Abstract
The Us2 gene is conserved among alphaherpesviruses, but its function is not known. We demonstrate here that the pseudorabies virus (PRV) Us2 protein is synthesized early after infection and localizes to cytoplasmic vesicles and to the plasma membrane, despite the lack of a recognizable signal sequence or membrane-spanning domain. Us2 protein is also packaged as part of the tegument of mature virions. The Us2 carboxy-terminal four amino acids comprise a CAAX motif, a well-characterized signal for protein prenylation. Treatment of infected cells with lovastatin, a drug that disrupts protein prenylation, changed the relative electrophoretic mobility of Us2 in sodium dodecyl sulfate-polyacrylamide gels. In addition, lovastatin treatment caused a dramatic relocalization of Us2 to cytoplasmic punctate structures associated with microtubules, which appeared to concentrate over the microtubule organizing center. When the CAAX motif was changed to GAAX and the mutant protein was synthesized from an expression plasmid, it concentrated in punctate cytoplasmic structures reminiscent of Us2 localization in infected cells treated with lovastatin. We suggest that prenylation of PRV Us2 protein is required for proper membrane association. Curiously, the Us2 protein isolated from purified virions does not appear to be prenylated. This is the first report to describe the prenylation of an alphaherpesvirus protein.
The Us2 gene is encoded in the genome of many alphaherpesviruses, including those of human, equine, canine, feline, bovine, avian, and porcine origin (17, 24, 28, 38, 45, 46, 69, 73, 75). Despite this conservation, no function has been assigned and, when tested, the protein is not required for virus growth in cultured cells. The herpes simplex virus type 2 (HSV-2) (23, 26, 29), HSV-1 (42, 47, 71), and equine herpesvirus type 1 (EHV-1) (48) homologs are virion structural proteins. Late after infection of Vero cells with HSV-2, the Us2 protein localizes to the nucleus and cytoplasm (29). EHV-1 Us2 protein localizes to the plasma membrane despite lacking a classical N-terminal signal sequence. Moreover, EHV-1 altered Us2 proteins lacking a conserved stretch of 16 hydrophobic amino acids at the N terminus also localized to the plasma membrane, indicating that this region is not required for membrane localization (48). The authors of that study suggested that Us2 protein is a peripheral membrane protein, but the mechanism of localization was not specified. EHV-1 Us2-null mutants are attenuated in mice after intranasal inoculation (48). In contrast, HSV-2 Us2-null mutants were as virulent as wild-type virus in mice after footpad inoculation (29) or intravaginal infection (26). EHV-1 Us2-null mutants formed small plaques on monolayers of rabbit kidney cells despite having normal kinetics in single-step growth studies (48).
The PRV Us2 gene encodes a 263-amino-acid protein with a predicted molecular mass of 28 kDa (69). PRV Us2-null mutants show wild-type virulence after intranasal inoculation of swine (33), whereas a different Us2 deletion mutant had delayed cell penetration kinetics in nasal mucosa explant cultures (70). EHV-1 Us2-null mutants also displayed delayed penetration kinetics in rabbit kidney cells (48). The genome of the attenuated PRV vaccine strain, Bartha, harbors a deletion in the unique short region encompassing the glycoprotein I (gI), gE, Us9, and Us2 genes (40, 51, 53). Although the roles of gE, gI, and Us9 in the virulence of PRV have been well documented (2, 3, 8, 11-13, 27, 33, 36, 37, 41, 50, 51, 59, 68, 72), no phenotype has been attributed to the lack of Us2 coding sequences. We have found that when the Bartha gE/gI/Us9/Us2 deletion is repaired, virulence is almost completely restored in a chicken embryo eye infection model. Importantly, when the Us2 gene was then deleted from this repaired Bartha genome, virulence decreased dramatically (A. C. Clase and B. W. Banfield, unpublished observations). These observations prompted us to characterize the PRV Us2 protein. We show here that the PRV Us2 protein is expressed early in infection and, like the EHV-1 Us2 protein, is found at the plasma membrane (48). Such localization requires prenylation of a C-terminal CAAX motif. When membrane localization of PRV Us2 protein was inhibited, the protein localized to microtubules and concentrated over the microtubule-organizing center. The Us2 protein incorporated into virions is not prenylated. We speculate that a function of nonprenylated Us2 protein in the virion is to facilitate binding of capsids to the microtubule cytoskeleton during entry.
MATERIALS AND METHODS
Cells and viruses.
PRV strains were propagated on PK15 cells growing in Dulbecco modified Eagle medium (DMEM)-10% fetal calf serum (FCS) at 37°C in a 5% CO2 environment. PRV174 is a Us2-null mutant derived from the wild-type PRV Becker strain. PRV174 was constructed as follows. A PpuMI-NotI fragment derived from the BamHI 7 fragment of PRV Becker containing sequences encoding the C terminus of Us9 and the entire Us2 open reading frame (ORF) was cloned into pGEM5ZF+ to construct the plasmid pTD11. The StyI-SphI fragment of enhanced green fluorescent protein (EGFP) was cloned into the StyI and SphI sites within Us2 to yield pTD13. pTD13 was cotransfected with Becker genomic DNA into PK15 cells. Virus resulting from the cotransfection was plated at low density on PK15 cell monolayers. Recombinant virus plaques expressing EGFP were identified with the aid of an inverted epifluorescence microscope. Recombinants were purified to homogeneity by three successive rounds of plaque purification. Southern blot analysis was performed to verify that the expected recombination events had occurred.
PK15 cells stably expressing an EGFP-tubulin fusion protein were isolated after transfection of the plasmid pEGFP-Tub (Clontech, Palo Alto, Calif.). At 48 h after transfection, cells were passaged into DMEM-10% FCS containing 1 mg of G418/ml. A G418-resistant colony of cells stably expressing EGFP-tubulin was isolated and expanded for further analysis.
PRV-GS443, a wild-type PRV Becker derivative that expresses a VP26-EGFP fusion protein (64), was kindly provided by Greg Smith, Northwestern University.
Production of antiserum.
The Us2 ORF was fused to the C terminus of the glutathione S-transferase (GST) gene in plasmid pGEX-3X (Amersham Biosciences, Piscataway, N.J.), resulting in plasmid pJR36. An insoluble GST-Us2 fusion protein was isolated from IPTG (isopropyl-β-d-thiogalactopyranoside)-induced BL21(DE3) Escherichia coli transformed with pJR36 by using the B-Per Bacterial Protein Extraction Reagent (Pierce, Rockford, Ill.). The partially purified protein was electrophoresed on a preparative sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) gel. A slab of polyacrylamide containing the GST-Us2 fusion was cut from the preparative gel and sent to Bethyl Laboratories (Montgomery, Tex.) for production of goat polyclonal antiserum.
The UL34 ORF was fused to the C terminus of the GST gene in plasmid pGEX-3X resulting in plasmid pJR33. A soluble GST-UL34 fusion protein was purified from IPTG-induced BL21(DE3) E. coli transformed with pJR33 by glutathione-Sepharose chromatography, as described by the manufacturer (Amersham Biosciences). Purified GST-UL34 was sent to Bethyl Laboratories for production of goat polyclonal antiserum.
Us2 expression kinetics.
Confluent monolayers of PK15 cells growing in 60-mm dishes were infected with PRV Becker at a multiplicity of infection (MOI) of 10. At the indicated times postinfection, the medium was removed and the cells washed three times with phosphate-buffered saline (PBS). Cells were scraped into 165 μl of PBS and transferred to a 1.5-ml microfuge tube. Then, 85 μl of SDS-PAGE sample buffer was added, and the sample was passed through a 28-gauge syringe needle to reduce the viscosity of the sample. Aliquots were heated to 100°C for 5 min, electrophoresed through SDS-10% PAGE gels, and analyzed by Western blotting with polyclonal antiserum to Us2, gB, or gC. The polyvalent goat PRV gC (antibody 282) and gB (antibody 284) antisera have been described previously (55, 58).
PAA treatment.
Confluent monolayers of PK15 cells growing in 60-mm dishes were used for these experiments. At 1 h prior to infection, cell culture medium was replaced with DMEM-10% FCS either with or without 200 μg of phosphonoacetic acid (PAA; Sigma, St. Louis, Mo.)/ml. Cells were infected with PRV Becker at an MOI of 10 and incubated in the presence or absence of 200 μg of PAA/ml for 6 h. Cell extracts were prepared as described above and analyzed by SDS-10% PAGE. Western blots were performed with goat polyclonal antiserum raised against Us2, gB, or gC.
Indirect immunofluorescence microscopy.
PK15 cells were seeded onto glass coverslips and grown to 30 to 40% confluence prior to infection with PRV or transfection with PRV Us2 expression plasmids. At the end of the experiment, cells were rinsed three times with PBS and then fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. Cells were rinsed with PBS and permeabilized in PBS-1% bovine serum albumin (PBS-BSA) containing 0.1% Triton X-100 at room temperature for 3 min. Cells were rinsed three times with PBS and incubated for 45 min with Us2, gB, or UL34 polyclonal antiserum diluted in PBS-BSA. Cells were rinsed three times with PBS-BSA and incubated for 30 min with Alexa 568-conjugated secondary antibodies (Molecular Probes, Eugene, Oreg.) diluted in PBS-BSA. The cells were washed three times with PBS-BSA, followed by three washes with PBS. Coverslips were mounted on glass slides, and images were captured by using a Zeiss Axiophot epifluorescence microscope equipped with a motorized stage and a cooled charge-coupled device camera. Series of Z-images were deconvolved by using Slidebook 3.0.7.3 software (Intelligent Imaging Innovations, Inc., Denver, Colo.), and images of 0.5-μm optical sections through the middle of cells were exported into Adobe Photoshop 6.0 (Adobe Systems, Inc., San Jose, Calif.) for the construction of image composites. Confocal images were obtained by using a Zeiss 510 laser scanning confocal microsope.
Staining cells with anti-farnesyl antibodies.
At 17 h posttransfection or 5 h postinfection, cells were rinsed three times with PBS and fixed in acetone-methanol (1:1) at −20°C for 10 min. The acetone-methanol was removed, and PBS was added to the cells. Rabbit anti-farnesyl antiserum (Exalpha Biologicals, Inc., Watertown, Mass.) and goat anti-Us2 antiserum diluted in PBS-BSA were added to the cells and incubated at room temperature for 45 min. Cells were rinsed three times with PBS-BSA and then incubated for 30 min with Alexa 568- or Alexa 488-conjugated secondary antibodies (Molecular Probes) diluted in PBS-BSA. The cells were washed three times with PBS-BSA, followed by three washes with PBS. Coverslips were mounted on glass slides, and images were captured by using a Zeiss 510 laser scanning confocal microsope.
Virion purification.
Virions were purified as described previously (43). Briefly, three confluent 150-mm dishes of PK15 cells were infected at an MOI of 10. At 16 h postinfection, the medium was collected and centrifuged at 3,000 rpm in a Sorvall ST-H750 rotor to remove cellular debris. The clarified supernatant was layered on a 30% sucrose-PBS cushion and centrifuged in a Beckman SW28 rotor at 23,000 rpm for 3 h. The pellet containing virions was resuspended in 0.5 ml of PBS and then centrifuged through 1 ml of 30% sucrose-PBS cushion at 28,000 rpm for 90 min in a Beckman SW55 Ti rotor. The pelleted virions were resuspended in PBS and stored in aliquots at −80°C.
Protease treatment of virions.
Isolated virions were treated with 10 μg of proteinase K (PK; Fisher, Fair Lawn, N.J.) per ml in either the presence or the absence of 1% SDS. After incubation for 60 min at room temperature, phenylmethylsulfonyl fluoride was added to each sample to a final concentration of 2 mM to inhibit further proteolysis (7). Samples were immediately loaded onto a SDS-12% polyacrylamide gel, electrophoresed, and transferred to Immobilon-P membranes (Millipore, Bedford, Mass.). Us2 and gB were detected by Western blotting.
Us2 expression plasmids.
The entire coding sequence of Us2 was amplified by PCR from Becker genomic DNA with the forward primer Us2ER1/Gst-F (5′-CACCGGAATTCCATGGGGGTGACGGCCATCACC-3′), containing an EcoRI site, and the reverse primer Us2Sal-R (5′-CATAGTCGACCTAGGAGATGGTACATCGCGG-3′) containing a SalI site. The product was cloned into pCR-Blunt II TOPO vector by using the zero Blunt TOPO PCR cloning kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. The cloned PCR product was subcloned into pCINeo at the EcoRI and SalI sites to produce the plasmid pCC34. pCC35 was constructed in the same manner as pCC34 except that for PCR the reverse primer used was Us2mut-R (5′-CATAGTCGACCTAGGAGATGGTACCTCGCGGGGCGCGC-3′) containing a SalI site. This primer introduces a mutation (underlined) that changes the cysteine codon at position 259 to a glycine codon. All PCR products were sequenced to ensure that no unexpected mutations were introduced during amplification. The plasmids pCC34 and pCC35 were transfected into PK15 cells by using the FuGene 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, Ind.) according to the manufacturer's instructions. At 18 h after transfection, cells were fixed and stained for Us2 protein, and images were captured as described above.
Immunoprecipitation.
PK15 cells growing on 100-mm dishes were infected with PRV Becker at an MOI of 10. At 5 h postinfection cells were lysed on ice in 1 ml of 10 mM Tris (pH 7.4)-150 mM NaCl-1% NP-40-1% sodium deoxycholate. Nuclei were removed by centrifugation for 5 min at 1,000 × g. The supernatant was delipidated by addition of 4 volumes of ice-cold acetone and incubated overnight at −20°C. Samples were centrifuged at 10,000 × g for 10 min. Protein pellets were resuspended in 800 μl of 10 mM Tris (pH 7.4)-150 mM NaCl-1% NP-40-1% sodium deoxycholate, and 80 μl of 10% NP-40-5% sodium deoxycholate-1% SDS was added. Then, 400 μl was transferred to a new microfuge tube containing 5 μl of rabbit preimmune serum and the samples rocked for 1 h at 4°C. Fifty microliters of immobilized protein-G (Pierce) was added and the sample rocked at 4°C for 1 h. Immune complexes were removed by centrifugation at 10,000 × g for 5 min. The supernatant was transferred to a fresh microfuge tube containing 3 μl of rabbit anti-farnesyl antiserum (Exalpha Biologicals) and incubated overnight on ice. A total of 50 μl of immobilized protein G was added, and the sample was rocked at 4°C for 1 h. Immune complexes were isolated by centrifugation at 10,000 × g for 5 min. The supernatant was discarded, and the pellet was washed with 500 μl of ice-cold 20 mM Tris (pH 7.5)-150 mM NaCl-1% NP-40. Immune complexes were pelleted by centrifugation at 10,000 × g for 5 min. The supernatant was discarded, and the pellet was washed with 500 μl of ice-cold 20 mM Tris (pH 8.8)-150 mM NaCl-1% NP-40-0.2% SDS. Immune complexes were pelleted by centrifugation at 10,000 × g for 5 min. The supernatant was discarded, and the pellet was washed with 500 μl of ice-cold 20 mM Tris (pH 6.8)-150 mM NaCl-1% NP-40-0.2% SDS. Immune complexes were pelleted by centrifugation at 10,000 × g for 5 min, resuspended in 50 μl of SDS-PAGE sample buffer, and boiled for 5 min. Samples were centrifuged at 10,000 × g for 5 min, and the supernatant was transferred to a new microfuge tube. Samples were analyzed by SDS-PAGE and Western blotting as described above.
RESULTS
Identification of Us2 protein in PRV-infected cell lysates.
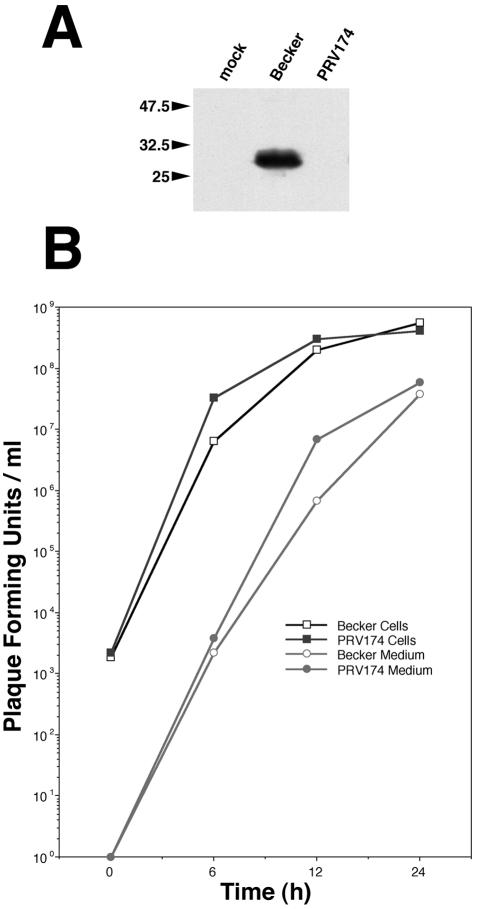
We produced a polyvalent antiserum against a GST-Us2 fusion protein as described in Materials and Methods. This Us2 antiserum recognized a single protein by Western blotting in PRV Becker-infected PK15 cell lysates that was not detected in lysates from cells infected with the Becker Us2-null mutant, PRV174 (Fig. 1A). The estimated molecular mass of PRV Us2 protein determined by gel electrophoresis was 28 kDa. This estimate is consistent with the predicted molecular mass based on the primary amino acid sequence, as well at that reported previously by van Zijl et al. (69).
FIG. 1.
Us2 is a 28-kDa protein dispensable for replication of virus in cell culture. (A) Western blot of PRV Becker- and PRV174-infected cell lysates. PRV Becker is a wild-type virus strain and PRV174 is a Us2-null mutant. Us2 was visualized by using a polyvalent antiserum raised in a goat. (B) Single-step growth analysis of Becker strain and PRV174 in PK15 cells. Virus associated with cells and virus released into the culture supernatant are plotted separately.
Deletion of the Us2 gene does not affect virus replication.
Single-step growth analysis was performed to determine whether deletion of the Us2 gene affected viral replication. Figure 1B illustrates the kinetics of virus replication of the parental Becker strain and PRV174 in PK15 cells, as well as the time course of extracellular virus production. Both the rate of virus production and the final titers achieved were similar for both the PRV174 and PRV Becker strains. These data indicate, that like HSV-2 (29) and EHV-1 (48), the Us2 protein is not required for efficient replication in cultured cells.
Virulence properties of PRV174.
The Us2 ORF is disrupted in a large genomic deletion of the attenuated PRV Bartha strain that also encompasses the Us9, gE, and gI genes (49). Although it is known that the Us9, gE, and gI genes contribute to the neurovirulence of the wild-type Becker strain (8, 10, 72), the contribution of Us2 to neurovirulence is unknown. Accordingly, the wild-type strain Becker and the Becker Us2-null virus, PRV174, were tested in a rat eye model of infection as described previously (19). PRV174 exhibited virulence and neuronal spread properties that were indistinguishable from the parental wild-type Becker strain (data not shown).
Kinetics of PRV Us2 protein expression.
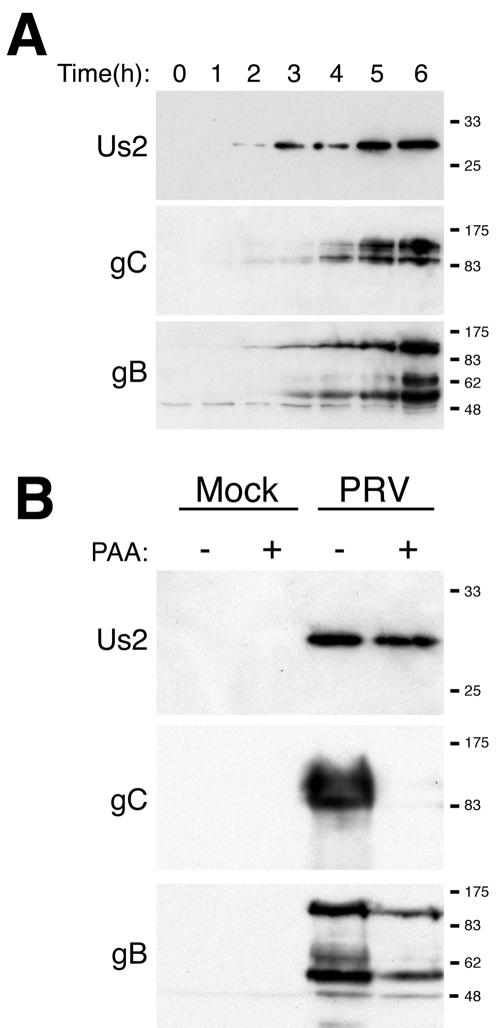
Us2 protein was first detected in cell lysates by Western blotting by 2 h after infection (Fig. 2A). In contrast, the viral gB and gC were not detected until 3 and 4 h after infection, respectively. Herpesvirus genes are grouped into three kinetic classes: immediate early, early, and late. Immediate-early genes are the first viral genes expressed, early genes are expressed prior to viral DNA replication, and late genes are dependent on viral DNA replication for their expression (reviewed in reference 56). To determine whether Us2 protein was expressed with early or late kinetics, we examined its synthesis in the presence of PAA, an inhibitor of viral DNA synthesis, compared to gC (a known late gene product) and gB (an early-late gene product). Expression of PRV Us2 protein was unaffected by PAA, whereas gC was not detected and gB was reduced in the presence of PAA (Fig. 2B). We conclude that the PRV Us2 protein is expressed with early kinetics, which stands in contrast to findings that the Us2 protein of HSV-2 (29) and EHV-1 (6) are expressed with late kinetics.
FIG. 2.
PRV Us2 is an early gene product. (A) Kinetics of Us2 synthesis in Becker strain-infected cell lysates. At the indicated times postinfection, Us2, gC, and gB in infected cell lysates were analyzed by Western blotting. (B) Synthesis of Us2, gC, and gB in the presence or absence of 200 μg of PAA/ml. At 6 h after infection, cell lysates were prepared and Western blots performed. Extracts from mock-infected cells were also analyzed.
Subcellular localization of Us2 protein in infected cells.
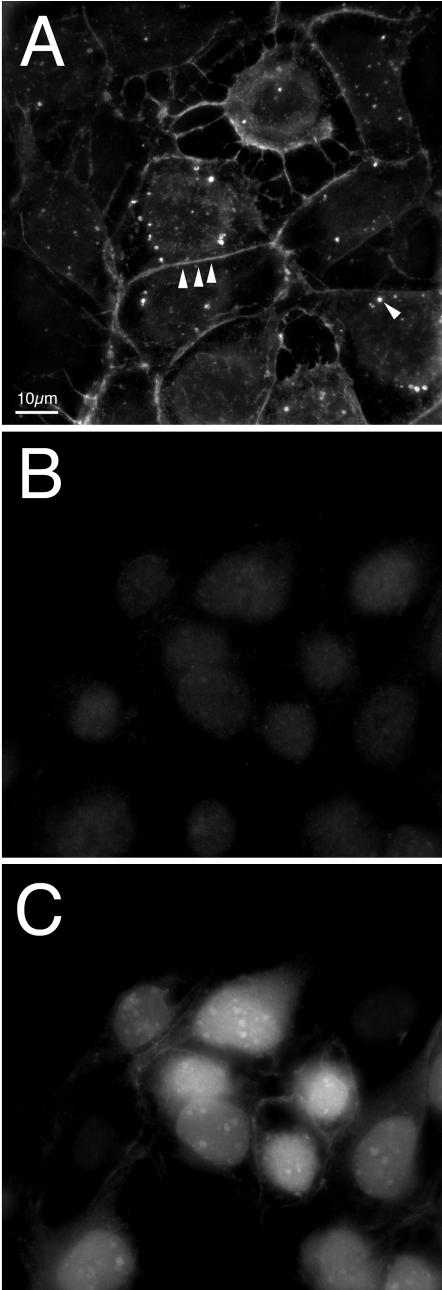
PRV Us2 protein is found in close proximity to the plasma membrane and cytoplasmic vesicles at 5 h after PRV Becker infection of PK15 cells (Fig. 3A). As a negative control, PK15 cells were infected with PRV174 (Us2-null virus) and then stained for Us2 protein expression at 5 h postinfection (Fig. 3B). PRV174-infected cells showed no Us2 expression despite efficient infection of these cells, as evidenced by the expression of EGFP from the Us2 locus in PRV174 (Fig. 3C). We conclude that our Us2 antiserum reacts specifically with Us2 protein in PRV Becker-infected cells.
FIG. 3.
PRV Us2 localization in infected cells. Indirect immunofluorescence microscopy of PK15 cells 5 h after infection with Becker and PRV174. Us2 localization was detected with Us2 antisera. (A) Becker strain-infected cells stained for Us2. Arrowheads point to staining at the plasma membrane and cytoplasmic vesicular structures. (B) PRV174-infected cells stained for Us2 expression. (C) The same field of PRV174-infected cells shown in panel B analyzed for EGFP expression.
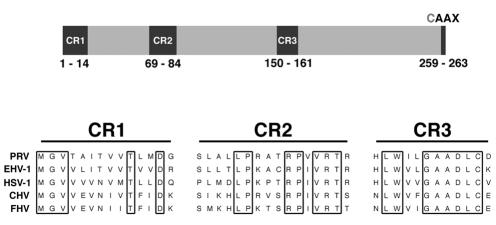
Previous reports have shown that EHV-1 Us2 protein also localized to the plasma membrane despite lacking a classical N-terminal signal sequence or a putative transmembrane domain (48). Sequence analysis of the PRV Us2 coding sequence also failed to identify any obvious signal sequence or potential transmembrane domain. However, a C-terminal CAAX motif was identified that could provide a site for lipid modification, thereby facilitating association with the plasma membrane (Fig. 4). The CAAX motif is a signal for protein prenylation where a farnesyl group or a geranylgeranyl group are covalently linked to an invariant cysteine residue four amino acids from the C terminus of the protein (reviewed in references 14 and 16). Accordingly, we determined whether the C-terminal CAAX motif in PRV Us2 was modified by prenylation and whether this modification was required for plasma membrane association.
FIG. 4.
PRV Us2. The top panel shows a diagram of the PRV Us2 protein, indicating the positions of conserved regions (CR) and the C-terminal CAAX motif. Lower panel shows amino acid sequence alignments of the Us2 CRs from PRV, EHV-1, HSV-1, canine herpesvirus (CHV) and feline herpesvirus (FHV).
PRV Us2 protein prenylation.
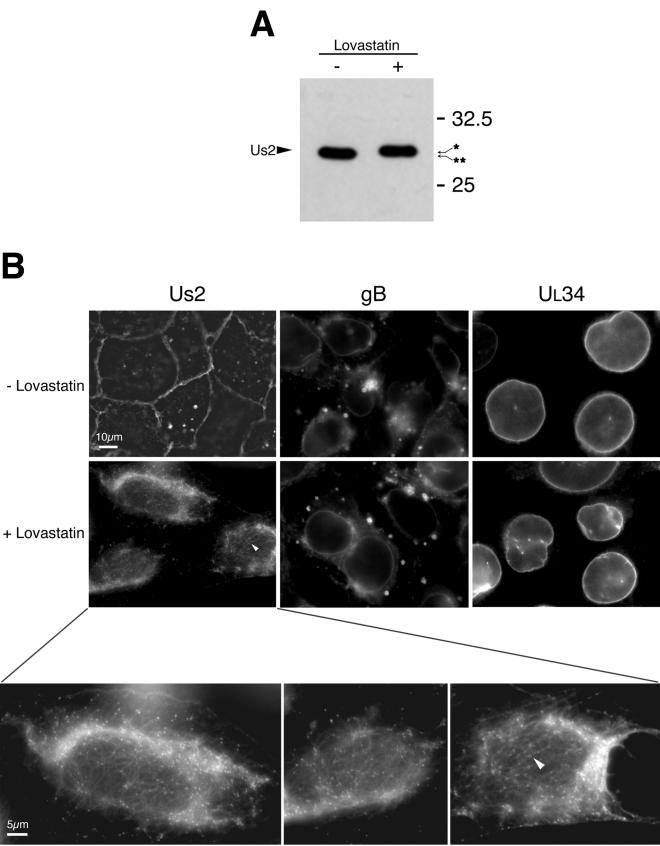
To determine whether the CAAX motif in PRV Us2 was functional and required for plasma membrane localization, we infected cells with PRV Becker in the presence or absence of the drug lovastatin. Lovastatin inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase, preventing the synthesis of farnesylpyrophosphate and geranylgeranylpyrophosphate in the cell (1, 22), which are the substrates for the prenylation reaction. If PRV Us2 protein was indeed prenylated, treatment with lovastatin should prevent modification of the cysteine residue of the CAAX motif, resulting in an altered mobility of Us2 on SDS-PAGE gels and potentially altering the localization of Us2 in PRV-infected cells.
First, it was important to verify that lovastatin treatment did not affect viral replication. PK15 cells were infected with PRV Becker at an MOI of 10 in the presence of a variety of lovastatin concentrations (0, 0.3, 1, 3, 10, and 30 μM). At 24 h after infection, culture supernatants and cells were harvested together, and the amount of infectious virus produced was determined by plaque assay. Similar amounts of infectious virus were obtained regardless of the concentration of lovastatin present in the culture medium (data not shown). Next, we determined whether lovastatin treatment affected the relative mobility of Us2 on SDS-PAGE gels. PK15 cells were infected with PRV Becker in the presence or absence of 30 μM lovastatin. Cell lysates were harvested at 6 h after infection, and Us2 expression was analyzed by Western blotting. Prenylation of CAAX motifs involves the proteolytic cleavage of the three C-terminal amino acids concomitant with the addition of lipid to the invariant cysteine residue (reviewed in references 14, 18, and 57). Thus, prenylation typically results in an increased mobility of the modified protein on SDS-PAGE gels. As predicted, the Us2 protein synthesized in the presence of lovastatin had a lower relative mobility compared to the protein synthesized in the absence of drug (Fig. 5A). Figure 5B shows immunofluorescence micrographs of cells infected with PRV Becker in the presence or absence of lovastatin. In the presence of lovastatin, Us2 protein did not associate with the plasma membrane but instead was detected in numerous punctate spots of uniform size. Areas in the micrograph that appear as regions of diffuse Us2 protein staining actually represent the accumulation of large numbers of these tiny punctate structures concentrated in a perinuclear location. Interestingly, the majority of these structures appeared to be associated with filamentous cellular structures, which also reacted with our Us2 antiserum in lovastatin-treated cells (white arrowhead). As expected, the localization of gB, a type I membrane protein associated with the secretory pathway (55), and UL34, a type II membrane protein that associates primarily with the nuclear membrane (35, 62, 74), were not affected by lovastatin treatment (Fig. 5B). These data indicate that lovastatin treatment did not alter membrane protein localization nonspecifically.
FIG. 5.
Lovastatin treatment of PRV-infected cells. (A) PK15 cells were infected with Becker strain in the presence or absence of lovastatin, and extracts were analyzed for Us2 expression by Western blotting. The arrows on the right of the blot point to the different mobility of Us2 proteins extracted from cells treated with lovastatin versus control. ✽, nonprenylated form of Us2; ✽✽, prenylated form of Us2. (B) Indirect immunofluorescence microscopy of PK15 cells infected with PRV Becker in the presence or absence of lovastatin at 5 h postinfection. Cells were stained with antiserum directed against Us2, gB, or UL34. The lower panels show higher-magnification images of Us2 staining in lovastatin-treated cells. Arrowheads indicate filamentous cellular structures that are associated with the punctate Us2 structures that also reacted with Us2 antiserum.
To ensure that our observations in lovastatin-treated cells were due to the inhibition of protein prenylation and not to a reduction in cholesterol biosynthesis, which is also affected by lovastatin, we treated infected cells with 30 μM lovastatin in the presence of 250 μM mevalonate. Under these conditions, synthesis of farnesylpyrophosphate and geranylgeranylpyrophosphate is maintained and protein prenylation continues, whereas cholesterol biosynthesis remains inhibited (9, 32). The addition of mevalonate, in the presence of lovastatin, restored Us2 protein localization to the plasma membrane, supporting the notion that it is the inhibition of protein prenylation and not the depletion of cholesterol that caused the disruption of Us2 protein localization in lovastatin-treated cells (data not shown).
Packaging of Us2 into PRV virions.
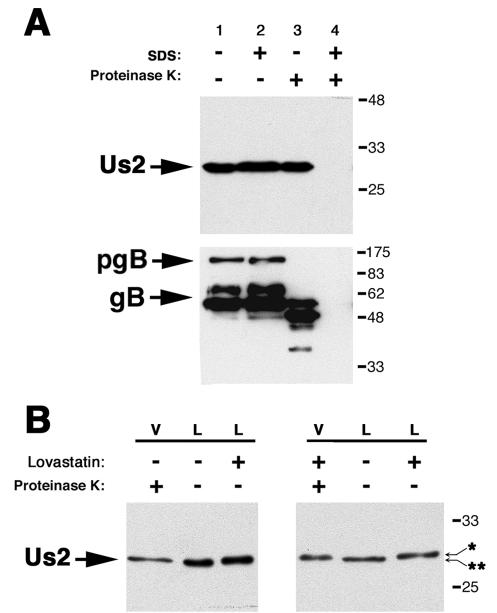
The Us2 homologs from both EHV-1 and HSV-2 are incorporated into mature virions (29, 48). To determine whether PRV Us2 was packaged into virions, we subjected purified extracellular virions to a protease protection assay described previously (7, 43). Purified virions were treated with PBS, SDS, PK, or both SDS and PK. Treated virions were subjected to SDS-PAGE, and Us2 was detected by Western blotting (Fig. 6A). As shown in Fig. 6A, lanes 1 to 3, Us2 protein was unaffected by treatment with PBS, SDS alone, or PK alone. In lane 4, where virions were treated with both SDS and PK, Us2 protein was susceptible to digestion. In these experiments, PRV gB was used as a loading control and as a control for PK activity in the absence of SDS treatment (43). These data indicate that Us2 protein is a component of PRV Becker virus particles and is only digested by PK when the lipid envelope is compromised by SDS treatment. Thus, Us2 protein is very likely a tegument component.
FIG. 6.
Us2 is incorporated into the tegument of PRV virions. PK15 cells were infected with PRV Becker at an MOI of 10. At 16 h after infection, virus was harvested, purified, and treated with or without SDS and/or PK. (A) Western blot of purified virus analyzed for Us2 and gB. In lane 1, purified virions were treated with PBS alone to serve as a control for virion integrity. In lane 2, virions were treated with SDS alone to solubilize the lipid envelope. In lane 3, virions were treated with PK alone to degrade proteins exterior to the lipid envelope, namely, the ectodomains of viral glycoproteins. Us2 was degraded only after treatment with both SDS to solubilize the lipid envelope and PK to degrade proteins (lane 4). (B) Cells were infected with PRV Becker in the presence or absence of lovastatin. Western blots of purified virus (V) and cell lysates (L) were probed for Us2. Us2 from purified virus particles had the same relative mobility as the Us2 in cell lysates treated with lovastatin. ✽, nonprenylated form of Us2; ✽✽, prenylated form of Us2.
Prenylation is not required for Us2 incorporation into virions.
We next determined whether prenylation was necessary for incorporation of Us2 protein into extracellular virus particles, and we investigated whether the prenylated or nonprenylated form of Us2 protein was packaged into virions. PK15 cells were infected with PRV Becker in the presence or absence of lovastatin. Virus was harvested at 16 h postinfection, purified as described in Materials and Methods, and analyzed by SDS-PAGE and Western blotting, alongside of PRV Becker-infected cell lysates prepared in the absence or presence of lovastatin. Virion preparations were treated with PK to digest any contaminating Us2 protein not found within virus particles. Interestingly, as shown in Fig. 6B, Us2 protein packaged into PRV Becker virions in the absence of drug had the same mobility as protein harvested from Becker cell lysate treated with lovastatin. These data suggest that a nonprenylated form of Us2 protein is preferentially incorporated into virions. In support of this conclusion, we found that in the presence of lovastatin Us2 protein was packaged into virions efficiently and the protein had the same electrophoretic mobility as that found in PRV Becker-infected cell lysates treated with lovastatin (Fig. 6B).
Nonprenylated Us2 associates with microtubules.
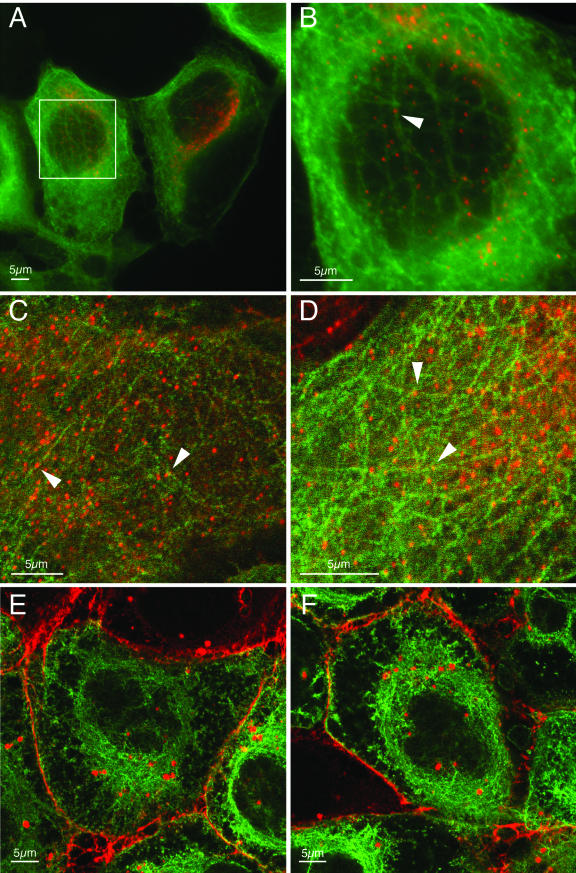
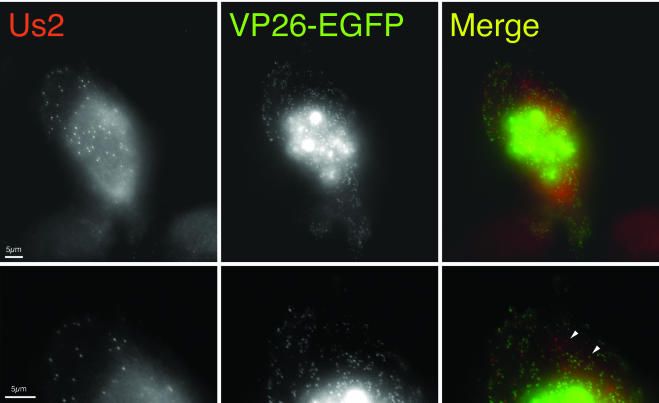
Herpes virions comprise a DNA genome surrounded by an icosahedral capsid. This capsid is surrounded by the tegument layer, which we have shown contains the Us2 protein. Recently, it has been reported that in neurons, alphaherpesvirus capsids travel in axons away from the cell body by using microtubule motors. Using a PRV strain that expresses an EGFP-VP26 fusion protein, PRV-GS443, Smith et al. were able to visualize and track the movement of individual viral capsids in neurons (64). In addition, it has been reported that incoming nucleocapsids of PRV (30) and HSV-1 (65) are transported to the nucleus via microtubules and that intact microtubules enhance HSV-1 infection (44) (recently reviewed in reference 63). In the presence of lovastatin, intracellular Us2 protein localizes to very uniform punctate spots that appear to associate with the cytoskeleton. We speculated that the nonprenylated Us2 protein in the tegument might function to associate viral capsids with microtubules. To investigate this hypothesis, we infected a PK15-derived cell line that stably expresses an EGFP-tubulin fusion protein in the presence of lovastatin and then determined the location of Us2 protein. Figure 7 shows that the uniform punctate Us2 structures observed in the presence of lovastatin associate with microtubules. However, when we infected PK15 cells with PRV-GS443 in the presence of lovastatin we found that the Us2 punctate structures did not colocalize with virus capsids in the cytoplasm (Fig. 8). These data caused us to question whether other viral proteins were required for localization of Us2 protein to these punctate structures.
FIG. 7.
Localization of Us2 in PK15 cells expressing EGFP-tubulin. (A to D) Fluorescence microscopy of EGFP-tubulin-expressing cells infected with PRV Becker strain in the presence of lovastatin and stained for Us2. Panel B is a higher-magnification image of the boxed area in panel A. The punctate Us2 structures in lovastatin-treated cells (red) colocalize with EGFP-labeled microtubules (green). Arrowheads point to Us2 puncta associated with microtubules. (E and F) Fluorescence microscopy of EGFP-tubulin-expressing cells infected with Becker in the absence of lovastatin and then stained for Us2. In the absence of lovastatin, Us2 localizes to the plasma membrane and to cytoplasmic vesicles. Panels A and B are digitally deconvolved images. Panels C to F are laser scanning confocal images.
FIG. 8.
Punctate Us2 structures do not colocalize with viral capsids. Cells were infected with PRV-GS443, which expresses a VP26-EGFP fusion protein. The left panels show Us2 staining. The middle panels show EGFP signal. The right panels show a merged image illustrating that the Us2 punctate structures (red) do not colocalize with viral capsids (green). The bottom series of panels are higher-magnification images of the upper panels. White arrowheads illustrate individual red and green puncta. The images were digitally deconvolved.
Localization of Us2 protein in transfected cells.
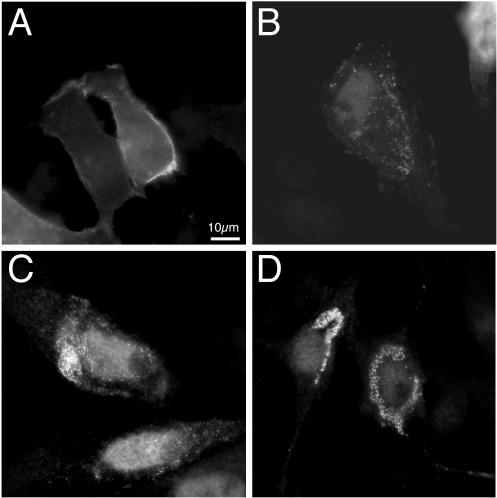
To examine Us2 protein localization in the absence of other viral proteins, we transfected PK15 cells with a Us2 expression plasmid. Figure 9A and B show the localization of Us2 protein in transfected cells in the absence and in the presence, respectively, of lovastatin. Without lovastatin treatment, Us2 protein was found primarily on the plasma membrane, as observed in infected cells. However, the protein did not localize to the cytoplasmic vesicles seen in infected cells. In transfected cells treated with lovastatin, Us2 protein was found in uniform, tiny punctate structures reminiscent of those observed in PRV-infected cells. These data indicate that no other viral proteins are required to localize Us2 to the plasma membrane or to the uniform punctate structures seen in the presence of prenylation inhibitors.
FIG. 9.
Localization of Us2 in transfected cells. Wild-type Us2 (pCC34) transfected into PK15 cells in the absence (A) or presence (B) of lovastatin. Without drug, Us2 localized to the plasma membrane, as shown in panel A. With drug, Us2 localized to discrete punctate structures, as shown in panel B. An expression plasmid containing Us2 with the cysteine residue at amino acid 259 mutated to a glycine (pCC35) was transfected into cells in the absence (C) or presence (D) of lovastatin. Mutation of the cysteine residue disrupted the localization of Us2 from the plasma membrane to uniform punctate structures similar to those seen after transfection of wild-type Us2 in the presence of lovastatin (see panel B). Transfection of the cysteine mutant in the presence of lovastatin does not substantially alter its localization, as shown in panel D. The images were digitally deconvolved.
The C-terminal cysteine is required for Us2 localization to the plasma membrane.
To confirm that the C-terminal CAAX motif is required for localization of Us2 protein to the plasma membrane, we changed the critical cysteine residue to a glycine and cloned this mutant Us2 gene into an expression plasmid. This plasmid, pCC35, was transfected into PK15 cells, and Us2 protein localization was examined by indirect immunofluorescence microscopy. The altered Us2 protein was found primarily in punctate structures similar to those observed in transfected and infected cells treated with lovastatin (Fig. 9C). Transfection of the plasmid carrying the Us2 cysteine mutant gene in the presence of lovastatin (Fig. 9D) did not substantially alter the localization of the Us2 protein. These data suggest that the CAAX motif is functional and required for localization of the Us2 protein to the plasma membrane.
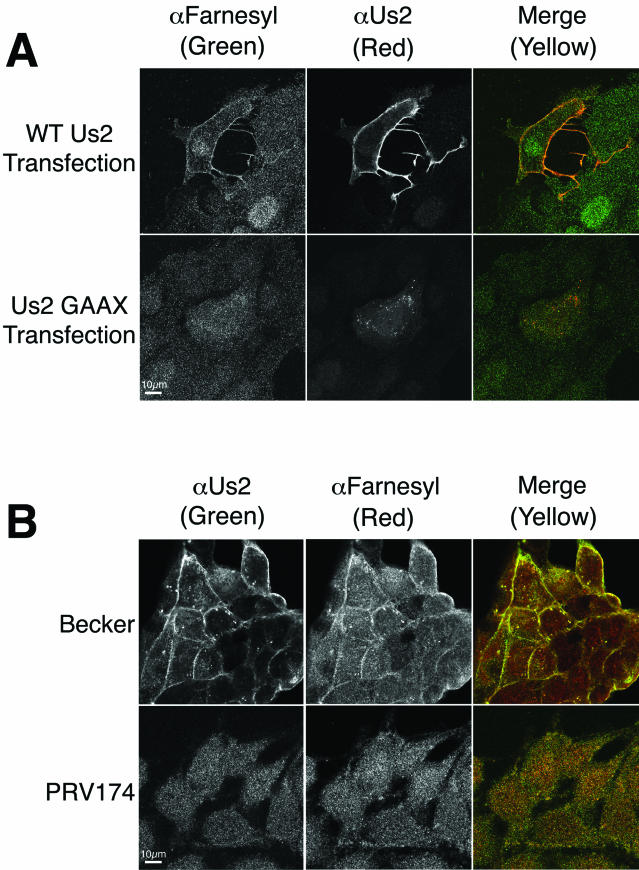
Us2 reacts specifically with anti-farnesyl antibodies.
To directly demonstrate that Us2 protein is prenylated, we took advantage of an antibody raised against farnesyl-cysteine (4, 39). PRV Us2 protein in transfected cells colocalized with anti-farnesyl antibodies at the plasma membrane (Fig. 10A). A mutant Us2 protein, where the CAAX motif had been changed to GAAX, did not react with the anti-farnesyl antiserum. Likewise, Us2 colocalized with anti-farnesyl antibodies at the plasma membrane and cytoplasmic vesicular structures in PRV Becker-infected cells (Fig. 10B). However, the anti-farnesyl antibodies failed to react with these structures in cells infected with PRV174, which is deleted for Us2.
FIG. 10.
Anti-farnesyl antiserum reacts specifically with Us2 in transfected and infected cells. (A) Cells were transfected with a wild-type Us2 expression plasmid or a plasmid that expresses a mutant Us2 in which the CAAX motif has been changed to GAAX. Cells were stained with rabbit anti-farnesyl antiserum and goat anti-Us2 antiserum at 17 h posttransfection. (B) Cells were infected with PRV Becker or the Us2-null mutant, PRV174, and stained for farnesyl and Us2 at 5 h postinfection. Images were obtained by using a Zeiss 510 laser scanning confocal microscope.
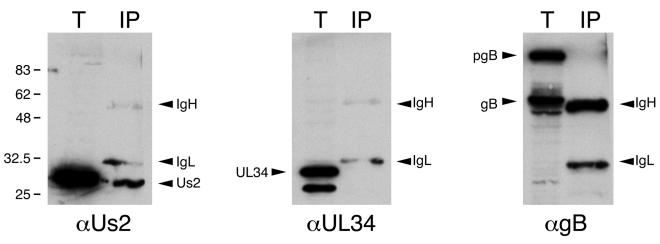
We next determined whether anti-farnesyl antibodies could specifically immunoprecipitate Us2 from infected cell extracts (Fig. 11). Immunoprecipitates were subjected to Western blotting with antisera to Us2, gB, and UL34. Whereas Us2 was readily detected in the immunoprecipitate, we could not detect UL34 or gB in these samples. Because the anti-farnesyl antiserum also cross-reacts with geranylgeranyl groups, we cannot conclude that Us2 is farnesylated as opposed to geranylgeranylated. However, these data conclusively indicate that Us2 is prenylated in infected and transfected cells.
FIG. 11.
Anti-farnesyl antibodies specifically immunoprecipitate Us2. Prenylated proteins were immunoprecipitated from PRV Becker-infected cell extracts prepared at 5 h postinfection. Total extracts (T) and immunoprecipitates (IP) were analyzed by Western blotting with antiserum raised against Us2, UL34, and gB. IgH, immunoglobulin heavy chain; IgL, immunoglobulin light chain.
DISCUSSION
The gene encoding the Us2 protein is conserved among the alphaherpesvirus family members with the notable exception of varicella-zoster virus. Pairwise, amino acid sequence alignments between Us2 homologs from PRV, EHV-1, and HSV-2 indicate 23% similarity between PRV and EHV-1, 21% similarity between PRV and HSV-2, and 26% similarity between EHV-1 and HSV-2. Moreover, three highly conserved blocks of amino acids between amino acids 1 to 14, 69 to 84, and 150 to 161 are maintained in all three viruses, as well as in the canine and feline alphaherpesvirus Us2 homologs (Fig. 4). The conservation of the Us2 ORF in diverse herpesviral genomes is consistent with the proposal that the gene appeared early in the herpesvirus lineage. The conservation of motifs indicates that a common structure or function will be found. Despite its wide conservation of sequence, no alphaherpesvirus requires Us2 protein for replication in cultured cells. Even in animals, Us2-null viruses have only modest attenuation, at best.
In this report we provide a detailed characterization of the PRV Us2 homolog. Our data support previous studies, indicating that PRV Us2 is a 28-kDa protein dispensable for replication in cell culture (69). Unlike HSV-2 Us2 (29) and EHV-1 Us2 (6), we found that PRV Us2 expression is not sensitive to the DNA synthesis inhibitor PAA. These data suggest that PRV Us2 is an early gene product.
By indirect immunofluorescence, the PRV Us2 protein was visible on the plasma membrane and cytoplasmic vesicles in infected cells. Similarly, the EHV-1 protein also localized to the plasma membrane despite lacking a signal sequence or transmembrane domain (48). In contrast, the HSV-2 protein was not localized to the plasma membrane but instead localized to the cytoplasm and to discrete granules within and at the periphery of the nucleus (29).
The PRV Us2 coding sequence has no obvious N-terminal signal sequence or a transmembrane domain and yet was associated with membranes in infected cells. Therefore, we searched for other sequences that could promote association of the protein with the membranes. We found a CAAX motif at the extreme C terminus of the protein, a common site for protein prenylation. Prenylated proteins are modified with a lipid moiety, either farnesyl or geranylgeranyl, by covalent linkage of the lipid to the invariant cysteine after proteolytic digestion of the C-terminal three amino acids. Prenylated cellular proteins play important roles in membrane attachment, signal transduction, and intracellular trafficking pathways (5, 21, 31, 34, 52, 67). Despite their relatively recent discovery in mammalian cells (61), many cellular proteins that undergo this type of modification have been identified, and several comprehensive reviews on their biology have been published (20, 54, 76). Previously, it was believed that prenylation functioned only as a membrane anchor for these modified proteins (21). However, prenylation and farnesylation, in particular, play important roles in protein maturation, cellular signaling and trafficking, and protein-protein interactions. For example, prelamin A, the mammalian lamin A precursor, must be farnesylated prior to the endoproteolysis that produces the mature form of lamin A (5). The Ras family of proteins is the best studied of the prenylated proteins. Kato et al. have shown that the oncogenic transformation activity of Ras is dependent on its farnesylation (31). Moreover, GFP proteins bearing Ras CAAX motifs are localized to endomembranes, suggesting that farnesylation of Ras plays a role in the targeting of Ras to appropriate cellular compartments (15). In addition, prenylation is required for protein-protein interactions in both yeast (60) and mammalian (25, 66, 67) cells.
By treating infected cells with the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor lovastatin, we first deduced that PRV Us2 protein is prenylated at its C-terminal CAAX motif. Typically, when X is a serine residue, as it is in PRV Us2, the protein is modified with a farnesyl group rather than a geranylgeranyl group. Thus, it is most likely that PRV Us2 protein is farnesylated. Our data showing strong reactivity of PRV Us2 protein with anti-farnesyl antibodies further support this notion. The role of this modification is not clear, but prenylated Us2 may play a role in signal transduction or protein-protein interactions at the plasma membrane. Interestingly, tubulin has been shown to bind prenylated peptides with high affinity, suggesting a possible role of farnesylation in protein trafficking via the cytoskeleton. Of the sequenced Us2 homologs, only the PRV and BHV-1.2 Us2 proteins contain C-terminal CAAX motifs. How other alphaherpesvirus Us2 homologs that lack CAAX motifs, such as the EHV-1 Us2 protein, localize to membranes is unclear at present (48).
The Us2 protein is a component of the tegument. Curiously, we found that the form of Us2 that is packaged into virions has the same relative mobility as the nonprenylated form of the protein. Furthermore, our data indicate that prenylation is not required for packaging of Us2 into virions. It may be that prenylation marks the Us2 protein for separate functions. Our finding that the Us2 protein in virions is not prenylated suggests that nonprenylated Us2 may have a unique function during initial stages of infection.
An interesting possibility is that the nonprenylated Us2 protein in the tegument helps associate the newly uncoated tegument-capsid complex with microtubules after virion uncoating. This supposition is based on our observation that, in the presence of lovastatin, Us2 protein associated with microtubules. Previous reports have shown that herpesvirus capsids associate with and traffic via microtubules (64, 65) and that microtubules enhance HSV-1 infection (44). Sodeik and others have reported that, when capsids were associated with microtubules, there was typically a distance of ca. 50 nm between the capsid and the microtubule. These researchers suggested that these data indicated the presence of some unspecified tethering components. They also report that although much of the tegument layer is removed from the capsid upon entry at the plasma membrane, some tegument proteins, perhaps Us2, remain associated with the migrating capsids (65). More work is required to determine whether this speculation has merit. The delayed penetration phenotypes of some Us2 mutants provide some credence to the idea that Us2 protein facilitates movement of capsids to the nucleus. However, given the fact that Us2 protein is not required for replication in cultured cells and has modest to no effects on virulence of wild-type strains, it is likely that other viral proteins compensate for the loss of Us2 function. These mechanisms are likely to be employed by varicella-zoster virus, which has no counterpart to Us2 encoded in its genome.
All alphaherpesvirus Us2 homologs sequenced to date share three highly conserved regions termed CR1, CR2, and CR3 (Fig. 4). The precise functions of these motifs are not clear; however, screening of a yeast two-hybrid HeLa cell library using the HSV-2 Us2 protein as bait identified cytokeratin 18 as an interacting protein (23). A more detailed analysis indicated that HSV-2 Us2 protein sequences that comprise CR2 were sufficient for the cytokeratin 18-Us2 protein interaction. Cytokeratin 18 is an intermediate filament protein found primarily in epithelial cells that line internal body cavities—the gastrointestinal tract, liver, and pancreas in particular. Based on the tissue distribution of cytokeratin 18, it is not clear what the relevance of the Us2 protein interaction is in the context of natural HSV-2 infections. It may be that the HSV-2 Us2 CR2 motif is capable of interacting with other intermediate filament components. Nonetheless, it is intriguing that we also observed an interaction of Us2 protein with a cytoskeletal component.
Lastly, we investigated the effects of lovastatin on Us2 protein localization in uninfected cells. Transient transfections in the presence of lovastatin with a plasmid that expresses the PRV Us2 protein produced the identical mislocalization of Us2 that was observed in infected cells. We conclude that the punctate regions did not contain any viral proteins other than Us2. To demonstrate that the CAAX motif of Us2 is the mechanism by which the protein is associated with the plasma membrane, we replaced the cysteine residue with a glycine and therefore blocked prenylation. In transfected cells, this altered protein is not associated with membranes, a finding consistent with our proposal that the CAAX motif is required for prenylation, and this modification is sufficient to localize the protein to membranes.
To our knowledge, Us2 is the first alphaherpesvirus protein shown to be prenylated. Prenylation of cellular proteins is known to be essential for localization and function. Our observations indicate that prenylation not only affects Us2 localization but also distinguishes the infected cell protein from the virion protein. However, we have only begun to characterize the structure and enigmatic functions of the PRV Us2 protein.
Acknowledgments
We thank Renée Finnen for helpful comments on the manuscript, Greg Smith of Northwestern University for kindly providing strain PRV-GS443, Steve Fadul (UCHSC light microscopy facility) for help with confocal microscopy, and Ken Teter for recommending the anti-farnesyl antiserum.
This work was supported in part by research grant number 5-FY00-631 from the March of Dimes Birth Defects Foundation and NIH grants AI48626 to B.W.B. and NS33506 to L.W.E. M.G.L. was supported in part by NIH training grant AI52066.
REFERENCES
- 1.Alberts, A. W., J. Chen, G. Kuron, V. Hunt, J. Huff, C. Hoffman, J. Rothrock, M. Lopez, H. Joshua, E. Harris, A. Patchett, R. Monaghan, S. Currie, E. Stapley, G. Albers-Schonberg, O. Hensens, J. Hirshfield, K. Hoogsteen, J. Liesch, and J. Springer. 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 77:3957-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic, N., B. Klupp, A. Brack, T. C. Mettenleiter, G. Ugolini, and A. Flamand. 1996. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology 219:279-284. [DOI] [PubMed] [Google Scholar]
- 3.Banfield, B. W., G. S. Yap, A. C. Knapp, and L. W. Enquist. 1998. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J. Virol. 72:4580-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron, R., E. Fourcade, I. Lajoie-Mazenc, C. Allal, B. Couderc, R. Barbaras, G. Favre, J. C. Faye, and A. Pradines. 2000. RhoB prenylation is driven by the three carboxyl-terminal amino acids of the protein: evidenced in vivo by an anti-farnesyl cysteine antibody. Proc. Natl. Acad. Sci. USA 97:11626-11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck, L. A., T. J. Hosick, and M. Sinensky. 1990. Isoprenylation is required for the processing of the lamin A precursor. J. Cell Biol. 110:1489-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breeden, C. A., R. R. Yalamanchili, C. F. Colle, and D. J. O'Callaghan. 1992. Identification and transcriptional mapping of genes encoded at the IR/Us junction of equine herpesvirus type 1. Virology 191:649-660. [DOI] [PubMed] [Google Scholar]
- 7.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 72:4560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brideau, A. D., J. P. Card, and L. W. Enquist. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 74:834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, M. S., and J. L. Goldstein. 1980. Multivalent feedback regulation of HMG-CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J. Lipid Res. 21:505-517. [PubMed] [Google Scholar]
- 10.Card, J. P., P. Levitt, and L. W. Enquist. 1998. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J. Virol. 72:4434-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Card, J. P., L. Rinaman, J. S. Schwaber, R. R. Miselis, M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1990. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J. Neurosci. 10:1974-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 66:3032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957-969. [DOI] [PubMed] [Google Scholar]
- 14.Casey, P. J., and M. C. Seabra. 1996. Protein prenyltransferases. J. Biol. Chem. 271:5289-5292. [DOI] [PubMed] [Google Scholar]
- 15.Choy, E., V. K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98:69-80. [DOI] [PubMed] [Google Scholar]
- 16.Clarke, S. 1992. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61:355-386. [DOI] [PubMed] [Google Scholar]
- 17.Colle, C. F., III, and D. J. O'Callaghan. 1995. Transcriptional analyses of the unique short segment of EHV-1 strain Kentucky A. Virus Genes 9:257-268. [DOI] [PubMed] [Google Scholar]
- 18.Cox, A. D., and C. J. Der. 1992. The ras/cholesterol connection: implications for ras oncogenicity. Crit. Rev. Oncog. 3:365-400. [PubMed] [Google Scholar]
- 19.del Rio, T., H. C. Werner, and L. W. Enquist. 2002. The pseudorabies virus VP22 homologue (UL49) is dispensable for virus growth in vitro and has no effect on virulence and neuronal spread in rodents. J. Virol. 76:774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelb, M. H., J. D. Scholten, and J. S. Sebolt-Leopold. 1998. Protein prenylation: from discovery to prospects for cancer treatment. Curr. Opin. Chem. Biol. 2:40-48. [DOI] [PubMed] [Google Scholar]
- 21.Glomset, J. A., M. H. Gelb, and C. C. Farnsworth. 1990. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem. Sci. 15:139-142. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, J. L., and M. S. Brown. 1990. Regulation of the mevalonate pathway. Nature 343:425-430. [DOI] [PubMed] [Google Scholar]
- 23.Goshima, F., D. Watanabe, H. Suzuki, H. Takakuwa, H. Yamada, and Y. Nishiyama. 2001. The US2 gene product of herpes simplex virus type 2 interacts with cytokeratin 18. Arch. Virol. 146:2201-2209. [DOI] [PubMed] [Google Scholar]
- 24.Haanes, E. J., and C. C. Tomlinson. 1998. Genomic organization of the canine herpesvirus US region. Virus Res. 53:151-162. [DOI] [PubMed] [Google Scholar]
- 25.Haklai, R., M. G. Weisz, G. Elad, A. Paz, D. Marciano, Y. Egozi, G. Ben-Baruch, and Y. Kloog. 1998. Dislodgment and accelerated degradation of Ras. Biochemistry 37:1306-1314. [DOI] [PubMed] [Google Scholar]
- 26.Inagaki-Ohara, K., T. Iwasaki, D. Watanabe, T. Kurata, and Y. Nishiyama. 2001. Effect of the deletion of US2 and US3 from herpes simplex virus type 2 on immune responses in the murine vagina following intravaginal infection. Vaccine 20:98-104. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs, L., H. J. Rziha, T. G. Kimman, A. L. Gielkens, and J. T. Van Oirschot. 1993. Deleting valine-125 and cysteine-126 in glycoprotein gI of pseudorabies virus strain NIA-3 decreases plaque size and reduces virulence in mice. Arch. Virol. 131:251-264. [DOI] [PubMed] [Google Scholar]
- 28.Jang, H. K., M. Ono, T. J. Kim, Y. Izumiya, A. M. Damiani, T. Matsumura, M. Niikura, C. Kai, and T. Mikami. 1998. The genetic organization and transcriptional analysis of the short unique region in the genome of nononcogenic Marek's disease virus serotype 2. Virus Res. 58:137-147. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, Y. M., H. Yamada, F. Goshima, T. Daikoku, S. Oshima, K. Wada, and Y. Nishiyama. 1998. Characterization of the herpes simplex virus type 2 (HSV-2) US2 gene product and a US2-deficient HSV-2 mutant. J. Gen. Virol. 79:2777-2784. [DOI] [PubMed] [Google Scholar]
- 30.Kaelin, K., S. Dezelee, M. J. Masse, F. Bras, and A. Flamand. 2000. The UL25 protein of pseudorabies virus associates with capsids and localizes to the nucleus and to microtubules. J. Virol. 74:474-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, K., A. D. Cox, M. M. Hisaka, S. M. Graham, J. E. Buss, and C. J. Der. 1992. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc. Natl. Acad. Sci. USA 89:6403-6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimman, T. G., N. de Wind, N. Oei-Lie, J. M. Pol, A. J. Berns, and A. L. Gielkens. 1992. Contribution of single genes within the unique short region of Aujeszky's disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J. Gen. Virol. 73:243-251. [DOI] [PubMed] [Google Scholar]
- 34.Kitten, G. T., and E. A. Nigg. 1991. The CaaX motif is required for isoprenylation, carboxyl methylation, and nuclear membrane association of lamin B2. J. Cell Biol. 113:13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knapp, A. C., and L. W. Enquist. 1997. Pseudorabies virus recombinants expressing functional virulence determinants gE and gI from bovine herpesvirus 1.1. J. Virol. 71:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kritas, S. K., H. J. Nauwynck, and M. B. Pensaert. 1995. Dissemination of wild-type and gC-, gE-, and gI-deleted mutants of Aujeszky's disease virus in the maxillary nerve and trigeminal ganglion of pigs after intranasal inoculation. J. Gen. Virol. 76:2063-2066. [DOI] [PubMed] [Google Scholar]
- 38.Leung-Tack, P., J. C. Audonnet, and M. Riviere. 1994. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain). Virology 199:409-421. [DOI] [PubMed] [Google Scholar]
- 39.Lin, H. P., S. C. Hsu, J. C. Wu, I. J. Sheen, B. S. Yan, and W. J. Syu. 1999. Localization of isoprenylated antigen of hepatitis delta virus by anti-farnesyl antibodies. J. Gen. Virol. 80:91-96. [DOI] [PubMed] [Google Scholar]
- 40.Lomniczi, B., M. L. Blankenship, and T. Ben-Porat. 1984. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J. Virol. 49:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomniczi, B., S. Watanabe, T. Ben-Porat, and A. S. Kaplan. 1984. Genetic basis of the neurovirulence of pseudorabies virus. J. Virol. 52:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longnecker, R., and B. Roizman. 1987. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science 236:573-576. [DOI] [PubMed] [Google Scholar]
- 43.Lyman, M. G., G. L. Demmin, and B. W. Banfield. 2003. The attenuated pseudorabies virus strain Bartha fails to package the tegument proteins Us3 and VP22. J. Virol. 77:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mabit, H., M. Y. Nakano, U. Prank, B. Saam, K. Dohner, B. Sodeik, and U. F. Greber. 2002. Intact microtubules support adenovirus and herpes simplex virus infections. J. Virol. 76:9962-9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGeoch, D. J., A. Dolan, S. Donald, and D. H. Brauer. 1986. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 14:1727-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGeoch, D. J., H. W. Moss, D. McNab, and M. C. Frame. 1987. DNA sequence and genetic content of the HindIII l region in the short unique component of the herpes simplex virus type 2 genome: identification of the gene encoding glycoprotein G, and evolutionary comparisons. J. Gen. Virol. 68:19-38. [DOI] [PubMed] [Google Scholar]
- 47.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 48.Meindl, A., and N. Osterrieder. 1999. The equine herpesvirus 1 Us2 homolog encodes a nonessential membrane-associated virion component. J. Virol. 73:3430-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 50.Mettenleiter, T. C., B. Lomniczi, N. Sugg, C. Schreurs, and T. Ben-Porat. 1988. Host cell-specific growth advantage of pseudorabies virus with a deletion in the genome sequences encoding a structural glycoprotein. J. Virol. 62:12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mettenleiter, T. C., N. Lukacs, and H. J. Rziha. 1985. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J. Virol. 56:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mical, T. I., and M. J. Monteiro. 1998. The role of sequences unique to nuclear intermediate filaments in the targeting and assembly of human lamin B: evidence for lack of interaction of lamin B with its putative receptor. J. Cell Sci. 111:3471-3485. [DOI] [PubMed] [Google Scholar]
- 53.Petrovskis, E. A., J. G. Timmins, T. M. Gierman, and L. E. Post. 1986. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J. Virol. 60:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rando, R. R. 1996. Chemical biology of protein isoprenylation/methylation. Biochim. Biophys. Acta 1300:5-16. [DOI] [PubMed] [Google Scholar]
- 55.Robbins, A. K., D. J. Dorney, M. W. Wathen, M. E. Whealy, C. Gold, R. J. Watson, L. E. Holland, S. D. Weed, M. Levine, J. C. Glorioso, et al. 1987. The pseudorabies virus gII gene is closely related to the gB glycoprotein gene of herpes simplex virus. J. Virol. 61:2691-2701. [DOI] [PMC free article] [PubMed]
- 56.Roizman, B., and E. Sears. 1996. Herpes simplex viruses and their replication, p. 1043-1107. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 57.Rowinsky, E. K., J. J. Windle, and D. D. Von Hoff. 1999. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J. Clin. Oncol. 17:3631-3652. [DOI] [PubMed] [Google Scholar]
- 58.Ryan, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1987. Analysis of pseudorabies virus glycoprotein gIII localization and modification by using novel infectious viral mutants carrying unique EcoRI sites. J. Virol. 61:2962-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rziha, H. J., T. C. Mettenleiter, V. Ohlinger, and G. Wittmann. 1986. Herpesvirus (pseudorabies virus) latency in swine: occurrence and physical state of viral DNA in neural tissues. Virology 155:600-613. [DOI] [PubMed] [Google Scholar]
- 60.Sapperstein, S., C. Berkower, and S. Michaelis. 1994. Nucleotide sequence of the yeast STE14 gene, which encodes farnesylcysteine carboxyl methyltransferase, and demonstration of its essential role in a-factor export. Mol. Cell. Biol. 14:1438-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt, R. A., C. J. Schneider, and J. A. Glomset. 1984. Evidence for posttranslational incorporation of a product of mevalonic acid into Swiss 3T3 cell proteins. J. Biol. Chem. 259:10175-10180. [PubMed] [Google Scholar]
- 62.Shiba, C., T. Daikoku, F. Goshima, H. Takakuwa, Y. Yamauchi, O. Koiwai, and Y. Nishiyama. 2000. The UL34 gene product of herpes simplex virus type 2 is a tail-anchored type II membrane protein that is significant for virus envelopment. J. Gen. Virol. 81:2397-2405. [DOI] [PubMed]
- 63.Smith, G. A., and L. W. Enquist. 2002. Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 18:135-161. [DOI] [PubMed] [Google Scholar]
- 64.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki, N., and T. Kataoka. 1993. The effect of posttranslational modifications on the interaction of Ras2 with adenylyl cyclase. Science 259:683-686. [DOI] [PubMed] [Google Scholar]
- 67.Thissen, J. A., J. M. Gross, K. Subramanian, T. Meyer, and P. J. Casey. 1997. Prenylation-dependent association of Ki-Ras with microtubules: evidence for a role in subcellular trafficking. J. Biol. Chem. 272:30362-30370. [DOI] [PubMed] [Google Scholar]
- 68.Tomishima, M. J., and L. W. Enquist. 2001. A conserved α-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Zijl, M., H. van der Gulden, N. de Wind, A. Gielkens, and A. Berns. 1990. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J. Gen. Virol. 71:1747-1755. [DOI] [PubMed] [Google Scholar]
- 70.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 71.Weber, P. C., M. Levine, and J. C. Glorioso. 1987. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science 236:576-579. [DOI] [PubMed] [Google Scholar]
- 72.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 67:3786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wild, M. A., S. Cook, and M. Cochran. 1996. A genomic map of infectious laryngotracheitis virus and the sequence and organization of genes present in the unique short and flanking regions. Virus Genes 12:107-116. [DOI] [PubMed] [Google Scholar]
- 74.Ye, G. J., K. T. Vaughan, R. B. Vallee, and B. Roizman. 2000. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J. Virol. 74:1355-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zelnik, V., R. Darteil, J. C. Audonnet, G. D. Smith, M. Riviere, J. Pastorek, and L. J. Ross. 1993. The complete sequence and gene organization of the short unique region of herpesvirus of turkeys. J. Gen. Virol. 74:2151-2162. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, F. L., and P. J. Casey. 1996. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 65:241-269. [DOI] [PubMed] [Google Scholar]