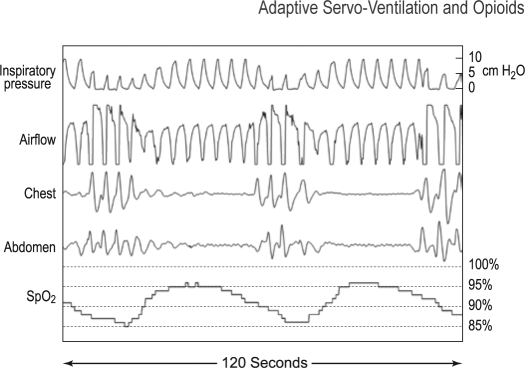
Figure 6.
Central apnea during 120-sec epoch with ASV support. This 120-sec sample of raw data obtained with EEP of 9.0 cm H2O shows respiratory events that are indicated by desaturation and the absence of thoracic and abdominal motion in spite of the air flow signals by PTAF corresponding with the backup rate of 15/h and the evidence of increasing pressure by the ASV. Increasing inspiratory pressure is associated with an increasing airflow signal without a corresponding increase in chest volume reflected by the thoracic abdominal signal. These events were scored as central apneas. It is conceivable that airway obstruction is present but this can only be inferred.
DISCUSSION
The major conclusions from this study are that in patients with sleep disordered breathing associated with chronic opioids, CPAP seems to worsen sleep apnea, and ASV, as applied within the original recommended parameters, is not sufficient therapy (Table 3). All of these subjects were referred for assessment of obstructive sleep apnea and had had moderate to severe sleep disordered breathing on baseline polysomnography (minimum AHI 20/h). The predominant type of disturbances found on baseline was equally divided between central and obstructive apnea; the obstructive apneas present were usually complex, consisting of both central and obstructive elements. The frequency of obstructive apneas decreased with ASV with a corresponding increase in hypopneas, while the frequency of central apneas remained unchanged.
Some of the changes in respiratory variables can be related to the complexities of defining events when patients are treated with variable inspiratory pressure support and with a timed backup rate of 15 breaths/min. Apneas and hypopneas are by definition determined by analysis of the airflow signal, however the ASV device introduces an artifactual flow signal measured by an air pressure transducer. Without measurement of intrathoracic pressure or evidence of thoracic and abdominal motion with absent airflow, airway obstruction can not be recognized by usual criteria. The presence of an obstructed airway can only be surmised. Furthermore, using the only ASV device by ResMed currently available in the United States, there is no software with which to download respiratory events. Even so, the validity of such events would be doubtful. At present, the detection of apnea with ASV depends upon analysis of the thoraco-abdominal signals that reflect volume changes. When there was no volume change (i.e., absent thoraco-abdominal motion ≥10 sec) in spite of an airflow signal, we scored these events as central although there could obviously have been increased upper airway resistance and persistent airway obstruction (Figure 6). Since there was usually some airflow signal and evidence of thoraco-abdominal movement, most of the events associated with desaturations were necessarily scored as hypopnea. Current standards do not differentiate obstructive and central hypopnea. Irrespective of the classification of respiratory events, clinically adequate treatment was not achieved using CPAP or ASV.
We fully appreciate the possibility that by using default settings, upper airway obstruction may not have been adequately eliminated and that the current practice is to titrate end-expiratory pressure (EEP). Adaptive servoventilation was originally intended to treat patients with central apneas (i.e., Cheyne-Stokes respiration) in whom it was assumed that the airway was patent and that high end-expiratory pressure would be unnecessary. There was also concern that high positive airway pressure might adversely affect venous return. The original conservative recommendations, therefore, are probably not appropriate for patients with increased upper airway resistance or frank airway obstruction. Unfortunately, we are lacking sufficiently validated guidelines or algorithms for identifying airway obstruction, distinguishing central and obstructive events and for making adjustments in therapy using ASV. Since the need for increasing EEP has been appreciated, we have modified our protocol so that as long as respiratory events persist, regardless of their morphology, EEP is increased by 1-cm increments to the maximum value (currently 10.0 cm H2O). In the last 3 patients reported here (including the case presentation), end-expiratory pressure was titrated to 7, 9, and 10 cm H2O respectively. There seemed to be some improvement in severity of desaturations and frequency of events (particularly in 2 cases), but even with increasing pressures, we could not normalize ventilation and SpO2. Adaptive servoventilation clearly modulated the respiratory pattern but in no case was the frequency of residual respiratory disturbances, the degree of hypoxemia, and the arousal pattern clinically acceptable.
With respect to central events, the failure of ASV to stabilize breathing as compared to ASV therapy for CSR is likely due to differences in the pathophysiology of these 2 processes. The normal stability of respiratory rate and tidal volume during NREM sleep relies on an intact metabolic control system.22–24 Peripheral chemoreceptors sensitive to PO2 and PCO2, mainly located in the carotid bodies, are responsible for the breath-by-breath variations in ventilation. Cheyne-Stokes breathing also depends upon the presence of a negative feedback control system but appears to develop due to increased loop gain and phase delays resulting in miscommunication between the peripheral chemoreceptors and the central pattern generator.11 Central apneas associated with CSR occur when phasic hyperpnea reduces PCO2 below the apneic threshold. Apart from these occasions, the fundamental breathing pattern during CSR is not erratic but resembles a highly stable amplitude modulated sinusoid radio-frequency signal. CSR and central apneas are greatly attenuated during REM sleep because the regulation of ventilation is less dependent on the properties of negative feedback control systems in this sleep state.
The pathophysiology of opioid induced sleep disordered breathing is not known; however, the presence of an underlying erratic breathing pattern compared to the extremely uniform sinusoidal oscillations of CSR points to fundamentally different mechanisms. The chaotic nature of the breathing pattern during NREM sleep seen in patients taking opioids resembles the breathing pattern during REM sleep. This property suggests that metabolic control systems are less involved in these cases. In contrast to CSR or CompSAS, the pauses in these patients were often highly variable. Ataxic breathing of a moderate to severe category was present in 82% on baseline, 94% with CPAP, and 82% with ASV. It is not clear if the failure of ASV to consistently stabilize ventilation in these patients was related to the presence of an underlying highly irregular rhythm.
In general, both physiologic and pathologic respiratory patterns can be classified as regular or irregular depending upon the tidal volume (VT) and frequency (f). For example, the breathing pattern in slow wave NREM and tonic REM sleep is stable, while the breathing pattern during alert wakefulness and phasic REM sleep is characterized by variable VT and f. Various terms exist in the literature to describe different types of pathologic variable breathing patterns. These include Cheyne-Stokes respiration, periodic breathing, Biot's breathing, ataxia, apneustic breathing, cluster breathing, as well as central and obstructive sleep apnea. In 1876, Biot25 described a completely irregular breathing pattern distinct from CSR which occurred in a 16-year-old male patient with tuberculous meningitis. His original illustration shows breaths of differing amplitude, without any regular rhythm, and with random apneic periods of variable duration (breathing ataxia). Biot's type breathing pattern has been associated with diseases of the central nervous system and high-altitude pulmonary edema26 (note that high altitude pulmonary edema does not occur at moderate elevation of 1,500 meters in Salt Lake City, Utah). In 1981, Webber27 reported on a model for inducing an irregular spontaneous breathing pattern in pentobarbital anesthetized cats which he erroneously called “Biot breathing.” However, the respiratory pattern in these animals consisted of groups of breaths that “start and stop abruptly with intervening apneic holds (cluster pattern).” In view of our experience, in which CPAP seems to worsen central sleep apnea in patients treated with opioids, the following observation by Webber is very intriguing: “pneumotaxic lesioned cats not breathing periodically can be brought into the Biot pattern [actually cluster breathing] by exposing the airways to positive end-expiratory pressure (PEEP).” As a result of the different definitions of “Biot breathing,” there has been confusion in the literature regarding the terms Biot's, ataxic, and cluster breathing.
Breathing abnormalities during NREM sleep in patients receiving long-term opioid medications were first reported in 2003.6 We have found in the present series of patients, the ataxic irregular breathing pattern as described by Biot as well as the cluster breathing described by others.27–29 Figure 4 of the case illustration in this report shows cluster breathing with central apneas while breathing room air, CPAP, and ASV. Note that with ASV, the pattern is most clearly seen in the chest leads because the airflow signal is altered by air pressure signals from the ASV device. In addition to the cluster pattern, one can also appreciate a subtle variability in the length of apneas between clusters. Figure 5 shows the same patient breathing supplemental oxygen, but now classic Biot's respiration (ataxia) is obviously present. Although technically, Biot's respiration should probably refer only to breathing patterns with markedly variable VT, random apneas, and without any regular frequency or rhythm as he reported, we have included cluster breathing in the definition, since both patterns are seen in patients taking opioids, and there is no other term which would suffice. Our impression is that classic Biot's breathing and cluster breathing may be due to similar pathophysiologic processes, with cluster breathing representing the most extreme degree.
In summary, we have found that patients being treated with chronic opioids who are seen for evaluation of possible obstructive sleep apnea syndrome, the respiratory abnormalities are complex, difficult to define using standard criteria, and ataxic breathing or Biot's respiration is characteristic. These cases are invariably complicated by their comorbidities, numerous medications and pain requiring chronic opioid therapy. In our experience, these patients are becoming increasingly common as the prescription rate for chronic opioids increases.30,31 Conventional therapy with CPAP is typically unsuccessful or actually worsens respiratory events. Adaptive servoventilation modulates the breathing patterns but does not eliminate ataxic breathing or hypoxemia, and it does not consistently reduce apneas and hypopneas. It appears that increasing EEP may improve the effectiveness of ASV if residual airway obstruction is the primary basis for failure. Central respiratory disturbances associated with opioids may be difficult to eliminate due to the ataxic breathing. Future investigations will need to explore the effects of adjusting various ASV parameters (minimum and maximum inspiratory pressures, end-expiratory pressure, and respiratory rate). Until more effective therapy can be provided, our current practice is to use ASV with a backup rate of 15, the maximum EEP determined by titration with polysomnography and supplemental oxygen to as much as 10 L/min if necessary to prevent hypoxemia.
ACKNOWLEDGMENTS
We greatly appreciate the artistic and technical skills, and especially the patience of our medical illustrator, Jill Rhead MA.
Data from this work was presented in abstract form at the European Respiratory Society Annual Congress in Stockholm, Sweden on September 17, 2007.
Financial Support was provided by the Deseret Foundation, LDS Hospital.
ABBREVIATIONS
- ASV
Adaptive servoventilation
- CPAP
Continuous positive airway pressure
- CSR
Cheyne-Stokes respiration
- CompSAS
Complex sleep apnea syndrome
- AHI
Apnea-hypopnea index
- CAI
Central apnea index
- OAI
Obstructive apnea index
- HI
Hypopnea index
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Teschler H, Döhring J, Wang Y, et al. Adaptive pressure support servo-ventilation. A novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164:614–19. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 2.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–42. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepperell JCT, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 4.Allam JS, Olson EJ, Gay PC, et al. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132:1839–46. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 5.Morgenthaler TI, Gay PC, Gordon N, et al. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 6.Farney RJ, Walker JM, Cloward TV, et al. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–9. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- 7.Teichtahl H, Wang D, Cunnington D, et al. Ventilatory responses to hypoxia and hypercapnia in stable methadone maintenance treatment patients. Chest. 2005;128:1339–47. doi: 10.1378/chest.128.3.1339. [DOI] [PubMed] [Google Scholar]
- 8.Walker JM, Farney RJ, Rhondeau SM, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3:455–62. [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128:1348–56. doi: 10.1378/chest.128.3.1348. [DOI] [PubMed] [Google Scholar]
- 10.Webster LR, Choi Y, Desaih H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9:425–32. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 11.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 12.Howard RS, Rudd AG, Wolfe CD, et al. Pathophysiological and clinical aspects of breathing after stroke. Postgrad Med J. 2001;77:700–2. doi: 10.1136/pmj.77.913.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naughton MT. Pathophysiology and treatment of Cheyne-Stokes respiration. Thorax. 1998;53:514–8. doi: 10.1136/thx.53.6.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley TD. Nonhypercapnic central sleep apnea. In: McNicholas WT, Phillipson EA, editors. Breathing disorders in sleep. London: W.B. Saunders; 2002. pp. 246–64. [Google Scholar]
- 15.Eckert DJ, Jordan AS, Marchia P, et al. Central sleep apnea. Pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey JE, Skatrud JB. A sleep-induced apneic threshold and its consequences. Am Rev Respir Dis. 1986;133:1163–70. doi: 10.1164/arrd.1986.133.6.1163. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XS, Rowley JA, Demirovic F, et al. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol. 2003;94:101–7. doi: 10.1152/japplphysiol.00264.2002. [DOI] [PubMed] [Google Scholar]
- 18.Rowley JA, Zhou XS, Diamond MP, et al. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama H, Smith CA, Rodman JR, et al. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–9. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales AD. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 21.Flemons WW., (Chair) Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118:909–39. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan CE. Breathing in sleep. In: Orem J, Barnes CD, editors. Physiology in sleep. New York: Academic Press; 1980. pp. 213–72. [Google Scholar]
- 24.Krimsky WR, Leiter JC. Physiology of breathing and respiratory control during sleep. Semin Respir Crit Care Med. 2005;26:5–12. doi: 10.1055/s-2005-864197. [DOI] [PubMed] [Google Scholar]
- 25.Biot MC. Contribution a l'ètude de phènomène respiratoire de Cheyne-Stokes. Lyon Mèd. 1876;23:517–528. 561-567. [Google Scholar]
- 26.Fujimoto K, Matsuzawa Y, Hirai K, et al. Irregular nocturnal breathing patterns at high altitude in subjects susceptible to high-altitude pulmonary edema (HAPE): A preliminary study. Aviat Space Environ Med. 1989;60:786–9. [PubMed] [Google Scholar]
- 27.Webber CL, Jr, Speck DF. Experimental Biot periodic breathing in cats: effects of changes in PIO2 and PICO2. Respir Physiol. 1981;46:327–44. doi: 10.1016/0034-5687(81)90130-4. [DOI] [PubMed] [Google Scholar]
- 28.Posner JB, Saper CB, Schiff ND, Plum F, editors. Plum and Posner's diagnosis of stupor and coma. New York: Oxford University Press; 2007. Examination of the comatose patient; pp. 46–53. [Google Scholar]
- 29.Freeman WD, Sen S, Roy TK, et al. Cluster breathing associated with bihemispheric infarction and sparing of the brainstem. Arch Neurol. 2006;63:1487–90. doi: 10.1001/archneur.63.10.1487. [DOI] [PubMed] [Google Scholar]
- 30.ARCOS, US Department of Justice, Drug Enforcement Administration, Office of Diversion Control. http://www.deadiversion.usdoj.gov/arcos/retail_drug_summary.
- 31.CDC. Increase in poisoning deaths caused by non-illicit drugs–Utah, 1991-2003. MMWR Morb Mortal Wkly Rep. 2005;54(21):33–6. [PubMed] [Google Scholar]