Abstract
Receptor priming of low-pH-triggered virus entry has been described for an enveloped virus (15). Here we show with major group human rhinoviruses (HRV) and its intercellular adhesion molecule-1 receptor that nonenveloped viruses follow this novel cell entry principle. In vitro the receptor primed HRV for efficient uncoating at mild low pH (5.5 to 6.0). Agents preventing endosomal acidification reduced or blocked rhinovirus cell infection, while nocodazole had no effect on infection of any serotype tested. The entry inhibitory effect of lysosomotropic agents was overcome by exposing cell-internalized HRV to mild low pH (5.5 to 6.0). We therefore conclude that receptor priming of major group HRV must occur in vivo as well. Cooperation of a cellular receptor and low pH in virus uncoating will polarize the exit of the genome to the receptor-bound, membrane-proximal region of the virus particle during acidification of endosomes. This process must be required for efficient penetration of the cellular membrane by viruses.
Entry of viruses into host cells requires binding to one or several cell surface receptors and subsequent penetration of the cellular membrane. The penetration or entry process in enveloped viruses occurs by fusion of virus and cell membranes (21), while in nonenveloped viruses the process requires local disruption of the bilayer (3). For most viruses, cell entry is mediated by either receptor (pH independent) or low pH. Recently, however, it was shown that entry of the retrovirus avian leukosis virus (ALV) required both receptor and low pH, providing a novel principle for entry of enveloped viruses (15). Binding of ALV to its cellular receptor converts the viral envelope protein into a metastable form sensitive to low pH so that subsequent virus internalization and endosomal acidification triggers membrane fusion and release of the viral capsid into the cell cytoplasm.
Cell entry and receptor recognition have been extensively studied in picornaviruses, a large family of nonenveloped viruses responsible of several human and animal diseases (19). Picornaviruses are constituted by an icosahedral protein capsid built by 60 protomers assembled in 12 pentamers, with a single-stranded RNA genome closely packed inside. Cell entry requires uncoating or exit of the RNA from the capsid, which presumably moves to the cell cytoplasm through a membrane pore generated by hydrophobic capsid polypeptides (3). Receptor-mediated uncoating of picornaviruses at neutral pH was first described for poliovirus (12). Poliovirus does not require a low-pH step or endocytosis for cell entry (8, 16), so multimeric binding of the virus to the receptor must trigger the molecular events leading to virus penetration (18). The poliovirus entry pathway differs from that described for human rhinovirus serotype 2 (HRV2), a member of the minor group of HRV, which bind to receptors belonging to the low-density lipoprotein (LDL) family (10). HRV2 requires both endocytosis and a low-pH step for efficient uncoating and cell entry (1, 2). The LDL receptor is used for attachment of minor group HRV to host cells, but it does not appear to mediate HRV2 uncoating, in contrast to the receptor for the major group of HRV, intercellular adhesion molecule-1 (ICAM-1) (11).
ICAM-1 binds to a depressive surface or canyon around the icosahedral fivefold axis of the HRV capsid (13). There are 60 receptor binding sites in the virus particle, although receptor molecules on the cell surface must interact with one or two neighboring pentamers. ICAM-1 mediates uncoating at neutral pH of some, but not all, of the major group of HRV (11, 23). These differences among HRV serotypes are not related to receptor binding affinity but to variations in receptor binding thermodynamics and particle stability (11, 23). The HRV3 and -14 serotypes are labile to receptor binding, presumably because they lack a fatty acid-like molecule (pocket factor) present in a hydrophobic cavity of the viral capsid protein 1 (VP1) in HRV16 (24). Receptor-mediated uncoating of HRV16 at neutral pH and physiological temperatures is highly inefficient, and the virus particles remain intact even after hours of receptor binding in solution (11). These results raised the issue of the factors involved in entry of HRV16 and related major group rhinoviruses.
Here we have investigated the role of low pH and receptor in uncoating and cell entry of major group serotypes having (HRV16) and lacking (HRV3 and HRV14) pocket factors. In solution and in HeLa cells the ICAM-1 receptor and mild low pH cooperate for rhinovirus uncoating and cell infection. Our report shows that receptor priming of low-pH-mediated entry occurs in nonenveloped viruses and discusses its implications for virus entry.
MATERIALS AND METHODS
Virus and receptor protein preparation.
HRV3 and HRV16 stocks came from the American Type Culture Collection, HRV14 came from the R. Rueckert laboratory, and HRV2 came from D. Blaas. Viruses were propagated in HeLa-H1 cells at 35°C, and stocks were prepared as described elsewhere (7). [35S]-Met-labeled viruses (10−6 cpm/virion) were kept at either 4 or −70°C for short- or long-term storage, respectively. Some variations in virion stability with time and among preparations were observed (see Fig. 1, 2, and 3). A soluble fragment of the ICAM-1 receptor having the N-terminal 190 residues and with similar virus binding activity compared to that of variants with the complete extracellular region was used (5).
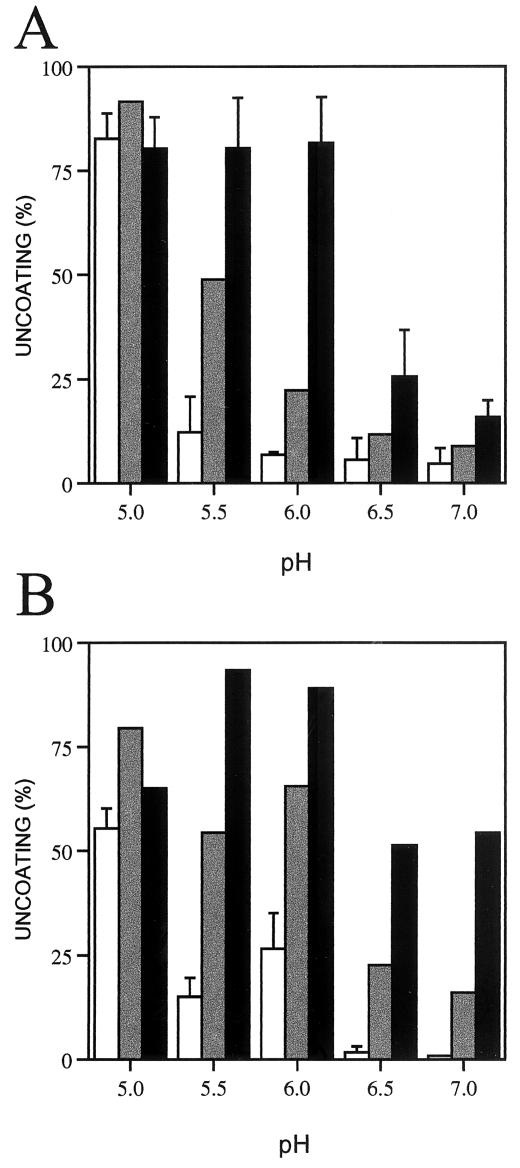
FIG. 1.
Uncoating of HRV-receptor complexes with the pH. Complexes of HRV16 (A) and HRV3 (B) with bound receptor were first prepared by incubations with 1 (grey bars) and 5 (black bars) μM ICAM-1 at 37°C and pH 8.0 for 30 (A) and 15 min (B) (see Materials and Methods). Viruses were also incubated in the absence (white bars) of the ICAM-1 receptor. Complexes or virions were then diluted five times with ice-cold buffers of the indicated pH and were incubated at 37°C for additional 30 min. The samples were then analyzed by sucrose gradient sedimentation and uncoating determined as described in Materials and Methods. Averages and standard deviations from four and three experiments done with three different virus preparations are presented for HRV16 in the absence of ICAM-1 and at high ICAM-1 concentration, respectively.
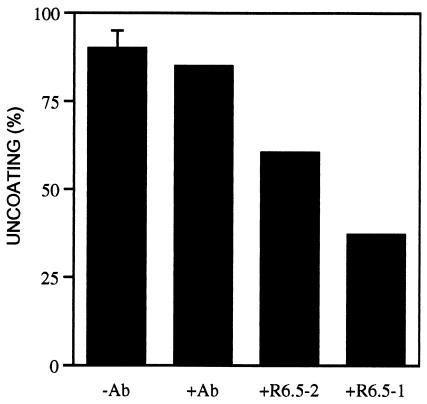
FIG. 2.
Inhibition of low-pH-mediated uncoating of HRV16-receptor complexes by an anti-ICAM-1 monoclonal antibody. Virus-receptor complexes were prepared at pH 8.0 as described in the legend to Fig. 1 in two independent experiments with HRV16 and 1 or 2 μM concentration of ICAM-1. The virus-receptor binding inhibitory antibody R6.5 (7 μM) (5) was added to the complexes prepared with 1 (+R6.5-1) or 2 μM ICAM-1 (+R6.5-2). After 15 min of incubation with either the R6.5 antibody, an anti-measles virus hemagglutinin antibody (+Ab), or phosphate-buffered saline (−Ab), the complexes were diluted in MES buffer (pH 6.0) and incubated for an additional 15 min at 37°C prior to sedimentation analysis. Uncoating was determined as described in the legend to Fig. 1. No uncoating was monitored in the absence of receptor (data not shown). A single virus preparation was used, which appeared more labile upon receptor binding that those viruses used in Fig. 1 and 3.
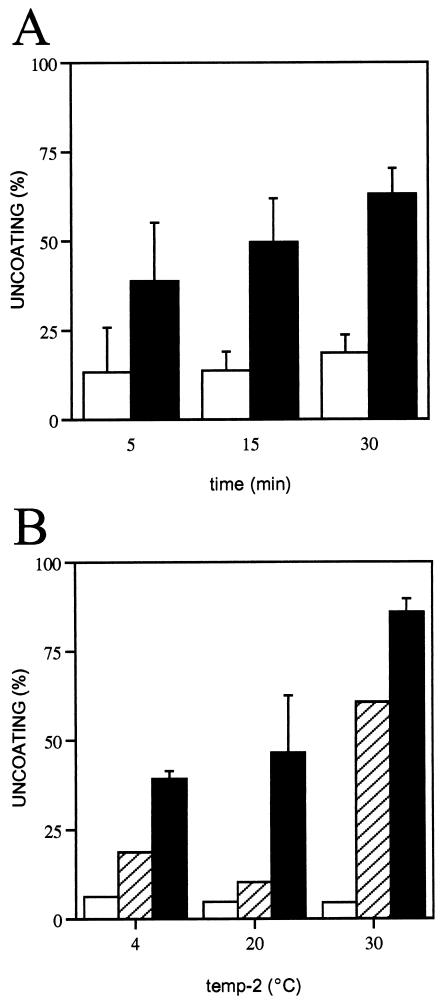
FIG. 3.
Low-pH-mediated uncoating of HRV16-receptor complexes with time and temperature. (A) HRV16 incubated in the absence (open bars) or presence (closed bars) of 5 μM ICAM-1, as described in the legend to Fig. 1, was diluted five times with MES buffer (pH 6.0) and was incubated for the indicated times at 37°C. Ice-cold samples were subjected to sucrose gradient sedimentation, and uncoating was determined as described in Materials and Methods. (B) HRV16 was first incubated with ICAM-1 (5 μM) for 30 min at 20 (open bars), 30 (hatched bars), and 37°C (closed bars). Samples were then diluted five times with MES buffer (pH 6.0) and were incubated for an additional 30 min at the indicated temperature (temp-2). Samples were processed as described for panel A, and uncoating was determined.
Analysis of virus conformation and determination of uncoating.
HRV uncoating decreases the amount of infectious virus particles with sedimentation around 150S and results in the appearance of empty particles sedimenting with a velocity of 80S (7). For analysis of virus conformation and uncoating, radiolabeled viruses were run through linear 5 to 20% sucrose gradients in phosphate-buffered saline. Virions were first treated with soluble receptor in 10 mM HEPES, pH 8.0, containing 150 mM NaCl and 5% fetal calf serum. This pH was optimal for ICAM-1 binding to HRV (6). Samples (10 μl) were diluted five times with ice-cold buffers having pH values of 7.0 (25 mM HEPES and 150 mM NaCl), 6.0 to 6.5 (25 mM morpholineethanesulfonic acid [MES] and 150 mM NaCl), or 5.0 to 5.5 (25 mM sodium acetate and 150 mM NaCl) and were processed as indicated. The pH of the dilutions was determined to be 0.1 greater than that of the dilution buffer. Treated virus samples were chilled for 5 min on ice, loaded onto 5-ml sucrose gradients, and centrifuged at 40,000 rpm and 4°C for 1 h in an SW50.1 rotor. About 20 fractions were collected from the bottom and were scintillation counted. Uncoating was determined from the decrease in the counts (cpm) of the infectious virus fractions in the treated viruses by using the following equation: (%cpm untreated − %cpm treated/%cpm untreated) × 100 (7).
Analysis of HeLa cell infection by rhinoviruses.
Subconfluent HeLa-H1 cell monolayers were first prepared in 96-well plates. Minimal essential medium (MEM; GIBCO) supplemented with glutamine and 2% fetal calf serum (infection medium) having or lacking the lysosomotropic agent bafilomycin A1 (Sigma), nocodazole (Sigma), or ammonium chloride was added to the wells and was incubated at 35°C for 30 min. Subsequently, an additional 50 μl of infection medium having HRV2, -3, -14, or -16 was added to the cell monolayers (infection time = 0). Virus inocula were removed after 1 h of incubation at 35°C, cells were washed, and infection medium (100 μl) lacking methionine and having or lacking the lysosomotropic agents was added. In the experiments presented in Fig. 7, ice-cold virus inocula were added to the cell monolayer and were maintained on ice for 1 h for virus binding to cells without internalization. After removal of inocula, cells were washed, medium lacking methionine was added, and plates with infected cells were transferred to 35°C (infection time = 0). For protein labeling, cell supernatants were replaced 6 h postinfection with methionine-free medium having 50 μCi of [35S]Met/ml. Infected cell monolayers were treated with electrophoresis sample buffer (50 μl) 3 and 16 h postlabeling. Cell lysates clarified by centrifugation and cell supernatants collected 16 h postlabeling were run in sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Gels were subjected to fluorography with 1 M sodium salicylate and were dried. Radioactivity counts corresponding to virus proteins were determined by using a Fujifilm phosphorimager and software and were used to calculate the infectivity ratio in the presence and absence of lysosomotropic agents. Count ratios between areas containing virus (viral capsid protein VP3) and host cell proteins (Rvirus) were first calculated from each electrophoresis track for either virus-infected cell lysates or supernatants. The same ratios for equivalent areas were determined for the track corresponding to uninfected cells (Rcell) and were subtracted from Rvirus. The infectivity ratio of each HRV serotype in the presence and absence of lysosomotropic agent was calculated with the following formula: (Rvirus + agent − Rcell + agent/Rvirus − agent − Rcell − agent) × 100.
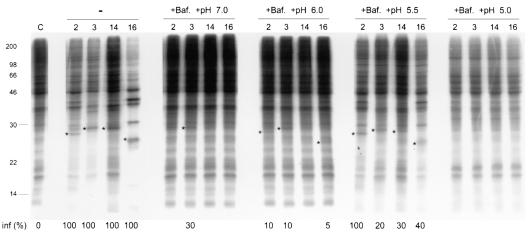
FIG. 7.
Mild low pH triggers entry of HRV into HeLa cells. SDS-PAGE of radiolabeled HeLa cell lysates collected 9 h postinfection with HRV2, -3, -14, and -16 or uninfected cells (C) in the absence (−) or presence of bafilomycin (+Baf.) and with additional cell washing. Cells were washed for 2 h postinfection and for 30 min with buffers having bafilomycin and pHs of 7.0, 6.0, 6.5, and 5.0 (see the legend to Fig. 1). Cells were infected at a virus multiplicity of 200 PFU/cell, and infection was synchronized as described in Materials and Methods. Asterisks indicate tracks where a significant amount of viral capsid protein VP3 and other viral proteins appeared. Infectivity ratios (inf %) determined as described in Table 1 have been included under tracks with expression of viral proteins.
RESULTS
Uncoating of HRV-receptor complexes with pH.
During cell entry, viruses first attach to receptors on the cell surface at neutral pH and are subsequently internalized and exposed to low pH in endosomal compartments. To mimic this process, HRV16 and HRV3 with and without their ICAM-1 receptor bound at physiological temperature and pH 8.0 were moved to solutions with pH ranging from 5.0 to 7.0 (Fig. 1). Uncoating or release of the viral genome from HRV-receptor complexes was monitored by formation of empty virus particles (see Materials and Methods).
The HRV16 serotype required exposure to pH 5.0 for uncoating in the absence of bound receptor (Fig. 1A). However, the RNA released from HRV16-receptor complexes at higher pH, 5.5 to 6.0 (Fig. 1A), showing that the ICAM-1 receptor primed the HRV16 virions for uncoating at mild low pH. Uncoating was very inefficient at neutral pH, as reported earlier for this serotype (11, 23), so that cooperation between receptor and mild low pH was quite evident. Higher receptor concentration, and therefore higher binding multivalency, gave similar uncoating at pH 5.5 or 6.0, while lower receptor concentration required lower pH to cause significant uncoating. Based on the calculated affinity of the ICAM-1 receptor for HRV16 at 37°C (165 nM) (23), we determined that 70% (3.4 sites/pentamer) and 96% (all 5 sites/pentamer) of the receptor binding sites in HRV16 were occupied at ICAM-1 concentrations of 1 and 5 μM, respectively. As reported for receptor-mediated uncoating of HRV3 at neutral pH in solution (7), binding to at least 3 sites/pentamer was required for low-pH-triggered uncoating of HRV-receptor complexes.
Mild low pH (5.5 to 6.0) was also optimum for uncoating of HRV3-receptor complexes at physiological temperatures (Fig. 1B), suggesting some receptor priming effect for this serotype as well. However, an additive rather than cooperative effect of receptor and pH was observed with HRV3. The lowest receptor concentration (1 μM) gave significantly higher uncoating at mild low pH than at pH 7.0. The receptor and low-pH additive effect in uncoating could be of significance at the cell surface, particularly for HRV3 particles binding to ICAM-1 with subsaturating multivalency. The receptor appears to have no effect in the uncoating of HRV16 at pH 5.0 and has a marginal effect with HRV3 (Fig. 1), essentially as reported for HRV14 (11). These differences could arise from the higher stability of HRV3 than HRV16 at pH 5.0 (Fig. 1). The low receptor priming effect at pH 5.0 suggests a fast dissociation of the receptor from the virus at this pH (6).
Release of the bound receptor from HRV16 by competition with an anti-ICAM-1 monoclonal antibody prior to acidification decreased uncoating (Fig. 2). Higher inhibition correlated with a higher antibody/receptor ratio. These results show that low-pH-mediated uncoating of virus-receptor complexes must precede release of the receptor by the decrease of the pH (6). Moreover, receptor priming of HRV at neutral pH appears reversible and must not trigger a significant release of the pocket factor molecule present in HRV16.
Low-pH-mediated uncoating of HRV16-receptor complexes with time and temperature.
The cooperative effect of mild low pH in uncoating of HRV16-receptor complexes was assessed with time. A significant amount of uncoating (60% of that observed after 30 min) occurred within the initial 5-min incubation of the complexes at pH 6.0 (Fig. 3A), showing that low pH triggers a fast exit of the RNA genome from the virus-receptor complexes.
Receptor-mediated HRV uncoating occurs at temperatures above 25°C (7, 11). Similarly, the cooperative effect of pH and receptor was temperature dependent (Fig. 3B). No uncoating was monitored with HRV16 in experiments where initial receptor binding was done at 20°C, even after subsequent exposure of the acidified complexes at higher temperatures. The receptor binding temperature appears important for RNA exit at mild low pH, although the most efficient uncoating reaction was observed when both binding and acidification reactions were performed at temperatures above 25°C.
HRV infection at neutral endosomal environments.
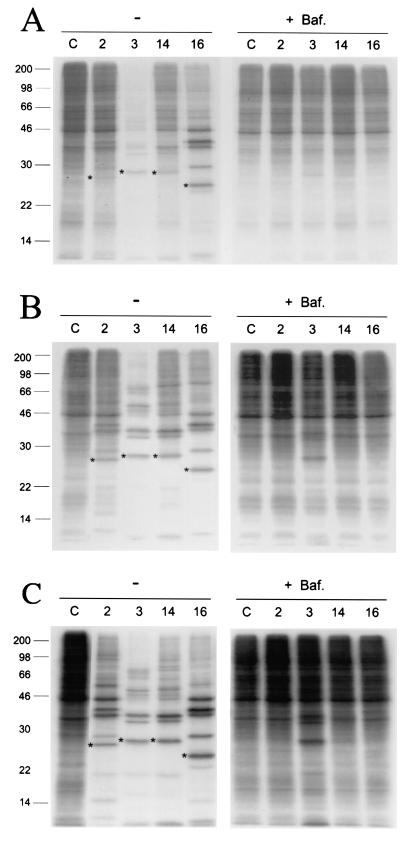
The lysosomotropic agent bafilomycin A1 (bafilomycin) prevents endosomal acidification by inhibition of the vacuolar H+-ATPase (4). In the presence of this agent at a concentration of 200 nM the average pH of the endosomes in HeLa cells has been determined to be neutral (2). Therefore, we used HeLa cells treated with bafilomycin to investigate how changes in the endosomal pH affect infection of several major group HRV serotypes (HRV3, -14 and -16). HRV2, a member of the minor group rhinoviruses extensively studied, was included as control (2). Infectivity was monitored by the appearance of viral proteins (Fig. 4 and Table 1). Bafilomycin blocked HRV2 infection of HeLa cells at low and high virus titers (Fig. 4) and had no effect on poliovirus infection (data not shown), as previously documented for those viruses (2, 16). Infectivity of the major group serotype HRV16 and HRV14 appeared affected by the agent as equally as HRV2 (Fig. 4). By contrast, HRV3 infectivity was partially reduced, and around 25% of the particles were infective at neutral pH (Table 1). Similar effects of bafilomycin in the infectivity of the HRV serotypes were observed by 9 and 22 h postinfection, after viral-mediated cell lysis (Fig. 4B and C). Experiments with bafilomycin showed that endosomal acidification was essential for efficient HRV entry, although a population of HRV3 particles could enter at neutral pH.
FIG. 4.
Effect of bafilomycin in HRV infection. (A and B) SDS-PAGE of radiolabeled HeLa cell lysates collected 9 h postinfection with HRV2, -3, -14, and -16 in the absence (−) or presence of 200 nM bafilomycin (+Baf.). (C) Cell lysates harvested 22 h postinfection. Lines corresponding to lysates from uninfected cells are labeled C. HeLa cells were infected with virus multiplicities of 10 (A) and 100 PFU/cell (B and C) as described in Materials and Methods. Asterisks indicate the viral capsid protein VP3, quantified for determination of virus infectivity (Table 1). Mobility and mass (kDa) of molecular size markers are indicated on the left side.
TABLE 1.
HRV infectivity in the presence of lysosomotropic agentsa
| Agent | MOI (PFU/cell) | Time (h) | Infectivity ratio (%) for serotype:
|
|||
|---|---|---|---|---|---|---|
| HRV2 | HRV3 | HRV14 | HRV16 | |||
| Bafilomycin A1 | 10 | 9 | NS | 10 | NS | NS |
| 100 | 9 | NS | 17 | NS | NS | |
| 100 | 22 | NS | 27 | NS | NS | |
| Nocodazole | 10 | 9 | 110 | 130 | 120 | 110 |
| 100 | 9 | 150 | 100 | 140 | 85 | |
| NH4Cl (5 mM) | 100 | 9 | 41 | 68 | 57 | 70 |
| NH4Cl (15 mM) | 100 | 9 | 8 | 39 | 25 | 13 |
| NH4Cl (25 mM) | 100 | 9 | NS | 13 | 10 | NS |
| NH4Cl (50 mM) | 100 | 9 | NS | NS | NS | NS |
HRV infectivity ratios were determined from the amount of viral protein (VP3) expressed in infected HeLa cells in the presence and absence of the lysosomotropic agents (see Materials and Methods and Fig. 4 to 6). Viral protein expression was determined from SDS-PAGE of cell lysates collected at the indicated time postinfection. Averages of two experiments are presented for bafilomycin (200 nM) and nocodazole (20 μM). Values under 5% were considered not significant (NS). Multiplicities of infection (MOI) are indicated.
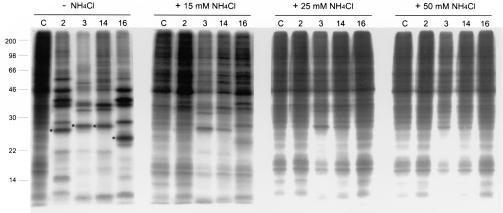
Weak bases, such as ammonium chloride, raise the endosomal pH and have been commonly used in the study of low-pH requirements for entry of viruses (9, 15, 17). We used increasing concentrations of ammonium chloride to investigate further the sensitivity of HRV serotype entry to a gradual increase in the endosomal pH (Fig. 5). High concentration of the base (70 mM) blocked infection of all HRV tested when added to HeLa cells prior to addition of the viruses, was partially inhibitory when added 1 h postinfection, and did not have any effect 5 h postinfection (data not shown). Thus, ammonium chloride appears to specifically affect HRV entry events, as shown with other viruses (15). Increasing concentrations of the agent decreased infectivity of all serotypes (Fig. 5 and Table 1). HRV2 appeared to be the serotype most sensitive to changes in the endosomal pH by ammonium chloride, followed by HRV16, -14, and -3. The partial inhibition of the HRV infectivity observed under certain conditions suggests differences in receptor binding multivalency or stability among viral particles. As shown by solution experiments (Fig. 1), viruses binding to the receptor with high multivalency could still be infective under a slight increase in the endosomal pH.
FIG. 5.
Sensitivity of HRV cell infection to increasing concentrations of ammonium chloride. SDS-PAGE of radiolabeled HeLa cell lysates collected 9 h postinfection with HRV2, -3, -14, and -16 or uninfected cells (C) in the absence (−NH4Cl) or presence (+) of the indicated concentrations of ammonium chloride. HeLa cells were infected with a virus multiplicity of 100 PFU/cell as described in Materials and Methods. Asterisks indicate the viral capsid protein VP3, quantified for determination of virus infectivity (Table 1). Mobility and mass (kDa) of molecular size markers are indicated on the left side.
HRV infection at mild low-pH endosomal environments.
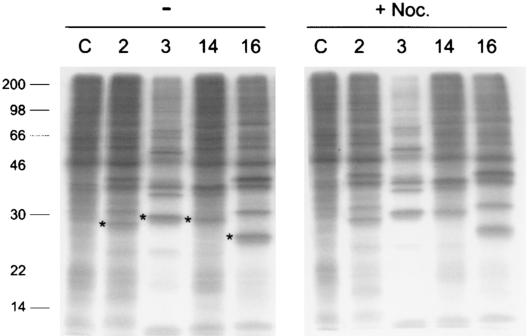
The drug nocodazole inhibits microtubule-associated transport of early to late endosomes and lysosomes, restricting the decrease of the intravesicular pH. The average pHs of endocytic compartments in HeLa cells treated with nocodazole have been determined to be around 6.0, although the pH of some vesicles could go down to 5.6 (2). Under conditions similar to those used by Bayer and coworkers (2) the infectivity of the HRV serotypes tested were not affected (Fig. 6 and Table 1), suggesting that exposure to mild low pH in vivo was sufficient for uncoating and efficient HRV entry.
FIG. 6.
Effect of nocodazole in HRV infection. SDS-PAGE of radiolabeled HeLa cell lysates collected 9 h postinfection with HRV2, -3, -14, and -16 or uninfected cells (C) in the absence (−) or presence of 20 μM nocodazole (+Noc.). HeLa cells were infected with a virus multiplicity of 10 PFU/cell. Asterisks indicate the viral capsid protein VP3, quantified for determination of virus infectivity (Table 1). Mobility and mass (kDa) of molecular size markers are indicated on the left side.
Mild low pH triggers entry of HRV into HeLa cells.
To investigate further the relevance of mild low pH (5.5 to 6.0) in cell entry of HRV serotypes, infected HeLa cells in the presence of bafilomycin were exposed to different pHs (Fig. 7), as was done for virus-receptor complexes in solution (Fig. 1). Similar methods were used for modulation of endosomal pH in cells and with purified intracellular vesicles (15, 17). Viruses first bound to the cells at low temperatures, and they were subsequently internalized at high temperatures in the presence of bafilomycin. Infected cell samples washed with buffer pH 7.0 gave results similar to those of unwashed samples (data not shown), essentially the same as those presented in Fig. 4 (+Baf.), with only HRV3 being partially infective (Fig. 7). Washing with buffer pH 5.5 significantly overcame the cell entry blocking effect of bafilomycin for all HRV serotypes tested (Fig. 7). Several viral proteins were visualized, and host protein shutoff related to viral infection was evident. Full recovery of the infectivity obtained without bafilomycin was only achieved for the minor group HRV2, while recovery of major group HRV infectivity was partial. Binding of HRV2 to the LDL receptor could be tighter than HRV binding to ICAM-1, preventing release of the virus from the receptor during the 2-h internalization step. Viral proteins appeared for some HRV serotypes even in infected cells treated with buffer pH 6.0 (Fig. 7), although no significant host protein shutoff was observed. Similar results were seen in an independent experiment with buffers pH 7.0, 6.0, and 5.5 (data not shown). Interestingly, no virus infection was observed in cells treated with buffer pH 5.0, conditions at which the receptor does not have any priming effect (Fig. 1). Some residual HRV3 infection was observed at this low pH in a separate experiment (data not shown), which correlates with the higher stability of this serotype at pH 5.0 and the slight priming effect of the receptor for uncoating at this pH (Fig. 1) that is not seen with HRV16. Overall, the results presented show that mild low pH (5.5 to 6.0) mediates uncoating of HRV-receptor complexes in solution and HRV entry into host cells.
DISCUSSION
In this report we show that the ICAM-1 receptor primes major group HRV serotypes for efficient uncoating by mild low pH (5.5 to 6.0) and productive entry into host cells. Receptor priming must provide a signal for a polarized, membrane-proximal HRV uncoating during virus internalization, which appears significant for efficient penetration of the viral genome into the cell cytoplasm.
The requirements of endosomal uptake and acidification presented here for entry of several HRV serotypes agree with previous reports showing both clathrin-mediated endocytosis and bafilomycin inhibition of HRV14 entry (8, 16, 20). The ICAM-1 receptor appears to play an active role in uncoating and entry of all major-group serotypes included in this study. However, there are differences in pH requirements for uncoating and cell entry among serotypes, suggesting that they must penetrate the membrane in different cellular compartments, between the cell surface (neutral pH) and endosomal carrier vesicles (with pH 5.5 to 6.0), which accumulate in HeLa cells treated with nocodazole (2). Differences in uncoating and entry requirements among serotypes must be related to differences in the stability of the virus particles and to differences in receptor binding multivalency, dependent on the receptor expression level at the cell surface.
The large differences observed between rates for dissociation of monomeric ICAM-1 receptor from HRV and HRV particles from cells suggested multimeric attachment of HRV viruses to their host cells (6). Multimeric binding of ICAM-1 to HRV in solution is critical for receptor-mediated uncoating (7, 11) and for receptor priming of low-pH-triggered uncoating (Fig. 1). Multimeric receptor binding on the cell surface must then be critical for efficient uncoating of HRV virus particles and subsequent genome penetration of the membrane. However, there must be heterogeneity in receptor binding multivalency among a virus population, as suggested by the partial inhibitory effect of HRV infection with some lysosomotropic agents (Table 1). HRV3 particles (25%) entering at neutral pH in the presence of bafilomycin could bind with a higher multivalency to ICAM-1 than those particles (75%) requiring a low-pH step for cell entry. Uncoating experiments with HRV16 (Fig. 1) showed a significantly higher receptor priming effect at pH 5.5 than at pH 6.0 at the low receptor concentration tested, while no differences were observed at saturating concentrations. The requirement of pH 5.5 for efficient major-group HRV entry (Fig. 7) suggests that most infective particles must bind with subsaturating multivalency to receptors on the cell surface, quite likely as those achieved in solution at 1 μM concentration of ICAM-1 (3 to 4 sites/pentamer). Virus particles having a lower receptor binding multivalency must not be primed for uncoating at mild low pH during endocytosis. Low pH (≤6.0) enhances the rate of dissociation of monomeric ICAM-1 receptor (6) so that particles bound to few receptor molecules could dissociate prior to uncoating upon endosomal acidification. The dissociation rate from the receptor will increase in late endosomes (pH 5.0). Uncoating of released particles will be distal from the membrane and will be unproductive, explaining why a large number of virus particles are required for having a single infectious unit in HRV and related picornaviruses. Infectious particles could then be those bound to multiple receptor molecules, primed for mild low pH uncoating proximal to the membrane prior to receptor dissociation. Membrane interaction of hydrophobic virus polypeptides (3) exposed by receptor binding (22) could slow down dissociation from the receptor.
Experiments with bafilomycin and ammonium chloride (Fig. 4 and 5 and Table 1) showed that HRV serotypes that are stable upon receptor binding at neutral pH, HRV16 and HRV2, are the most sensitive to lysosomotropic agents during cell entry, while labile serotypes (HRV3) are less sensitive. Indeed, about 25% of the HRV3 particles appear to enter by receptor-mediated uncoating at neutral pH. A low-pH step in addition to the receptor is required for uncoating and entry of HRV particles remaining stable after receptor binding on the cell surface. The ICAM-1 receptor binds with binding kinetics similar to those of the HRV3 and HRV16 serotypes (23) so that the observed differences in cell entry among those serotypes could be related to differences in particle stability, associated with the noncovalently bound pocket factor molecule present in HRV16 (11).
It has been proposed that major group HRV uncoating requires initial release of the pocket factor by the receptor (13). This process must be quite slow at neutral pH, because receptor-mediated uncoating of HRV16 is highly inefficient (Fig. 1) (11, 23). We also show here that receptor priming for low-pH-mediated uncoating is reversible (Fig. 2), so that the ICAM-1 receptor must not trigger a significant release of the pocket factor present in HRV16. The highly cooperative effect of receptor and low pH in HRV16 uncoating (Fig. 1A) suggests that exposure of virus-receptor complexes to mild low pH must enhance the rate of pocket factor release in HRV, decreasing particle stability and leading to the exit of the viral RNA. Receptor-mediated conformational changes in the virus binding canyon region might open the door for an acid-triggered release of the pocket factor molecule at physiological temperatures. This two-step uncoating process could be followed by viruses having pocket factors, including picornavirus and reoviruses (14, 19).
The cooperative or additive effect of mild low pH and receptor in major group HRV uncoating shown here has important implications for virus entry. During endocytosis, viruses attached to cell surface receptors are exposed to gradually lower pH, which in many cases initiates the membrane penetration events. In the absence of cooperation between the receptor and the low pH in virus uncoating, the release of the viral genome could occur through multiple sites in the highly symmetric virus particles. However, the receptor priming effect described here will trigger initial low-pH-mediated capsid opening (22) at the receptor-bound region. Exit of the viral genome will then be polarized toward the membrane-proximal region of the viral particle, facilitating genome penetration into the cell cytoplasm through a locally disrupted membrane by viral polypeptides (3, 14). Receptor priming for subsequent low-pH-driven virus entry is a principle more extended that initially expected (15). This process might lead to the polarized and efficient penetration of cellular membranes by nonenveloped viruses.
Acknowledgments
This work has been sponsored by grants from Vetenskapsrådet (MFR-12637, NFR-11994) to J.M.C. Support from the Karolinska Institute-NOVUM and a recent grant from MCyT (BIO2002-03281) to J.M.C. are also acknowledged.
REFERENCES
- 1.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85:7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casasnovas, J. M., J. K. Bickford, and T. A. Springer. 1998. The domain structure of ICAM-1 and the kinetics of binding to rhinovirus. J. Virol. 72:6244-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casasnovas, J. M., and T. A. Springer. 1995. Kinetics and thermodynamics of virus binding to receptor: studies with rhinovirus, intercellular adhesion molecule-1 (ICAM-1), and surface plasmon resonance. J. Biol. Chem. 270:13216-13224. [DOI] [PubMed] [Google Scholar]
- 7.Casasnovas, J. M., and T. A. Springer. 1994. The pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediate in which ICAM-1 is bound and RNA is released. J. Virol. 68:5882-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeTuello, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier, M., and K. Wetz. 1990. Kinetics of poliovirus uncoating in HeLa cells in a nonacidic environment. J. Virol. 64:3590-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer, F., M. Gruenberger, H. Kowalski, H. Machat, M. Huettinger, E. Kuechler, and D. Blaas. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. USA 91:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoover-Litty, H., and J. M. Greve. 1993. Formation of rhinovirus-soluble ICAM-1 complexes and conformational changes in the virion. J. Virol. 67:390-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan, G., M. S. Freistadt, and V. R. Racaniello. 1990. Neutralization of poliovirus by cell receptors expressed in insect cells. J. Virol. 64:4697-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolatkar, P. R., J. Bella, N. H. Olson, C. M. Bator, T. S. Baker, and M. G. Rossmann. 1999. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 18:6249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liemann, S., K. Chandran, T. S. Baker, M. L. Nibert, and S. C. Harrison. 2002. Structure of the reovirus membrane-penetration protein, μ1, in a complex with its protector protein, σ3. Cell 108:283-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. T. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 16.Perez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racaniello, V. R. 1996. The poliovirus receptor: a hook, or an unzipper? Structure 4:769-773. [DOI] [PubMed] [Google Scholar]
- 19.Rueckert, R. R. 1996. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Fields, D. M. Knipe, P. M. Howley, J. L. Melnick, R. M. Chanock, B. Roizman, and T. P. Monath (ed.), Virology, vol. 1, 3rd ed. Raven Press, New York, N.Y.
- 20.Schober, D., P. Kronenberger, E. Prchla, D. Blaas, and R. Fuchs. 1998. Major and minor receptor group human rhinoviruses penetrate from endosomes by different mechanisms. J. Virol. 72:1354-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69:531-569. [DOI] [PubMed] [Google Scholar]
- 22.Xing, L., J. M. Casasnovas, and R. H. Cheng. 2003. Structural analysis of human rhinovirus complexed with ICAM-1 reveals the dynamics of receptor-mediated virus uncoating. J. Virol. 77:6101-6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing, L., K. Tjarnlund, B. Lindqvist, G. Kaplan, D. Feigelstock, R. H. Cheng, and J. M. Casasnovas. 2000. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO J. 19:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, R., D. C. Pevear, M. J. Kremer, V. L. Giranda, J. A. Kofron, R. J. Kuhn, and M. G. Rossmann. 1996. Human rhinovirus 3 at 3.0 Å resolution. Structure 4:1205-1220. [DOI] [PubMed] [Google Scholar]