Abstract
We studied the effect of cholecalciferol (VD3) intake on VD3 status and markers of calcium (Ca) homeostasis in mice and rats. Serum 25 hydroxycholecalciferol (25OH-VD3) concentrations were increased in animals fed diets containing 400–20,000 international units (IU) VD3/kg (37 nmol·L−1·1000 IU VD3−1), but body weight, serum Ca, and duodenal gene expression were not altered. High-VD3 intake decreased serum 1, 25-dihydroxycholecalciferol [1,25(OH)2-VD3] and renal 25 hydroxycholecalciferol-1ahydroxylase (CYP27B1) mRNA, suggesting that rodents tolerate high-VD3 intake by suppressing the activity of the VD3 endocrine system. Serum 25OH-VD3 declined when animals were fed diets containing 1000 to 25 IU VD3/kg (9–11 wk, inflection at 200 IU/kg, 4-fold steeper slope below this). Neither body weight nor serum Ca were influenced by low-VD3 intake. However, mice fed the 25-IU/kg diet had lower serum 1,25(OH)2-VD3, duodenal calbindin D9k mRNA, bone mineral density, and renal 25 hydroxycholecalciferol-24 hydroxylase mRNA, whereas renal CYP27B1 mRNA was elevated when rodents were fed ,200 IU VD3/kg. These data reveal a stress on VD3 and Ca metabolism at low dietary VD3 intake. Dietary Ca restriction (0.25 vs. 0.5%, 9 wk) increased serum 1,25(OH)2-VD3 and was 30% greater in rats fed a 10,000-IU VD3/kg diet. High-VD3 intake did not prevent Ca restriction-induced bone loss. Our data show that modeling human VD3 status requires lower intake than the current NRC rodent requirement (1000-IU/kg diet). Also, although rodents are very tolerant of high-VD3 intake, it cannot compensate for moderate Ca restriction.
Introduction
Human population-based studies suggest that high-cholecalciferol (VD3)3 status [measured by serum concentrations of the prohormone 25-hydroxycholecalciferol or (25OH-VD3)] lowers the risk for several chronic diseases. This includes diseases that are influenced by the traditional role of VD3 in controlling calcium (Ca) metabolism, like osteoporosis, as well as those that relate to nonclassical VD3 functions, e.g. cancer prevention and autoimmune diseases like diabetes and inflammatory bowel diseases (1,2). These observations are surprising given that the hormonally active form of VD3, 1,25-dihydroxycholecalciferol [1,25(OH)2-VD3], is the metabolite that mediates transcriptional events through the vitamin D receptor (VDR) in traditional target tissues (e.g. intestine, kidney, osteoblasts) as well as the fact that 25OH-VD3 has low affinity for the VDR. Still, some of these epidemiologic studies are supported by clinical intervention trials. For example, Trivedi et al. (3) found that increasing VD3 status in elderly men and women from 55 to 75 nmol/L with 50,000 IU VD3 doses every 4 mo offered protection from fracture over a 5-y period. In addition, intestinal Ca absorption increased in VD3-replete humans within the reference range for serum 25OH-VD3 (4) and VD3 supplementation increased Ca absorption even though serum 1,25(OH)2-VD3 concentrations decreased (5). Collectively, these studies have lead to a call from some scientists to raise the cut-off for assessing VD3 insufficiency from the traditional serum 25OHVD3 concentration of 37.5 nmol/L to >80 nmol/L (6). Unfortunately, most intervention studies relevant to this issue are limited by the length of the intervention, are confounded by seasonal changes in VD3 status, or simply have not yet been conducted (e.g. for many cancers and diabetes risk). As a result, there are still many gaps in our understanding of the long-term benefits and potential long-term risks of this proposed policy. To define the physiologic importance and the mechanism mediating the protective effect of high-VD3 status, we need to develop animal models whose serum 25OH-VD3 concentrations mimic the range of VD3 status in humans.
Although rodents have been an important experimental model to study the mechanisms of VD3 action on Ca metabolism, the VD3 requirement for mice and rats has not been formally determined. The rat and mouse NRC requirement for VD3 is 1000 IU/kg diet, but this is defined from a “personal communication” (7). Whereas the AIN76 (8) and AIN93 (9) diets favored by nutrition scientists use the NRC requirement, commercial nonpurified diets routinely contain 3000–5000 IU/kg diet. However, without information on the sensitivity and effect of VD3 intake on VD3 status in rodents, the ability of researchers to use these models to understand the relationship between VD3 status and physiological endpoints is limited. To fill this gap, we have conducted several studies in growing rats and mice to examine the effect of changing VD3 concentrations on serum VD3 metabolites and indices of Ca metabolism. We chose the levels of VD3 to flank the NRC requirement for rodents (1000 IU/kg VD3 diet) and to provide a wide range of intakes relevant to the question of VD3 deficiency and toxicity. Our findings reveal the range of acceptable VD3 intakes in rats and mice for researchers who wish to explore the effect of this nutrient on various health outcomes.
Materials and Methods
Experimental design
Animals were housed in individual cages in an UV B light-free environment (Clear UV Tube Guards, Pegasus Associates) on a 12-h-light/-dark cycle and consumed food and water ad libitum. Diets were prepared by Research Diets. Animals were weighed at the beginning and end of each study and were deprived of food overnight prior to killing. All of the animal experiments were approved by the Purdue University Animal Care and Use Committee.
Expt. 1: the effect of high-VD3 intake on VD3 and Ca metabolism
Two experiments were conducted. In Expt. 1A, 51 (27 female and 24 male) weanling Sprague Dawley rats (Harlan) were obtained and fed an AIN93G diet modified to contain 400 IU VD3/kg diet until 10 wk of age. Afterwards, rats were randomized to AIN93M diets (9) containing 1 of 5 VD3 levels for 4 wk: 400 (10 μg), 1000 (25 μg), 5000 (125 μg), 10,000 (250 μg), or 20,000 (500 μg) IU VD3/kg diet [n = 4–5 (males) or 5–6 (females) per diet].4
In Expt. 1B, 30 female C57BL/6 mice (Jackson Labs) were fed AIN93G diets (9) containing 400, 1000, 5000, 10,000, or 20,000 IU VD3/kg diet from weaning until 10 wk of age (n = 6 per diet). At the end of these experiments, the animals were killed and serum and tissues were obtained.
Expt. 2: the effect of low-VD3 intake on VD3 and Ca metabolism
Two experiments were conducted. In Expt. 2A, 30 male weanling Sprague Dawley rats were fed AIN93G diets containing 50 (1.25 μg), 100 (2.5 μg), 200 (5 μg), 400 (10 μg), or 1000 (25 μg) IU VD3/kg diet from weaning until 12 wk of age (n = 6).
In Expt. 2B, 36 male C57BL/6 mice were fed AIN93G diets containing 25 (0.625 μg), 50, 100, 200, 400, or 1000 IU VD3/kg diet from weaning until 14 wk of age (n = 6). At the end of the experiments, the animals were killed and serum and tissues were obtained.
Expt 3: the effect of dietary Ca restriction on bone mineral density in rats fed high-VD3 diets
Thirty weanling male Sprague Dawley rats were placed on 1 of 6 experimental diets in a 3 levels of VD3 (400, 1000, or 10,000 IU VD3/kg diet) by 2 levels of Ca (0.5 or 0.25%) factorial design experiment. Phosphorus level was held constant at 0.4% of the diet. At 10 wk of age, the rats were killed and serum and bone were obtained.
Expt 4: regulation of 25-hydroxycholecalciferol-1ahydroxylase mRNA levels by changes in dietary Ca in mouse kidney and duodenum
Twenty-seven, male C57BL/6 mice were fed a commercial nonpurified diet (8664, Harlan Teklad) until 90 d of age and then switched to 1 of 3 AIN93G diets with low (0.02%), normal (0.5%), or high (2%) Ca levels for 7 d. Dietary phosphorus levels were 0.3, 0.3, and 1.25%, respectively. After consuming the diets for 1 wk, mice were killed, serum was obtained, and duodenal scrapings and kidney were saved for RNA analysis.
Sample analysis
Serum Ca and VD3 metabolite analysis
Serum 1,25(OH)2-VD3 and 25OH-VD3 were analyzed via enzyme immunoassays using commercial kits (Immunodiagnostic Systems). The inter-assay CV for each assay was 10 and 8%, respectively, whereas the intra-assay CV for each assay was 15 and 10%, respectively. The cross reactivity of the 1,25(OH)2-VD3 assay for 25OH-VD3 was 0.0092%. Serum total Ca was analyzed via a quantitative colorimetric assay using the QuantiChrom Ca assay kit (BioAssay Systems).
Real-time PCR
Total RNA was isolated from mucosal scrapings from the first 2 cm of proximal small intestine and minced kidney tissue using the TriReagent procedure (Molecular Research Center). The isolated RNA was reverse transcribed into cDNA as previously described (10). Real-time PCR was conducted on samples using the BioRad My iQ RTPCR system containing SYBR green (Byroad). Transient receptor potential vanilloid family member 6 (TRPV6), 25-hydroxycholecalciferol-1αhydroxylase (CYP27B1), 25-hydroxycholecalciferol-24-hydroxylase (CYP24), VDR, and vitamin D-dependent Ca-binding protein 9 kDa form (calbindin D9k) mRNA levels were determined from the threshold cycle value (11) and were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase within the sample. PCR conditions and primers for calbindin D9k, TRPV6, CYP24, and glyceraldehyde 3-phosphate dehydrogenase were previously reported by our group (12) and CYP27B1 mRNA levels were measured using primer sets and conditions previously reported by Healy et al. (13). Mouse VDR PCR conditions were: forward primer, 5′TACATCCGCTGCCGCCACCCGC3′, reverse primer, 5′TCAGGAGATCTCATTGCC3′, annealing temperature = 55 C. Rat VDR PCR conditions were: forward primer, 5′TCGTATGGACGGAAGTACAGG3′,reverse primer,5′CAGCATGGAGAGAGGAGACAG3′, annealing temperature = 56 °C.
Bone analyses
The right and left femurs from each mouse were stripped of all muscle. The left femur was examined using digital calipers for length and midshaft thickness. Afterwards, the femur was dried, ashed, and Ca content was examined by atomic absorption spectrometry as previously described (14).
The right femur was fixed in neutral buffered formalin for 7 d followed by fixation and storage in 70% ethanol for at least 1 wk. Fixed bones were scanned using a PIXImus II small animal densitometer under the condition of 100- × 80-mm imaging area, 0.25- × 0.25-mm focal spot size, 80 κV and 400 μA (Lunar, GE-Healthcare). Measured variables included bone mineral density (BMD; g/cm2) and bone mineral content [BMC; g = BMD/(skeletal area, cm2)].
Statistical analysis
All data are reported as means ± SEM. Expt. 1b, 2a, and 2b were analyzed by 1-way ANOVA and 2-way ANOVA was used to analyze Expt. 1b (main effects = sex, dietary VD3), Expt. 3 (main effects = dietary VD3, dietary Ca), and Expt. 4 (main effects tissue, dietary Ca) using the SYSTAT Statistical program (version 12, SYSTAT Software). In Expt. 1a using both male and female mice, there was no interaction between sex and dietary VD3 for any of the parameters; as a result, we used sex as a covariate and 1-way ANOVA to increase the power to detect differences. When predicted vs. residual plots indicated the data were not normally distributed, data were log-transformed prior to analysis. Differences between individual means were determined by Fisher's protected least significant difference. Response curves were analyzed by regression analysis using general linear models procedures. In all analyses, P , 0.05 was considered significant.
Results
Expt. 1: the effect of high dietary VD3 intake on rats and mice
Feeding diets with increasing amounts of dietary VD3 from 400 to 20,000 IU/kg diet caused a linear increase in serum 25OH-VD3 concentrations in both 10-wk-old rats fed the diets for 4 wk and in weanling mice fed the diets for 7 wk (Expt. 1a, Fig. 1A). The linear regression line defining this relationship showed an increase of 0.037 nmol·L−1·IU VD3−1·kg−1 (r2 = 0.995). This response was not affected by gender and a similar response to dietary VD3 was seen in mice (Expt. 1b, 0.02 nmol·L−1·IU VD3−1·kg−1; r2 = 0.996; Fig. 1A). Although the concentrations of serum 25OH VD3 ranged from those considered optimal in humans (92 nmol/L in the 400-IU VD3/kg group) to very high concentrations (>800 nmol/L), neither body weight (e.g. 400 IU = 300 ± 25 g; 20,000 IU = 316 ± 27 g in rats) nor serum total Ca concentrations (e.g. 400 IU = 3.00 ± 0.03 mmol/L; 20,000 IU = 2.93 ± 0.03 mmol/L in rats) were affected in either rats or mice.
FIGURE 1.

The effect of diets containing from 400 to 20,000 IU VD3/kg diet on serum VD3 metabolite concentrations and renal CYP27B1 mRNA levels in rats and mice (Expt. 1A and 1B). Serum and kidney were harvested and analyzed for serum 25OH-VD3 (A), serum 1,25(OH)2-VD3 (B), and renal CYP27B1 mRNA (C). Points represent the means ± SEM, rats, n = 10 (rats) or 6 (mice). Within a species, means without a common letter differ, P < 0.05.
Serum 1,25(OH)2 VD3 was suppressed with increasing dietary VD3 intake in rats and mice (Fig. 1B). Similar to a previous report by Johnson et al. (15), the serum concentration of the hormonally active form of VD3, 1,25(OH)2-VD3, was 58% lower in female rats but the effect of high dietary VD3 intake on serum 1,25(OH)2 VD3 was similar in both genders (∼80% reduced from high- to low-VD3 intake). As we have reported previously (16), high-VD3 intake also suppressed serum 1,25(OH)2-VD3 in mice (50% with 20,000 IU VD3/kg; Fig. 1B). Consistent with the suppression of serum 1,25(OH)2-VD3, high dietary VD3 also significantly reduced renal expression of the mRNA encoding the enzyme responsible for the conversion of 25OH-VD3 to 1,25(OH)2-VD3, CYP27B1 mRNA (reduced by <70%) (Fig. 1C). Despite this, the duodenal expression of the 1,25(OH)2-VD3-inducible genes encoding calbindin D9k and TRPV6 (proteins thought to be responsible for intracellular diffusion of Ca and apical membrane uptake of Ca in the enterocyte, respectively) were not affected. Similarly, there was no effect of dietary VD3 on bone ash, bone Ca, or BMD (data not shown).
Expt. 2: the effect of low dietary VD3 intake on rats and mice. From Expt
1a and 1b, we learned that the NRC requirement for VD3 (1000 IU VD3/kg diet) led to serum 25OH VD3 concentrations in rodents (120 nmol/L) that were above the proposed optimal concentrations for humans (80 nmol/L). As a result, our next experiments examined the effect of VD3 restriction. When rats and mice were fed diets with decreasing amounts of VD3 (from 1000 IU/kg to 50 IU/kg in rats or 25 IU/kg in mice), serum 25OH-VD3 concentrations fell in a curvilinear relationship with an inflection point ∼200 IU VD3/kg (Fig. 2A). Above 200 IU VD3/kg, the slope was more shallow (0.071–0.08 nmol·L−1·IU VD3−1) than below 200 IU/kg (0.31 nmol·L−1·IU VD3−1) (Fig. 2A).
FIGURE 2.

The effect of diets containing from 25 to 1000 IU VD3/kg diet on serum VD3 metabolite concentrations and renal mRNA levels (Expt. 2A and 2B). Serum and kidney were harvested and analyzed for serum 25OH-VD3 in mice (white circle) and rats (black circle) (A), serum 1,25(OH)2-VD3 in mice (B), and renal CYP27B1 and CYP24 mRNA in mice (C). Points represent the means ± SEM, n = 6. Within a species (A) or parameter (C), means without a common letter differ, P < 0.05.
Below 100 IU VD3/kg intake, serum 1,25(OH)2-VD3 was reduced in both mice (Fig. 2B) and rats (20% reduction 50 IU/kg vs. 100 IU/kg; P < 0.05). In contrast, low-VD3 intake increased renal CYP27B1 mRNA levels in both mice (Fig. 2C) and rats (300% at 50 IU/kg vs. 1000 IU/kg; P < 0.05). This suggests low serum 1,25(OH)2-VD3 was a consequence of inadequate substrate (the prohormone 25-OH-VD3), not enzyme capacity.
CYP24 is an enzyme that adds a hydroxyl group to the 24 position of 1,25(OH)2 VD3; the CYP24 gene is strongly regulated at the transcriptional level by 1,25(OH)2-VD3 (17). Compared with the low-VD3 diet, renal CYP24 mRNA level was >300% higher in mice (Fig. 2C) and 91% higher in rats fed >200 IU VD3/kg. The lower expression of CYP24 mRNA in the kidney of mice fed the low-VD3 diets reflects the reduced production of serum 1,25(OH)2-VD3 in kidney at low-VD3 intake. Renal expression of the VDR mRNA, encoding the protein that mediates the transcriptional regulation of genes by 1,25(OH)2-VD3, was not influenced by dietary VD3 intake in either rats or mice (data not shown) and, in contrast to a previous report by Vieth et al. (18,19), renal VDR mRNA level and serum 1,25(OH)2-VD3 concentrations were not associated.
Neither body weight nor serum total Ca were influenced by low-VD3 intake. In the intestine, only calbindin D9k mRNA [a putative 1,25(OH)2-VD3 target gene] was significantly reduced and only in mice (55% reduction at 25 and 50 IU/kg compared with 1000 IU/kg). Whereas bone parameters like femur BMD, percent ash, and percent Ca in dry femur were not reduced in rats, BMD and BMC were significantly reduced in mice fed the lowest level of VD3 (25 IU/kg) (Fig. 3).
FIGURE 3.
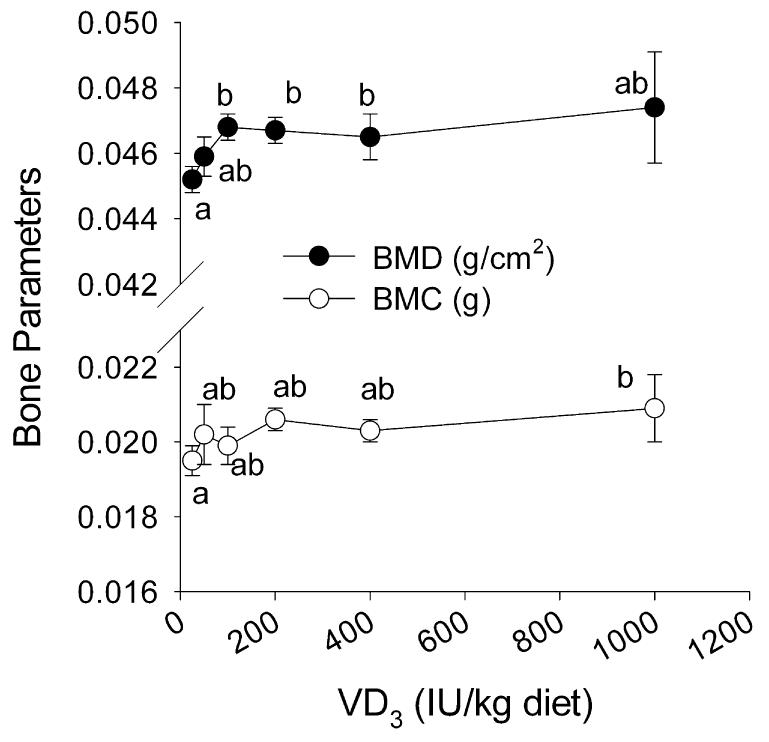
The effect of diets containing from 25 to 1000 IU VD3/kg diet on BMD and BMC in mice (Expt. 2B). Isolated femora were analyzed by dual X-ray absorptiometry. Points represent the means ± SEM, n = 6. Means without a common letter differ, P < 0.05.
Expt. 3: high dietary VD3 does not protect bone from the effects of Ca restriction in rats
Increasing VD3 intake elevated serum 25OH-VD3 concentrations similar to those observed in Expt. 1a (400 IU = 104 nmol/L; 1000 IU = 166 nmol/L; 10,000 IU = 589 nmol/L; P < 0.05 for main effect of VD3). Feeding a low-Ca diet from weaning increased serum 1,25(OH)2-VD3 (179% above 0.5% Ca diet group level; P < 0.05). Rats fed the 10,000-IU VD3/kg diet tended to have greater serum 1,25(OH)2-VD3 when dietary Ca was restricted (30% higher than the other low-Ca intake groups; P = 0.55). However, neither elevated serum 25OH-VD3 nor 1,25(OH)2-VD3 level protected BMD from the effects of Ca restriction (Fig. 4); i.e. only the main effect for dietary Ca was significant and BMD was reduced by 28% (P < 0.05).
FIGURE 4.
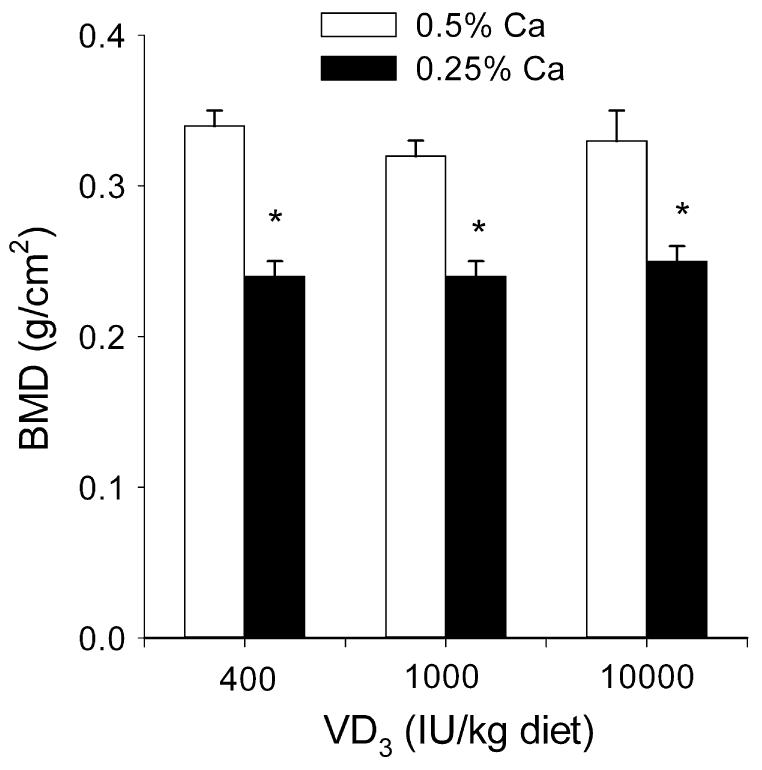
A high-VD3 diet containing 10,000 IU VD3/kg diet does not prevent low BMD caused by 50% dietary Ca restriction in rats (Expt. 3). Femora were isolated and BMD was assessed by dual X-ray absorptiometry. Bars represent the means + SEM, n = 6. *Different from 0.5% Ca, P < 0.05.
Expt. 4: changing dietary Ca intake modulates serum 1,25(OH)2-VD3 and CYP27B1 mRNA levels in kidney but not duodenum
We have previously reported that serum 1,25(OH)2-VD3 concentrations are inversely proportional to dietary Ca intake and other responses related to Ca metabolism, e.g. duodenal expression of TRVP6 and calbindin D9k mRNA and duodenal Ca absorption (12). Consistent with the elevation in serum 1,25(OH)2-VD3 concentrations, we found that renal CYP27B1 mRNA levels increased with dietary Ca restriction (Fig. 5). In contrast, duodenal CYP27B1 mRNA levels were lower than those in kidney (2% of levels on a 0.5% Ca AIN93G diet; P < 0.05) and they were not significantly altered by changes in dietary Ca (Fig. 5).
FIGURE 5.

Dietary Ca restriction increases renal but not duodenal CYP27B1 mRNA levels (Expt. 4). Serum and kidney were harvested and analyzed for serum 1,25(OH)2 VD3 concentrations (A) and renal and duodenal CYP27B1 mRNA levels (B). Bars represent the means + SEM, n = 9. Means without a common letter differ, P < 0.05.
Discussion
Many groups have evaluated the effect of VD3 deficiency on physiologic and disease states in rodents. For example, in addition to its traditional action controlling Ca and bone metabolism (20-22), VD3 deficiency in early life has been shown to accelerate type I diabetes in the nonobese diabetic mouse (23) and other groups have demonstrated that VD3 deficiency has a negative effect on tumor growth or burden in cancer models (24-26). Although these studies suggest an important role for VD3 in health, the use of such extremes is not relevant to the range of VD3 status currently being discussed as important for the protection of human health (2,6). In contrast, our data clearly show how dietary VD3 can be used to model human VD3 status in experimental mice and rats.
The serum 25OH-VD3 concentrations resulting from feeding a 1000-IU VD3/kg diet (the NRC requirement) to rats and mice is greater than the concentrations proposed to be optimal in humans (>130 nmol/L vs. 80 nmol/L) (6). By carefully examining the relationship between dietary VD3 and serum 25OH-VD3, we have determined that the dietary VD3 concentrations needed for modeling borderline deficiency (25–40 nmol/L) and average (50–60 nmol/L) and optimal (80–100 nmol/L) serum 25OH-VD3 concentrations are 25–50, 100, and 400 IU VD3/kg diet in growing rodents. Our data reveal a curvilinear response of serum 25OH-VD3 to increasing dietary VD3 concentrations (with an inflection point at 80–100 nmol 25OH-VD3/L with a 200-IU VD3/kg diet) that is similar to the relationships reported between serum 25OH-VD3 and serum VD3 in humans (27), and supplemental dietary VD3 in humans (28) and mature rats (29).
Our data show that only very low VD3 intake (<100 IU/kg diet resulting in serum 25OH-VD3 concentrations of <50 nmol/L) has negative effects on intestinal and renal gene expression and on BMD in growing rodents. Several other groups have examined the effect of reduced, but not deficient, VD3 intake on cancer-related endpoints in rodents (30-32). For example, mouse studies by Xue et al. (33,34) have used a Western diet with one-half as much dietary VD3 as the NRC requirement (500 vs. 1000 IU/kg diet) coupled with severe Ca depletion (0.05 vs. 0.5% of diet) to increase epithelial cell proliferation in mouse prostate, breast, and pancreas and to induce colonic neoplasms (35). However, these studies have not assessed serum 25OH-VD3 nor evaluated bone outcomes. In contrast, a recent 12-wk study in mature (10 wk old) rats showed that reducing VD3 intake below 200 ng VD3/d (∼300 IU VD3/kg diet) increased serum parathyroid hormone and renal CYP27B1 mRNA levels even while serum 1,25(OH)2-VD3 levels were reduced, because 25OH-VD3 is limiting. Our data confirm these findings.
Others have argued that VD3 is less toxic than the current upper limit of 2000 IU/d for humans would suggest (36) and our data support that position. Neither serum Ca nor growth were negatively affected even at dietary VD3 intake levels that raised serum 25OH-VD3 to >800 nmol/L. This is consistent with previous reports where short-term (18,19) and long-term (37,38) exposure to very high doses of VD3 greatly increased serum 25OH-VD3 without hypercalcemia in rats.
As in our study, several reports show that high-VD3 intake suppresses the serum concentration of 1,25(OH)2-VD3 in rats (18,19) and humans (5,39) but without a negative effect on intestinal gene expression (e.g. TRPV6 and calbindin D9k mRNA levels were not altered from 200 to 20,000 IU VD3/kg diet). Previously, we showed that 10,000 IU VD3/kg diet can dramatically increase serum 25OH-VD3 concentrations, rescue the phenotype of CYP27B1 knockout mice, and modulate renal and duodenal gene expression (e.g. increase calbindin D9k mRNA) (16). This suggests that supraphysiological levels of 25OH-VD3 can interact with the VDR and activate the genes whose protein products control Ca metabolism in the kidney, intestine, and bone. Collectively, our data demonstrate that signaling through the traditional VD3-endocrine system and the effects resulting from high serum 25OH-VD3 are balanced, thus limiting potential toxic effects.
The benefit of improved VD3 status has been hypothesized to result from increased local production of 1,25(OH)2-VD3 from extra-renal CYP27B1 expression in both classical (e.g. intestine and bone) and nonclassical VD3 target tissues (e.g. epithelial cells of the prostate, breast, and colon) (40). Extra-renal CYP27B1 has been identified by immunohistochemistry in a wide variety of tissues, including colonic epithelial cells (41). A role for local production of 1,25(OH)2-VD3 in the intestine has been suggested by Heaney et al. (4) who showed that intestinal Ca absorption efficiency improved as serum 25OH VD3 increased within the normal range even though serum 1,25(OH)2 VD3 was not elevated. Our data demonstrate that CYP27B1 mRNA is expressed in the duodenum, the site of maximal VD3-regulated intestinal Ca absorption (42). We found that CYP27B1 mRNA is expressed at very low levels relative to the kidney and that it is not altered by dietary Ca restriction, a classical regulator of the renal CYP27B1. This is similar to the lack of regulation of CYP27B1 mRNA by parathyroid hormone in prostate epithelial cells (43). However, although CYP27B1 mRNA was detected in duodenum, higher serum 25OH-VD3 did not exert beneficial effects on duodenal gene expression nor were BMD or bone Ca content improved in growing rodents when serum 25OH-VD3 was >45 nmol/L. In addition, high-VD3 status did not protect the bone of growing rats fed moderately Ca-restricted diets (50% reduction from optimal). While these data do not support the position that improved VD3 status is beneficial to bone and Ca metabolism, our interpretation is limited only to the period of rapid skeletal growth where the role of vitamin D is augmented by the growth hormone-insulin-like growth factor 1 system (44) and therefore may be less sensitive to the benefits of improved VD3 status. Additional preclinical studies are needed to determine whether mature animals can respond favorably to improved VD3 status and thus provide support for the relationships observed in humans, i.e. whether adult bone and Ca metabolism can be improved in rats and mice as serum 25OH-VD3 is increased from 50 to 100 nmol/L.
In conclusion, our studies are a comprehensive examination of the relationship between dietary VD3 intake and serum 25OH-VD3 in experimental rodents. Others have examined parts of this issue and our work confirms, unifies, and extends these earlier findings. Overall, our data show that the growing rat and mouse are resistant to changes in dietary VD3 across a large range of intakes. Similar to humans, bone and Ca metabolism in these animals is sensitive to lowering serum 25OH-VD3 below 45 nmol/L. While our data do not support a benefit to improving vitamin D status and increasing serum 25OH-VD3 concentrations from 50 to >80 nmol/L, our studies are limited to periods of rapid growth. Still, our data can now serve as a foundation to use rats and mice as preclinical models to evaluate the effects of improving VD3 status on the development of various disease states that are now being linked to high serum 25OH-VD3 in human populations.
Footnotes
Supported by funds from the NIH awards CA101113 (to J.C.F.) and DK054111 (to J.C.F.). C. G. was supported by a research assistantship from the Agricultural Research Service of the USDA.
Abbreviations used: 1,25 (OH)2 VD3, 1,25 dihydroxycholecalciferol; 25OH VD3, 25 hydroxycholecalciferol; BMC, bone mineral content; BMD, bone mineral density; calbindin D9k, vitamin D-dependent calcium-binding protein 9 kDa form; CYP24, 25-hydroxycholecalciferol-24 hydroxylase; CYP27B1, 25-hydroxycholecalciferol-1αhydroxylase; IU, international units; TRPV6, transient receptor potential vanilloid transporter isoform 6; VDR, vitamin D receptor; VD3, cholecalciferol.
One microgram of cholecalciferol is equal to 40 international units (IU).
Literature Cited
- 1.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469–74. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF. Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab. 1997;82:4111–6. doi: 10.1210/jcem.82.12.4412. [DOI] [PubMed] [Google Scholar]
- 6.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 7.NRC . Nutrient requirements of laboratory animals. National Academy Press; Washington, DC: 1995. [Google Scholar]
- 8.AIN Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 9.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 10.Fleet JC, Wood RJ. Specific 1,25(OH)2 D3-mediated regulation of transcellular calcium transport in Caco-2 cells. Am J Physiol. 1999;276:G958–64. doi: 10.1152/ajpgi.1999.276.4.G958. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ Ct) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144:3885–94. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- 13.Healy KD, Zella JB, Prahl JM, DeLuca HF. Regulation of the murine renal vitamin D receptor by 1,25-dihydroxyvitamin D3 and calcium. Proc Natl Acad Sci USA. 2003;100:9733–7. doi: 10.1073/pnas.1633774100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Kato S, Fleet JC, Vitamin D. Receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003;133:374–80. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JA, Beckman MJ, Pansini-Porta A, Christakos S, Bruns ME, Beitz DC, Horst RL, Reinhardt TA. Age and gender effects on 1,25-dihydroxyvitamin D3-regulated gene expression. Exp Gerontol. 1995;30:631–43. doi: 10.1016/0531-5565(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 16.Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–15. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–21. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 18.Shephard RM, DeLuca HF. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys. 1980;202:43–53. doi: 10.1016/0003-9861(80)90404-x. [DOI] [PubMed] [Google Scholar]
- 19.Vieth R, Milojevic S, Peltekova V. Improved cholecalciferol nutrition in rats is noncalcemic, suppresses parathyroid hormone and increases responsiveness to 1, 25-dihydroxycholecalciferol. J Nutr. 2000;130:578–84. doi: 10.1093/jn/130.3.578. [DOI] [PubMed] [Google Scholar]
- 20.Haavaldsen R, Nicolaysen R. Studies in calcium metabolism in rats. I. A long term study in rats given an optimal diet with and without vitamin D. Acta Physiol Scand. 1956;36:102–7. doi: 10.1111/j.1748-1716.1956.tb01308.x. [DOI] [PubMed] [Google Scholar]
- 21.Harrand RB, Hartles RL. A study on the effect of vitamin D in rats maintained on diets with different calcium and phosphorus content but with the same high ratio of calcium to phosphorus. Br J Nutr. 1969;23:523–31. doi: 10.1079/bjn19690061. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi N, Sassa H, Sato R, Taniguchi K, Okura N, Sato S, Naito Y. Calcium metabolism of pregnant rats fed a vitamin D-depleted diet. J Vet Med Sci. 2007;69:441–3. doi: 10.1292/jvms.69.441. [DOI] [PubMed] [Google Scholar]
- 23.Giulietti A, Gysemans C, Stoffels K, Van Etten E, Decallonne B, Overbergh L, Bouillon R, Mathieu C. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451–62. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 24.Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608–13. [PubMed] [Google Scholar]
- 25.Llor X, Jacoby RF, Teng BB, Davidson NO, Sitrin MD, Brasitus TA. K-ras mutations in 1,2-dimethylhydrazine-induced colonic tumors: effects of supplemental dietary calcium and vitamin D deficiency. Cancer Res. 1991;51:4305–9. [PubMed] [Google Scholar]
- 26.Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350–4. doi: 10.1093/jn/135.10.2350. [DOI] [PubMed] [Google Scholar]
- 27.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–5. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 28.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 29.Anderson PH, Sawyer RK, May BK, O'Loughlin PD, Morris HA. 25-Hydroxyvitamin D requirement for maintaining skeletal health utilising a Sprague-Dawley rat model. J Steroid Biochem Mol Biol. 2007;103:592–5. doi: 10.1016/j.jsbmb.2006.12.086. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49:6300–3. [PubMed] [Google Scholar]
- 31.Yang K, Lipkin M, Newmark H, Rigas B, Daroqui C, Maier S, Augenlicht L. Molecular targets of calcium and vitamin D in mouse genetic models of intestinal cancer. Nutr Rev. 2007;65:S134–7. doi: 10.1111/j.1753-4887.2007.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 32.Cope MB, Steele VE, Eto I, Juliana MM, Hill DL, Grubbs CJ. Prevention of methylnitrosourea-induced mammary cancers by 9-cis-retinoic acid and/or vitamin D3. Oncol Rep. 2002;9:533–7. [PubMed] [Google Scholar]
- 33.Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91:176–81. doi: 10.1093/jnci/91.2.176. [DOI] [PubMed] [Google Scholar]
- 34.Xue L, Yang K, Newmark H, Lipkin M. Induced hyperproliferation in epithelial cells of mouse prostate by a Western-style diet. Carcinogenesis. 1997;18:995–9. doi: 10.1093/carcin/18.5.995. [DOI] [PubMed] [Google Scholar]
- 35.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki HA. Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–5. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 36.Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007;85:6–18. doi: 10.1093/ajcn/85.1.6. [DOI] [PubMed] [Google Scholar]
- 37.Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144–52. doi: 10.1093/jn/123.1.144. [DOI] [PubMed] [Google Scholar]
- 38.Comer PF, Clark TD, Glauert HP. Effect of dietary vitamin D3 (cholecalciferol) on colon carcinogenesis induced by 1,2-dimethylhydrazine in male Fischer 344 rats. Nutr Cancer. 1993;19:113–24. doi: 10.1080/01635589309514242. [DOI] [PubMed] [Google Scholar]
- 39.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8:222–30. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 40.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 41.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 42.Pansu D, Bellaton C, Roche C, Bronner F. Duodenal and ileal calcium absorption in the rat and effects of vitamin D. Am J Physiol. 1983;244:G695–700. doi: 10.1152/ajpgi.1983.244.6.G695. [DOI] [PubMed] [Google Scholar]
- 43.Young MV, Schwartz GG, Wang L, Jamieson DP, Whitlatch LW, Flanagan JN, Lokeshwar BL, Holick MF, Chen TC. The prostate 25-hydroxyvitamin D-1 alpha-hydroxylase is not influenced by parathyroid hormone and calcium: implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis. 2004;25:967–71. doi: 10.1093/carcin/bgh082. [DOI] [PubMed] [Google Scholar]
- 44.Kasukawa Y, Baylink DJ, Wergedal JE, Amaar Y, Srivastava AK, Guo R, Mohan S. Lack of insulin-like growth factor I exaggerates the effect of calcium deficiency on bone accretion in mice. Endocrinology. 2003;144:4682–9. doi: 10.1210/en.2003-0745. [DOI] [PubMed] [Google Scholar]


