Abstract
Microwave reaction of RuCl3 with 2,2’-bipyridinyl-4,4’-dicarboxylic acid diethyl ester (debpy) in ethylene glycol generated Ru(bpy)32+ instead of the expected Ru(debpy)32+. GCMS analysis of the head space revealed CO2, and Ru(bpy)32+ was recovered from the filtrate. Further experiments suggest that RuCl3 decarboxylates the debpy during microwave synthesis.
The use of microwave reactors is a rapidly expanding area of synthetic chemistry in both organic and inorganic synthesis. The technique offers several advantages over traditional synthesis especially in synthesis of ruthenium and osmium complexes which typically require many hours of refluxing in high boiling solvents to affect a reaction. Similar reactions, when performed in a microwave reactor can occur in a matter of minutes. Matsumura-Inoue and Tanabe reported efficient and rapid synthesis of ruthenium polypyridine complexes using microwave irradiation.1 This method was used to synthesize a variety of tris bidentate complexes as well as bis tridentate complexes. For example, Ru(bpy)32+ was prepared by microwave heating of a ruthenium chloride solution with three equivalents of 2,2’-bipyridine in ethylene glycol for 15 minutes in 95% yield, which is slightly higher than the literature value of 86%.1 A number of related examples have recently appeared in the literature.1–6
Over the past decade numerous ruthenium bipyridine and phenanthroline complexes have been created for sensing applications or for applications where enhanced photophysical properties or electrochemical properties are required. Many of the complexes rely on the creation of an ester or amid linkage to the carboxylic acid derivative of 2,2’-bipyridine or 1,10-phenanthroline.7–18 The ruthenium complex prepared from 2,2’-bipyridinyl-4,4’-dicarboxylic acid diethyl ester (debpy), for example, has a significantly enhanced excited state lifetime of 2000 ns measured in dichloromethane when compared to the lifetime of the parent complex, Ru(bpy)3 2+, 600 ns.7
The commonly reported synthesis for Ru(debpy)32+ is rather arduous and produces low yields. Specifically, [Ru(DMSO)4Cl2] is refluxed with ~4 equivalents of debpy in ethanol for 1 week with a reported 15% yield.19 We have tried other synthetic schemes to prepare this complex, but these also suffered from poor yields and complex mixtures of ruthenium compounds. Specifically, reaction of debpy with Ru(COD)Cl220 or [Ru(CO)2Cl2]n21–22 in 2-methoxyethanol produced mixtures and only small amounts of the desired product.
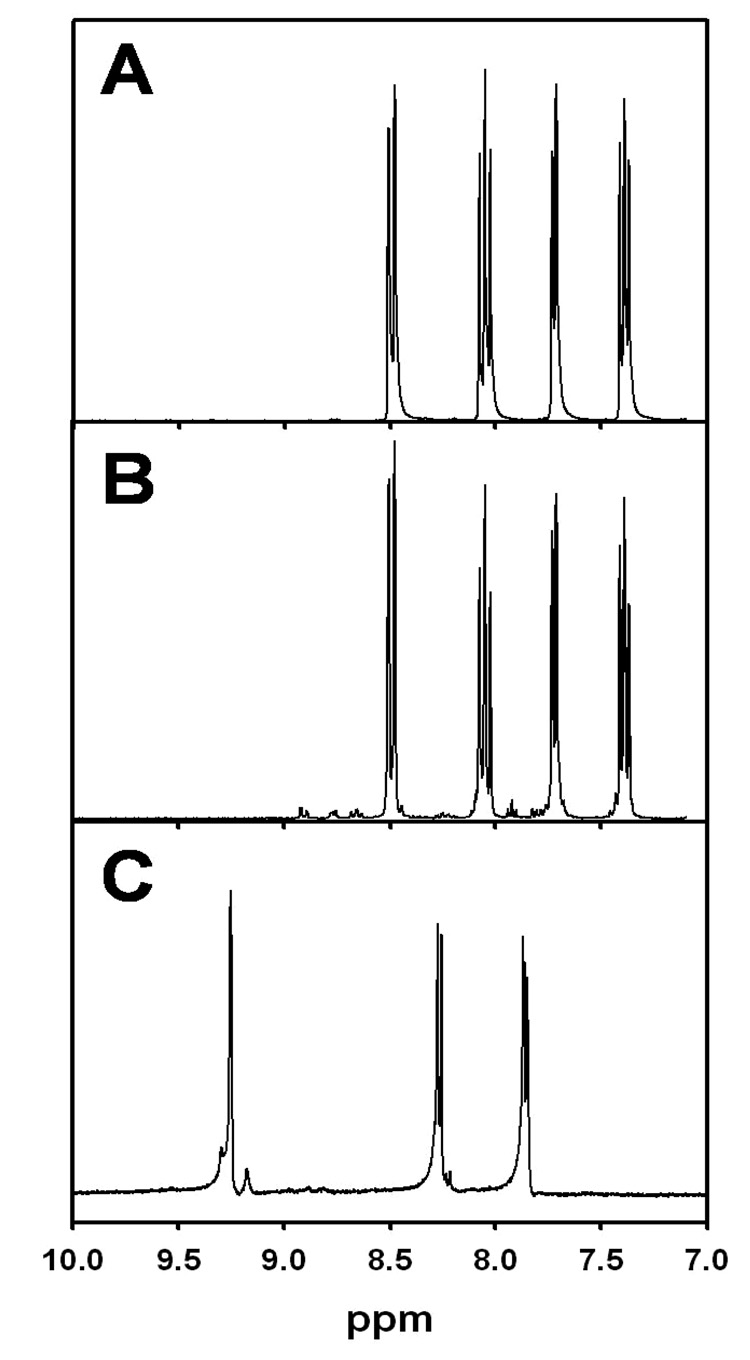
Attempted microwave synthesis of Ru(debpy)32+ in a CEM Explorer (Matthews, NC) reactor produced an unexpected product. A mixture of ruthenium trichloride trihydrate (13.1 mg, 0.0632 mmoles) and the ethyl ester (57.0 mg, 0.190 mmoles) in ~5 ml of ethylene glycol were reacted for 5 min. During the reaction, the pressure in the sealed vessel rose to >300 psi as the temperature was increased to 225 °C, at which point the instrument shutdown because the pressure limit had been exceeded. The solution was filtered to remove a small amount of black solid and then 2 ml of saturated aqueous ammonium hexafluorophosphate was added to the filtrate to recover an orange solid (60.5% yield). 1H NMR spectra of pure [Ru(bpy)3][PF6]2, as prepared by Nocera’s method,20 product from the microwave synthesis and [Ru(debpy)3][PF6]2, prepared as described by Murray et. al.19, are shown in Figure 1A–C, respectively. Comparison of the spectra in Figure 1, clearly indicate that the product obtained from the microwave reaction was Ru(bpy)32+. UV-Vis spectroscopy and electrospray mass spectrometry, when compared to high quality samples of Ru(bpy)32+ and the corresponding ethyl ester, also confirmed the product to be Ru(bpy)32+. The evidence clearly indicates that during the synthesis the ethyl ester undergoes a decarboxylation resulting in the loss of the entire ester side chain. The increase in pressure, which is not typically observed, is also consistent with the formation of volatile products. Analysis of the headspace gases by GC and GCMS revealed the presence of CO2 and ethanol.
Figure 1.
1H NMR spectra of (A) [Ru(bpy)3][PF6]2, (B) reaction product and (C) [Ru(debpy)3][PF6]2 all in CD3CN.
The control reaction of debpy in ethylene glycol in the absence of ruthenium trichloride was also performed. Under these conditions, GCMS analysis of solution indicated that the neither the debpy nor the ethylene glycol undergo reaction. Furthermore, there is no increase in pressure in the reactor during the reaction beyond what is typically observed due to heating of the solvent. In another control experiment, Ru(debpy)3(PF6)2, synthesized by another method19 was exposed to microwaves under the same reaction conditions and found to be unreactive.
To verify that properties specific to ruthenium chloride where required for reaction, a few other metal chlorides were examined. 1H NMR of the product isolated from the reaction of iron(III) chloride, under identical conditions, indicated that the complexed debpy remained intact. The reaction was repeated using osmium(III) chloride trihydrate and again, there was no evidence of decarboxylation. Thus, the trivial mechanism whereby the ruthenium trichloride behaves as a general Lewis acid can be eliminated.
In order to obtain further information on the role of the ruthenium trichloride, reactions were performed for 30 minutes with Ru(bpy)2Cl2. In this case, a series of tris-chelated complexes was obtained, as indicated by 1H NMR and electrospray mass spectroscopy. Specifically, no significant amounts of the ethyl ester products were obtained. Instead the corresponding ethylene glycol esters were present along with nearly equal amounts of the Ru(bpy)32+. In addition, complexes containing carboxylic acids were also present in similar amounts as were the various permutations of acids and esters23. These results, and the detection of CO2 and ethanol, strongly suggest that the reaction involves successive loss of the alcohol, with intermediate transesterification with ethylene glycol, and finally decarboxylation of the acid form. The reaction was also performed in ethylene glycol-d2. 1H NMR and 13C NMR revealed that the product, Ru(bpy)32+, was selectively deuterated at the 4 and 4’ positions. These results are also consistent with the decarboxylation of a carboxylic acid form of the coordinated ligand.
The fact that Ru(bpy)32+ is formed exclusively in the reaction with RuCl3 and Ru(debpy)32+ is inert under the same reaction conditions presented some mechanistic difficulties. However, reaction of Ru(bpy)Cl424 with excess dimethylbipyridine (dmbpy) under the same reaction conditions gave Ru(dmbpy)32+ as well as Ru(dmbpy)2(bpy)2+. This observation indicates that there is exchange of the ligands particularly in the early stages of the reaction. Thus there appears to be a stepwise reduction in the reactivity induced by the ruthenium as bipyridine ligands are added.
In summary, the debpy ligand undergoes a quantitative decarboxylation reaction under reaction conditions that are typically used in the microwave preparation of ruthenium complexes. The reaction involves the metal and results in the formation of the unsubstituted bipyridyl complexes. Thus microwave synthesis of ruthenium complexes using derivatives of the 4,4’-dicarboxybipyridine should be approached with caution. Although the reaction is not productive in this specific example, the ability to decarboxylate esters may have applications in organic synthesis where such reactions are difficult to perform. Further experiments with other esters and amides are planned.
ACKNOWLEDGMENT
Supported by National Institutes of Health Grant GM 20488 (to F.M. and B.D.) T J Anderson gratefully acknowledges funding provided through the Academic Center of Excellence Fellowship from the Idaho National Laboratory under DOE/NE Idaho Operations Office Contract DE-AC07-05ID14517.
REFERENCES
- 1.Matsumura-Inoue T, Tanabe M. Chemistry Letters. 1994;12:2443–2446. [Google Scholar]
- 2.Shen Y, Sullivan BP. Journal of Chemical Education. 1997;74:685–689. [Google Scholar]
- 3.Whittaker AG, Mingos DMP. Journal for the Chemical Society, Dalton Transactions. 2002:3967–3970. [Google Scholar]
- 4.Spinella A, Caruso T, Pastore U, Ricart S. Journal of Organometallic Chemistry. 2003;684:266–288. [Google Scholar]
- 5.Dabirmanesh Q, Roberts RMG. Journal of Organometallic Chemistry. 1997;542:99–103. [Google Scholar]
- 6.Liu L-C, Lee C-C, Hu AT. Journal of Porphyrins and Phthalocyanines. 2001;5:806–807. [Google Scholar]
- 7.Wacholtz WF, Auerbach RA, Schemehl RH. Inorganic Chemistry. 1986;25:227–234. [Google Scholar]
- 8.Pratt MD, Beer PD. Tetrahedron. 2004;60:11227–11238. [Google Scholar]
- 9.Sjudin M, Styring S, Wolpher H, Xu Y, Sun L, Hammarstrom L. Journal of the American Chemical Society. 2005;127:3855–3863. doi: 10.1021/ja044395o. [DOI] [PubMed] [Google Scholar]
- 10.Beer PD, Szemes F, Passaniti P, Maestri M. Inorganic Chemistry. 2004;43:3965–3975. doi: 10.1021/ic0499401. [DOI] [PubMed] [Google Scholar]
- 11.Bellusci A, Barberio G, Crispini A, Ghedini M, La Deda M, Pucci D. Inorganic Chemistry. 2005;44:1818–1825. doi: 10.1021/ic048951r. [DOI] [PubMed] [Google Scholar]
- 12.Chang K-H, Liao J-H, Chem C-T, Mehta BK, Chou P-T, Fang T-M. Journal of Organic Chemistry. 2005;70:2026–2032. doi: 10.1021/jo048368s. [DOI] [PubMed] [Google Scholar]
- 13.Odobel F, Zabri H. Inorganic Chemistry. 2005;44:5600–5611. doi: 10.1021/ic050078m. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Liu J, Jin K, Yang X, Peng Q, Sun L. Tetrahedron. 2005;61:5655–5662. [Google Scholar]
- 15.Geary EAM, Yellowless LJ, Jack LA, Oswald IDH, Parsons S, Nirata N, Durrant JR, Robertson N. Inorganic Chemistry. 2005;44:242–250. doi: 10.1021/ic048799t. [DOI] [PubMed] [Google Scholar]
- 16.Klein C, Nazeeruddin MdK, Di Censo D, Liska P, Gratzel M. Inorganic Chemistry. 2004;43:4216–4226. doi: 10.1021/ic049906m. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Song Y, Chem Y, Li X-Q, Ding F, Zhong R-Q. Chem. Eur. J. 2004;10:3685–3696. doi: 10.1002/chem.200305724. [DOI] [PubMed] [Google Scholar]
- 18.Vickers MS, Martindale KS, Beer PD. Journal of Materials Chemistry. 2005;15:2784–2790. [Google Scholar]
- 19.Murray RW, Masui H. Inorganic Chemistry. 1997;36:5118–5126. [Google Scholar]
- 20.Walker GW, Nocera DG. Inorganic Syntheses. 2004;34:66–68. [Google Scholar]
- 21.Aguirre P, Sariego R, Moya SA. J. Coord. Chem. 2001;54:401–413. [Google Scholar]
- 22.Thomas NC, Deacon GB. Inorganic Syntheses. 1989;25:107–110. [Google Scholar]
- 23.Electrospray mass spectroscopy of the products gave the following mass peaks and relative intensities 24% 285, Ru(bpy)32+; 2% 307 Ru(bpy)2(bpyCOOH)2+; 6% 329, (Ru(bpy)2(bpy(COOH)2)2+; 16% 351, Ru(bpy)2(bpyCOOHCOOCH2CH2OH)2+; 40% 373, Ru(bpy)2(bpy(COOCH2CH2OH)2)2+
- 24.Krause RA. Inorganica Chimica Acta. 1977;22:209–213. [Google Scholar]