Abstract
In Campylobacter jejuni the sugar 2,4-diacetamido-2,4,6-trideoxy-α-D-glucopyranose, termed N,N′-diacetylbacillosamine (Bac2,4diNAc), is the first carbohydrate in the glycoprotein N-linked heptasaccharide. Starting with uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) two enzymes of the general protein glycosylation (Pgl) pathway in C. jejuni (PglF and PglE) have been recently shown to modify this sugar nucleotide to form UDP-2-acetamido-4-amino-2,4,6-trideoxy-α-D-glycopyranose (UDP-4-amino-sugar) [Schoenhofen, I. C., et al. (2006) J Biol Chem 281, 723–732]. PglD has been proposed to catalyze the final step in N,N′-diacetylbacillosamine synthesis by N-acetylation of the UDP-4-amino-sugar at the C4 position. We have cloned, overexpressed and purified PglD from the pgl locus of C. jejuni NCTC 11168 and identified it as the acetyltransferase that modifies the UDP-4-amino-sugar to form UDP-N,N′-diacetylbacillosamine, utilizing acetyl coenzyme A as the acetyl group donor. The UDP-N,N′-diacetylbacillosamine product was purified from the reaction by reverse phase C18 HPLC and the structure determined by NMR analysis. Additionally, the full-length PglF was overexpressed and purified in the presence of detergent as a GST-fusion protein allowing for derivation of kinetic parameters. We found that the UDP-4-amino-sugar was readily synthesized from UDP-GlcNAc in a coupled reaction using PglF and PglE. We also demonstrate the in vitro biosynthesis of the complete heptasaccharide lipid-linked donor by coupling the action of eight enzymes (PglF, PglE, PglD, PglC, PglA, PglJ, PglH, and PglI) in the Pgl pathway in a single reaction vessel.
Campylobacter jejuni is the Gram-negative enteropathogen identified as the primary cause of gastroenteritis in humans and the most frequent infection to precede the peripheral neuropathy Guillain-Barré syndrome (1–3). Infection of humans generally occurs by ingestion of contaminated livestock or water. Although the mechanism of infection is not clearly understood, production of glycolipids and glycoproteins by the pathogen have been found to influence cell motility, host-cell interactions, and competence for DNA uptake (4–6). As resistance to antimicrobial agents rises, the potential for development of novel therapeutics against enzymes that produce glycoconjugates has intensified efforts targeted at the characterization of their biosynthetic pathways. In C. jejuni four major glycan structures have been identified: lipooligosaccharide (LOS), capsule, O-linked glycan, and N-linked glycan (7–10). The genes coding for enzymes that synthesize these glycan moieties have been found in clusters throughout the C. jejuni genome. The respective biochemical functions of the gene products have been assigned on the basis of biochemical data or sequence homology to glycosyltransferases and other enzymes known to modify sugars (11–13).
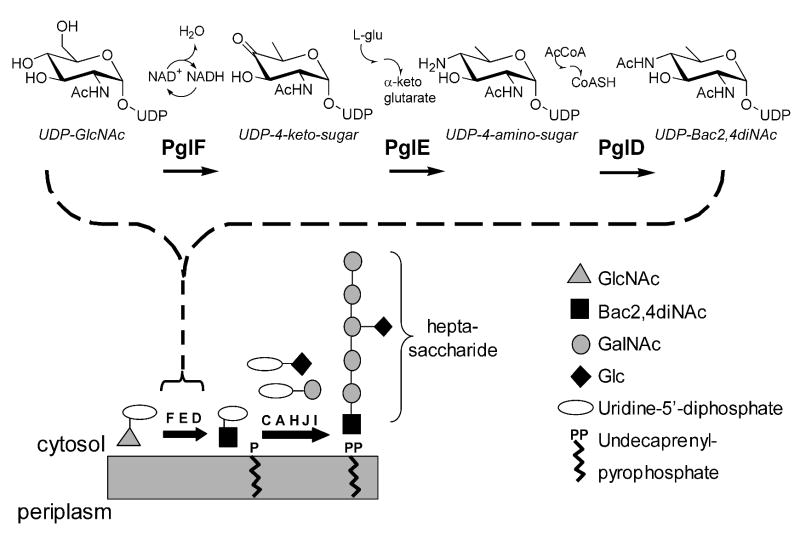
A common N-linked glycan has been detected in at least 22 periplasmic and cell-surface proteins (14, 10) in C. jejuni, and the Pgl pathway has been identified as the source of its biosynthesis. Genes of the pgl locus in the pathogen have been designated cj1119-cj1130 and the structure of the N-linked glycan has been shown to be the heptasaccharide GalNAc-α1,4-GalNAc-α1,4-(Glcβ1,3)-GalNAc-α1,4-GalNAc-α1,4-GalNAc-α1,3-Bac2,4diNAc-_1-Asn where GalNAc, GlcNAc, and Glc represent N-acetylgalactosamine, N-acetylglucosamine, and glucose, respectively, (Figure 1) (10). Assembly of this bacterial glycan begins with pyrophosphate bond formation between the Bac2,4diNAc phosphate and undecaprenylphosphate (Und-P) by Cj1124c (PglC) to form Bac2,4diNAc-α1-PP-Und (15). One N-acetylgalactosamine (GalNAc) is linked to the polyisoprenepyrophosphate-bound N,N′-diacetylbacillosamine by Cj1125c (PglA) to form GalNAc-α1,3-Bac2,4diNAc-α1-PP-Und (16). Next, four additional GalNAc and one branching glucose are added sequentially to the isoprene-linked disaccharide by PglJ, PglH, and PglI, to form the heptasaccharide (17). In N-linked glycoproteins the heptasaccharide is covalently attached at the N,N′-diacetylbacillosamine through a β-linkage to the amide nitrogen (N-linked) of an asparagine side chain in the canonical sequence Asn-Xaa-Ser/Thr motif, where Xaa can be any amino acid except proline (4).
Figure 1.
Pathway of the enzymatic synthesis of the bacterial glycoprotein heptasaccharide from UDP-GlcNAc by enzymes of the general protein glycosylation locus in C. jejuni. Enlarged is a detailed view of the pathway for UDP-Bac2,4diNAc synthesis.
Nearly all enzymes encoded in the pgl locus from C. jejuni strain NCTC 11168 responsible for synthesis of the heptasaccharide have been biochemically characterized (15, 17, 16, 18). Recently our laboratory reported biochemical evidence of enzyme-mediated glycosyltransferase activity, which strongly suggested that N,N′-diacetylbacillosamine was initially formed in C. jejuni as a uridine diphosphate derivative (Figure 2) (15). In these experiments a lengthy chemical synthesis was undertaken to produce UDP-Bac2,4diNAc and PglC was found to transfer Bac2,4diNAc phosphate to the undecaprenylphosphate lipid. The original bacillosamine, or 4-acetamido-2-amino-2,4,6-trideoxy-D-glucose was isolated and identified over thirty years ago from Bacillus licheniformis (19, 20). The difference between that bacillosamine and the derivative found in the C. jejuni N-linked heptasaccharide is an N-acetyl group at the C2 position. Figure 1 shows a scheme for the biosynthesis of UDP-N,N′-diacetylbacillosamine in C. jejuni beginning with UDP-GlcNAc. In this model Cj1120c (PglF) performs a cofactor-dependent hydride transfer from the C4 position of UDP-GlcNAc to C6, in conjunction with the elimination of water across the glucosyl C5 and C6 bond. The mechanism for the reaction is believed to follow a process similar to other C6 dehydratases such as dTDP-D-glucose-4,6-dehydratase (RmlB) from Salmonella enterica (21), resulting in the production of UDP-2-acetamido-2,6-dideoxy-α-D-4-ketohexulose (UDP-4-keto-sugar) (18). Cj1121c (PglE) catalyzes the pyridoxal-dependent transfer of an amino group from L-glutamate to the C4 position of the UDP-4-keto-sugar to form the UDP-4-amino-sugar (18). The final step in the enzymatic synthesis of UDP-Bac2,4diNAc is the proposed N-acetylation at the C4 position of the UDP-4-amino-sugar by Cj1123c (PglD).
Figure 2.
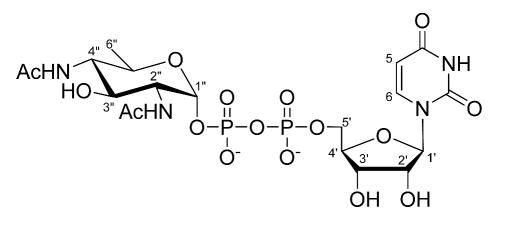
Structure of UDP-2,4-diacetamido-2,4,6-trideoxy-α-D-glycopyranose
The native dehydratase PglF has three domains: an N-terminal transmembrane domain, a C-terminal catalytic domain, and a linker between the transmembrane and catalytic domains. Recently, Logan and coworkers identified the biochemical activity of PglF by studying a construct that lacked the transmembrane domain (18). These investigators further reported that enzyme activity could not be detected in a coupled reaction between the truncated form of PglF and full-length PglE, suggesting that the transmembrane domain plays an important role in the coupled enzyme reaction. The truncation approach has also proven advantageous in the functional analysis of WbpM, a homologue of PglF, which is involved in pseudaminic acid biosynthesis in P. aeruginosa. In their experiments, Creuzenet and Lam found that the full-length WbpM retained activity, but only when isolated in the membrane fraction of the host expression system (22).
In this report we detail the functional characterization of PglD in addition to the biosynthesis and purification of UDP-Bac-2,4-diNAc. By harnessing the UDP-Bac2,4diNAc biosynthetic process in vitro we provide a rapid and cost-effective means to produce this rare sugar in milligram quantities. We also characterized the full-length PglF and demonstrate the in vitro enzymatic synthesis of the undecaprenylpyrophosphate-linked heptasaccharide in a coupled reaction that combined PglF, E, D, C, A, J, H, and I in a single reaction vessel. The results presented herein complete the functional identification of enzymes involved in the biosynthesis of the N-linked heptasaccharide in C. jejuni.
EXPERIMENTAL PROCEDURES
Molecular Biology
Amplification of pgl gene sequences from C. jejuni 11168 genomic DNA (ATCC 700819, designation NCTC 11168) were performed with oligonucleotides described in Table 1. BamH I and Xho I restriction sites were engineered to facilitate cloning of all amplicons into either pGEX4T-2 (GE Healthcare) to incorporate an N-terminal GST tag or pET-24a(+) (Novagen) to incorporate an N-terminal T7 and a C-terminal hexahistidine tag. Full-length pglD and pglE were subcloned into the pET vector. Full-length pglF and a truncated form of pglF coding for residues M130-V590 were subcloned into the pGEX vector to express GST-PglF and GST-PglF130, respectively. The full-length GST fusion PglF construct also included a C-terminal octahistidine tag. Amplifications were accomplished with the Stratagene Cloned Pfu Polymerase system as described by the manufacturer. Amplicons were purified and double-digested with BamH I and Xho I. Digested inserts and linearized vectors were fractionated by agarose gel electrophoresis and purified with the QIAquick Gel Extraction kit (Qiagen). Ligations were conducted with the T4 DNA Ligase kit (Promega) using an overnight incubation at 16 °C. Chemically competent DH5α strain of E. coli (Invitrogen) were transformed with the ligation reactions and colonies selected on Luria Bertani (LB) agar plates supplemented with either kanamycin or carbanicillin. Plasmids from colonies representing each construct were isolated and sequenced at the MIT CCR HHMI Biopolymers Laboratory.
Table 1.
Constructs and oligonucleotides used for this report
| Construct | Vector | Primer (5′-> 3′) |
|---|---|---|
| GST-PglF | pGEX4T-2 | Fwd: CGCGGATCCATGATTTTTTATAAAAGCAAAAGATTAGC
Rev: CGGCTCGAGTCAGTGATGATGATGATGATGATGGTGTACACCTTCTTTATTGTGTTTAAATTC |
| GST-PglF130 | pGEX4T-2 | Fwd: CGCGGATCCATGCTTGTGGATTTTAAACCTTC
Rev: CGGCTCGAGTCAGTGATGATGATGATGATGATGGTGTACACCTTCTTTATTGTGTTTAAATTC |
| PglE | pET24a(+) | Fwd: CGCGGATCCATGAGATTTTTTCTTTCTCC
Rev: CGGCTCGAGAGCCTTTATGCTCTTTAAG |
| PglD | pET24a(+) | Fwd: CGCGGATCCATGGCAAGAACTGAAAAAATTTATATTTATGGTGC
Rev: CGGCTCGAGCATCCTTTTTGCAGGTAC |
PglD and PglE Expression and Purification
Chemically competent BL21 (DE3) pLysS strain of E. coli (Invitrogen) were transformed for expression. For each construct 10 mL of an overnight culture was used to inoculate one liter of LB broth supplemented with 30 _g/mL kanamycin and 50 _g/mL chloramphenicol for selection, and incubated at 37 °C. At an OD600 of 0.6–1.0 the cultures were cooled to 16 °C prior to induction with 0.5 mM isopropyl _-D-1-thiogalactopyranoside (IPTG). After 22 hrs of incubation at 16 °C, the cells were harvested by centrifugation, resuspended in a solution of 0.9% NaCl and pelleted for storage at −80 °C. Pellets of cells that expressed PglD or PglE were resuspended in 50 mL ice cold lysis buffer (50 mM Tris-acetate, 100 mM NaCl, pH 7.5) supplemented with 30 mM imidazole, lysed by sonication, and lysate cleared by centrifugation at 145,000 × g for 45 minutes at 4 °C. Further handling of protein occurred at 4 °C unless otherwise stated. Cleared lysate was mixed with 4 mL Ni-NTA (Qiagen) resin per liter of original culture then tumbled for 4 hours. The Ni-NTA resin-protein mixture was packed into a K 9/15 column (GE Healthcare) and washed with 10 column volumes (cv) of lysis buffer supplemented with 30 mM imidazole at a flow rate of 3 mL/min. The resin bound protein was further washed with 20 cv of lysis buffer containing 40 mM imidazole then 10 cv of lysis buffer containing 50 mM imidazole. To elute the protein of interest a 30 cv gradient of imidazolelysis buffer from 50 mM to 500 mM was applied to the column. Protein concentration was determined using the Micro BCA kit (Pierce).
GST-PglF130 Expression and Purification
Chemically competent BL21 (DE3) pLysS cells were transformed for expression of the GST-PglF130 construct. A 10 mL volume of an overnight culture was used to inoculate one liter of LB broth and incubated at 37 °C with 50 _g/mL carbanicillin and 50 _g/mL chloramphenicol for selection. At an OD600 of 0.6–1.0 the culture was cooled to 30 °C then induced with 1 mM IPTG and incubated at 30 °C for three hours, then harvested and stored at −80 °C. Frozen cell pellets were resuspended in 50 mL of ice cold PBS buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.3), lysed by sonication, and the lysate cleared by centrifugation at 145,000 × g for 45 minutes. To purify, cleared lysate was mixed with 4 mL of glutathione Sepharose 4 Fast Flow resin (GS) (GE Healthcare) per liter of original culture, and tumbled to mix for 4 hours. The GS resin-protein mixture was packed into a K 9/15 column and washed with 25 cv of PBS at a flow rate of 3 ml/min. Protein was eluted with an isocratic flow of GS elution buffer (50 mM Tris, 10 mM L-glutathione, pH 8.0). Fractions containing purified material were identified by SDS-PAGE subjected to Coomassie staining or Western blot analysis probing for the affinity tag. Target fractions were pooled and exhaustively dialyzed against PBS. Protein concentration was determined using the Micro BCA kit.
Full-length PglF Expression and Purification
A 20 mL volume of an overnight culture of BL21 (DE3) pLysS cells transformed with the GST-PglF construct was used to inoculate 2 L of LB broth supplemented with 50 _g/mL carbenicillin and 50 _g/mL chloramphenicol, and cultured at 37 °C. At an OD600 of 0.6–1.0, the culture was cooled to 16 °C, induced with 0.5 mM IPTG, and incubated at 16 °C for 22 hrs. Cells were harvested and frozen at −80 °C until required. Frozen cell pellets were thawed in 100 mL ice cold PBS and lysed by sonication, then supplemented with 200 μM NAD+. Cellular debris was cleared by low-speed (8,000 × g) centrifugation for 45 minutes. The resulting supernatant was transferred to a clean centrifuge tube and subjected to high-speed centrifugation (145,000 × g) for 45 minutes to pellet the cell envelope fraction. The supernatant was discarded and the resulting pellet homogenized in 10 mL of PBS supplemented with 1.0% (v/v) Triton X-100 and 200 μM NAD+ (PBS-T). The detergent solution was tumbled at 4 °C for 2 hours then centrifuged at 145,000 × g for 1 hour. The supernatant containing detergent-solubilized GST-PglF was carefully removed so as not to disturb the pellet and then combined with 2 mL of GS resin pre-equilibrated in PBS-T. The GS resin-protein solution was tumbled for 4 hours then poured into a column and the resin was allowed to settle. Using gravity flow the resin-bound protein was washed with 25 cv of PBS-T containing 0.1% Triton X-100, then eluted with the GS elution buffer supplemented with 0.1% Triton X-100 and 200 μM NAD+. Fractions containing purified material were determined by SDS-PAGE (10%) subjected to Coomassie staining or Western blot analysis probing for the His-tag. To determine protein concentration a sample of the pooled fractions was exhaustively dialyzed against 0.1% Triton X-100 PBS-T then assayed with the Micro-BCA kit.
Enzymatic Reactions
To synthesize UDP-4-keto-sugar with purified GST-PglF, 1.5 mg (16.1 nmol) of enzyme in a 15 mL solution containing 5 mM L-glutathione, 50 mM Tris-acetate, 0.1% Triton X-100, 200 μM NAD+, 50 mM NaCl, 1 mM (15 μmol) UDP-GlcNAc, pH 8 was incubated for 10 hours at 28 °C. A sample of the reaction was filtered using a Microcon filtration unit [10 K molecular weight cut-off (MWC) – Millipore] for capillary electrophoresis (CE) analysis. A sample of the filtrate was equally mixed with methanol and injected into a Mariner Biospectrometry Workstation under a constant flow of 10 μL/min with data acquisition conducted in negative mode. To synthesize the UDP-4-amino-sugar by coupling the reactions of GST-PglF and PglE, the following components were added to a reaction as described above: 5 mg (108.5 nmol) purified PglE, L-glutamate and pyridoxal-5′-phosphate (PLP) to a final concentration of 15 mM and 100 μM, respectively, then incubated for 24 hours at 28 °C. The reaction volume was passed through a 10 K MWC filter then diluted 100 fold with H2O and loaded onto a 5 mL HiTrap Q FF anion exchange column (GE Healthcare) at a flow rate of 3 mL/min. The bound sugar was washed with 100 mL of H2O and eluted over a 120 mL gradient from 0 to 220 mM triethylammonium bicarbonate (TEAB), pH 8.6. Fractions containing purified UDP-4-amino-sugar were identified by CE then pooled, lyophilized, and analyzed by ESI-MS as described above. The UDP-4-amino-sugar product was quantified spectrophotometrically using the molar extinction coefficient of UDP (_262 = 10,000 M−1 cm−1 (23)). Approximately 2.3 mg (3.9 _mol) of UDP-4-amino-sugar were recovered from the coupled enzyme reaction. As an alternative to synthesize UDP-4-amino-sugar, approximately 15 mg (190.5 nmol) GST-PglF130 was bound to 4 mL of GS resin. PBS was added to the slurry such that the total volume measured 15 mL. 20 mg (33 μmol) UDP-GlcNAc was added and the reaction incubated at 28 °C overnight. The reaction was resuspended into a slurry, poured into a column, and the flow-through collected and then filtered with a 10K MWC membrane. In a total volume of 25 mL, the filtrate was combined with 15 mg (325.5 nmol) of purified PglE. L-glutamate and PLP were added to a final concentration of 20 mM and 100 μM, respectively. The reaction was incubated for 48 hours at 28 °C and the product purified as described for the coupled reaction. Recovery of UDP-4-amino-sugar by this method was approximately 9.5 mg (16 _mol). NMR chemical shifts and coupling constants of this material were virtually identical to published data (18). To synthesize UDP-Bac2,4diNAc enzymatically, in a total volume of 5 mL, 5 mg (212 nmol) of PglD was combined with 10 mg (17.0 μmol) UDP-4-amino-sugar and 14 mg (17.3 μmol) AcCoA (Sigma-Aldrich). The reaction was incubated at 37 °C for 3 hours then filtered with a 10K MWC membrane. The entire filtrate was loaded onto a preparative scale reverse phase C-18 column (Waters YMC Pack-Pro C18, 250 × 20 mm I.D., S-5 μm particle size, 12 nm pore size) equilibrated with 50 mM TEAB, pH 7.1 and eluted with a linear gradient of 0–25% acetonitrile over 300 mL. Eluting material was monitored for absorbance at 262 nm and fractions containing pure UDP-Bac2,4diNAc identified by CE. Target fractions were pooled and the solvent concentrated under reduced pressure to yield 6 mg of UDP-Bac2,4diNAc.
NMR Spectroscopy
A 4 mg sample of purified UDP-Bac2,4diNAc was resuspended in 600 μL of 99% D2O (Cambridge Isotopes) and placed in a 5-mm tube. All data was collected at 25 °C. A solution of 85% phosphoric acid in D2O was used as an external standard for 31P NMR and D2O (4.8 ppm) as the internal standard for 1H NMR spectrum calibration. The NMR data were processed with the MestRe-C (Mestrelab Research) software package. 1H and COSY spectra were obtained on a Bruker Avance-600 NMR spectrometer equipped with a pulse-field gradient triple resonance probe using standard pulse sequences from Bruker NMR. Homonuclear decoupling spectra were obtained on a Varian Inova-500 NMR spectrometer equipped with a pulse-field gradient triple resonance probe using standard pulse sequences from Varian. 31P spectra were measured with a Varian Mercury 300 NMR spectrometer with a pulse-field autoswitchable probe.
CE Analysis
CE was performed using a Hewlett Packard 3D CE system with UV detection and manual integration with the HP 3D CE software package. The running buffer was composed of 25 mM sodium tetraborate, pH 9.4 using a bare silica capillary (75 μm × 80 cm) with a detector distance of 72 cm. The capillary was conditioned before each run by washing with 0.4 M NaOH for 2 min, water for 2 min, and running buffer for 2 min. In general each sample was prepared by filtration with a 10 K MWC membrane, then diluted with water by a ratio of 1:4. Samples were introduced by pressure injection for 16 s at 30 mbar and the separation was performed at 22 kV.
Kinetic Measurements
For GST-PglF, 1.3 μg of enzyme was incubated in a reaction volume of 50 μL with 200 μM NAD+, 0.1% Triton X-100, 50 mM Tris-acetate, 50 mM NaCl, 5 mM L-glutathione, pH 7.7 with UDP-GlcNAc at 6 different concentrations varying from 0.8 mM to 31 mM. The reactions were incubated at 37 °C for 200 min, boiled for 2 min then filtered through a 10 K MWC membrane and analyzed by CE as described above. UDP-4-amino-sugar substrate for the PglD assays was enzymatically synthesized and quantified as described above. For each reaction 0.025 ng of enzyme was incubated in a total volume of 20 μL containing 50 mM Tris-acetate, 50 mM NaCl, 2 mM AcCoA, pH 7.7 and 5 μg bovine serum albumin to serve as a carrier protein with UDP-4-amino-sugar at 6 different concentrations varying from 0.1 mM to 2 mM. The reactions were incubated at 37 °C for 90 min, then boiled and filtered as done for the GST-PglF kinetics reactions.
Radioactive Assay Coupling PglD, C and A using labeled AcCoA
Und-P was synthesized as described elsewhere (24, 25). A 3 μL volume of DMSO and 7 μL volume of 14.3% (v/v) Triton X-100 were added to a tube containing 50 nmol Und-P. After vortexing and sonicating in a water bath, 1 μL of 1 M MgCl2, 10 μL of 10 mM UDP-GalNAc, 15 μL of 7 mM UDP-4-amino-sugar, 10 μL of PglC at 0.25 mg/mL, 10 μL of PglA at 0.5 mg/mL, 1.5 μL of unlabeled 14 mM AcCoA, and 1 μL of 20 μM [3H] AcCoA (American Radiolabeled Chemicals) were added, giving the reaction a specific activity of 48 nCi/nmol. The reaction was initiated by adding 10 μL of PglD at 0.5 mg/mL and incubated at room temperature. Aliquots of 12 μL were taken at 2, 4, 8, 30, and 60 min. Reactions were quenched and prepared for liquid scintillation counting as described elsewhere (15).
Heptasaccharide Biosynthesis
Components of the reaction were assembled in two tubes. Tube one contained Und-P (5 nmol), UDP-GlcNAc, UDP-GalNAc, and UDP-Glc (each at 1 mM, 1mM, and 0.2 mM final concentration, respectively) suspended with 3 μL of DMSO, and 7 μL of 14.3% Triton X-100 by vigorous vortexing and water bath sonication. Next, 4 μL of purified PglC, PglA, PglJ, and PglH (the latter two at 0.5 mg/ml stock concentration) were added, along with 10 μL of a PglI cell envelope fraction. Each prepared as described elsewhere (17). Combined in tube two were 3 μL of 15 mM NAD+, 10 μL of 200 mM L-glu, 10 μL of 2.7 mM PLP, 5 μL of 20 mM AcCoA, 10 μL of PglD, 25 μL of PglE, 37 μL GST-PglF (0.5 mg/mL, 0.5 mg/mL. 0.15 mg/mL protein stock concentration, respectively). The reaction was initiated by adding 64 μL of tube two to tube one then and then the mixture was incubated at room temperature for seven hours. The heptasaccharide was isolated and analyzed by HPLC and MALDI-MS as described elsewhere (15).
RESULTS
Cloning, Expression and Purification of PglD, PglE, and PglF
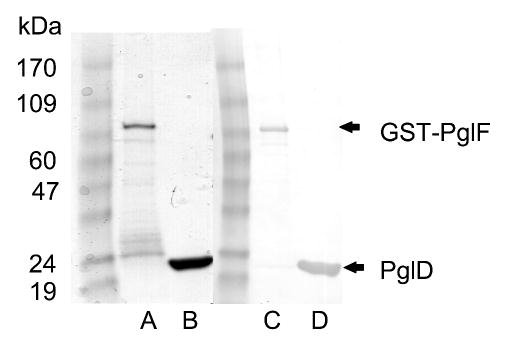
Full-length pglD, pglE and pglF werecloned from the C. jejuni strain NCTC 11168 genomic DNA and expressed in E. coli. Clones coding for PglE and PglD were expressed with an N-terminal T7-tag and a C-terminal His6-tag (Table 1). Both constructs overexpressed robustly in E. coli and purified by nickel-affinity chromatography with protein yields near 25 mg/L culture. Full-length PglF was expressed with an N-terminal glutathione S-transferase (GST) domain concurrently with a C-terminal octahistidine tag (GST-PglF). GST-PglF was purified in the presence of detergent and NAD+ by affinity chromatography with a yield of 1.5 mg/L culture. Similar to the construct reported by Logan and coworkers (18), we expressed GST-PglF130 (M130-V590) which omitted the putative transmembrane domain of full-length PglF. This truncated construct expressed well in E. coli yielding approximately 20 mg/L culture. Confirmation that purified material represented the target proteins was accomplished by SDS-PAGE and Western blot analysis to detect the affinity tags (Figure 3).
Figure 3.
(Lanes A & B) Coomassie stained 4–15% gradient SDS-PAGE gel of glutathione sepharose purified GST-PglF and Ni-NTA purified PglD. Calculated molecular mass for GST-PglF and PglD-His6 are 93 k Da and 23.5 k Da, respectively. (Lanes C & D) Corresponding Western blot probing for C-terminal His-tag. Arrows indicate band representing the respective construct.
Dehydratase activity of GST-PglF
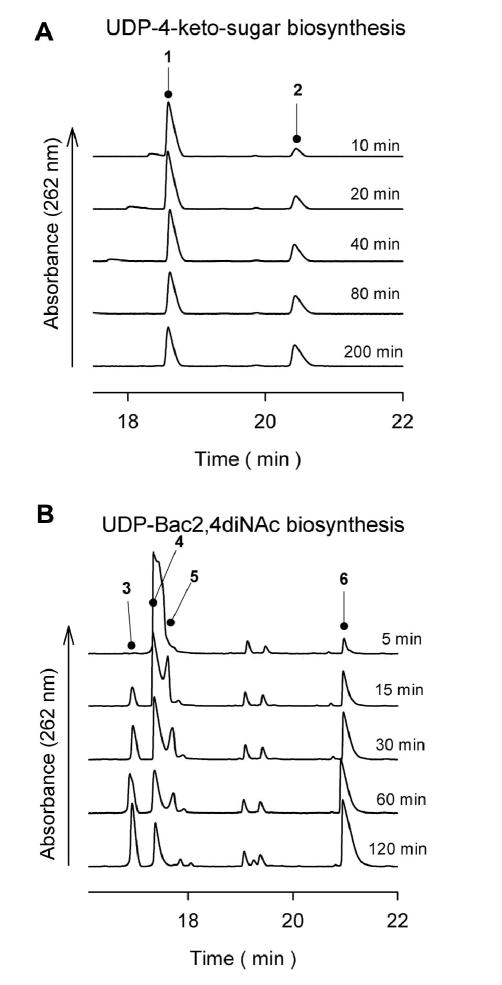
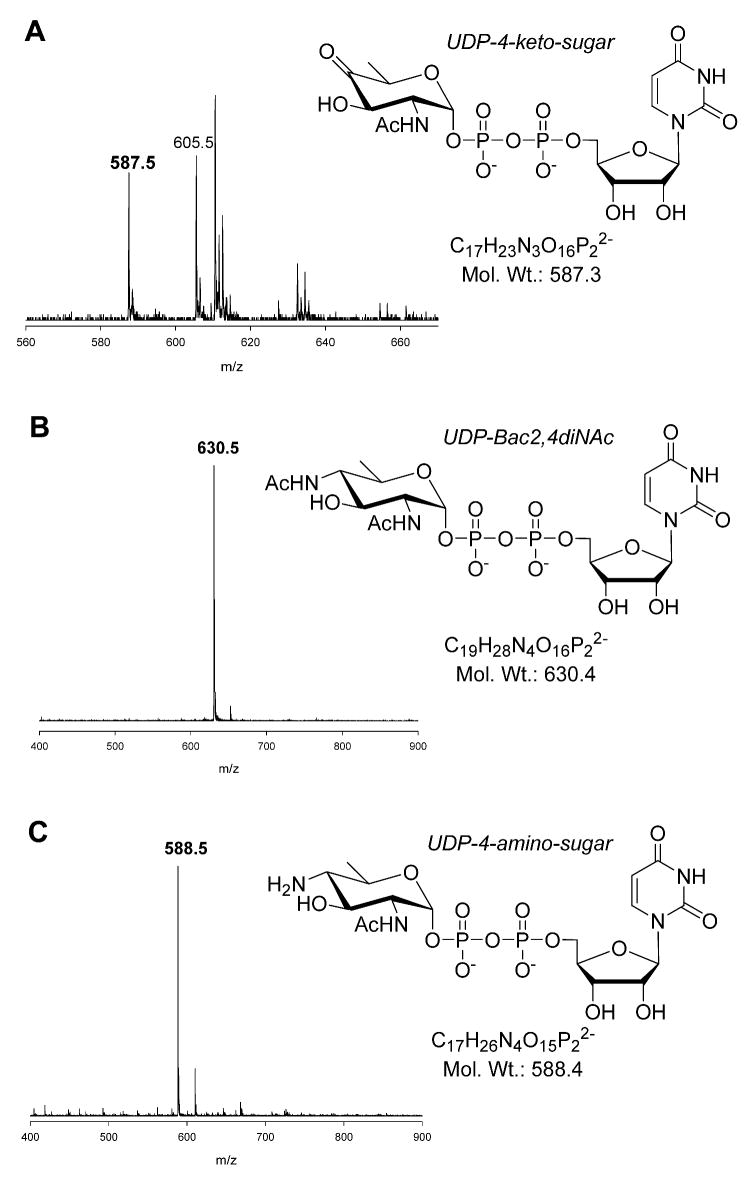
The reaction of GST-PglF was analyzed by CE using UV absorbance (262 nm) to detect the eluting species (Figure 4A). Although consumption of the cofactor was not apparent, catalytic activity by GST-PglF was enhanced in the presence of additional NAD+ but not NADP+ (data not shown). Electrospray ionization mass spectrometry (ESI-MS) analysis of the GST-PglF reaction indicated a mass of 587.5 Da, consistent with the formation of UDP-4-keto-sugar. CE analysis of the same reaction indicated the presence of starting material (data not shown), consistent with the ESI-MS observation of a species having a mass of 605.5 Da (Figure 5A). Alternatively, it is possible for the keto-sugar to exist in equilibrium with the corresponding diol form (26). The diol of the catalytic product of PglF would correspond to the addition of 18 atomic mass units (water) to the UDP-4-keto-sugar. Therefore the mass of 605.5 Da may be attributed, in part, to the diol form of the PglF product.
Figure 4.
CE Time-course analysis of (A) GST-PglF using 10 _g enzyme with UDP-GlcNAc at 1mM final concentration and (B) PglD using 1.3 ng of enzyme with UDP-4-amino and AcCoA at 1.2 mM and 1.0 mM final concentrations. Both reactions were conducted at 37 °C, pH 7.7 with aliquots taken at the times indicated. Numbered peaks are 1, UDP-GlcNAc; 2, UDP-4-keto; 3, UDP-Bac; 4, UDP-4-amino; 5, AcCoA; 6, CoASH.
Figure 5.
ESI-MS of (A) GST-PglF reaction mixture. (B) HPLC purified UDP-Bac. (C) Ion exchange purification of UDP-4-amino from GST-PglF and PglE coupled reaction.
Acetyltransferase activity of PglD
PglD is a putative acetyltransferase (6). Amino acid sequence analysis shows the C-terminal domain to be consistent with a hexapeptide repeat motif. Members from a subclass of the hexapeptide repeat enzymes, known as xenobiotic acetyltransferases, have been shown to use acetyl-Coenzyme A (AcCoA) for acetylation of a variety of substrates (27, 28). Purified PglD was incubated with the purified UDP-4-amino sugar and AcCoA in excess. The resultant CE elution profile showed two additional peaks that flanked the substrate and cofactor signals (Figure 4B). To confirm that an acetyl group was transferred to UDP-4-amino-sugar, the PglD reaction products were purified by reverse phase high performance liquid chromatography (RP-HPLC) and analyzed by ESI-MS and CE. Consistent with the loss of a proton and addition of an acetyl group to form UDP-Bac2,4diNAc, the ESI-MS revealed a mass peak of 630.5 (Figure 5B). The RP-HPLC purification step also separated a species with an atomic mass of 767, consistent with the Coenzyme A (CoASH) (data not shown).
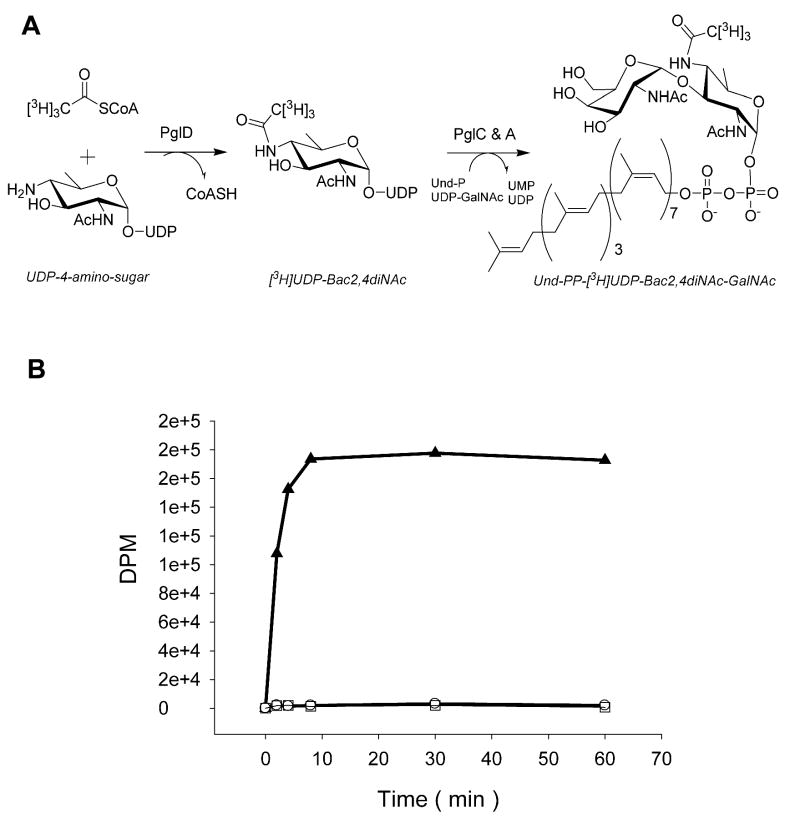
To further demonstrate acetyl group transfer by PglD to the UDP-4-amino-sugar, a coupled enzyme assay was employed using [3H]AcCoA. Isolation of the labeled UDP-Bac2,4diNAc was simplified by incorporating purified PglC, an integral membrane glycosyltransferase from C. jejuni responsible for transferring Bac2,4diNAc phosphate to the isoprene lipid carrier Und-P (15), into the assay. PlgA, which transfers the first GalNAc onto Bac2,4diNAc-PP-Und (17), was also included to consume the product of PglC, thus driving the reaction forward and facilitating isolation of the labeled product. In addition to cofactors and salts, reactants included purified the UDP-4-amino-sugar, Und-P, and UDP-GalNAc (Figure 6A). The organic phase was extracted and assayed for radioactivity. Because AcCoA was expected to remain in the aqueous layer, counts in the organic layer were attributed to incorporation of a radiolabled acetyl group into the polyisoprenepyrophosphate-linked substrate (Figure 6B). For control reactions, the Und-P or the UDP-4-amino-sugar was omitted. Both control reactions resulted in background levels of counts, strongly suggesting acetyl group transfer to UDP-4-amino by PglD (Figure 6B).
Figure 6.
(A) Reaction scheme for the transfer of 3H labeled acetyl group to UDP-4-amino and onto Und-P by the enzymes PglD and PglC. PglA adds GalNAc to Und-PP-[3H]Bac. Aliquots of the reaction were taken over time and the organic phase subjected to liquid scintillation counting. (B) Plot of the liquid scintillation counts. Reactants included UDP-GalNAc [3H] AcCoA, Und-P, and UDP-4-amino (▲). Control reactions omitted Und-P (○) or UDP-4-amino (□).
NMR analysis of the PglD product
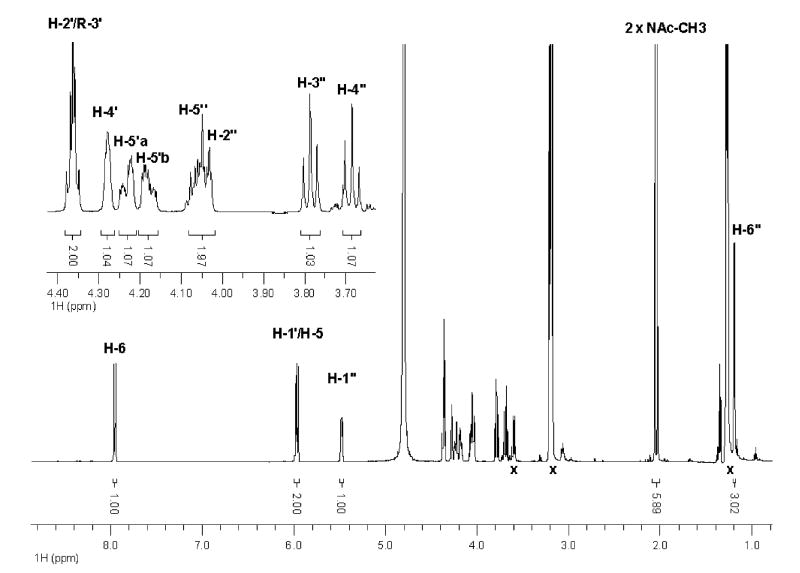
To further establish the identity of the enzymatic product of PglD, the proton NMR spectra of the purified PglD reaction product was analyzed. A series of homonuclear decoupling experiments was conducted to identify the chemical shifts for protons from the pyranose ring (supporting information, Figure S-1). In the proton spectrum two peaks centered at 2.05 ppm with a total peak area of six protons suggested the presence of two acetyl groups in the compound (Figure 7). In comparison to the observed chemical shift of proton H-4 on the UDP-4-amino-sugar (18), the H-4 apparent triplet shifted to a more de-shielded position of 3.69 ppm from 3.11 ppm (Figure 7, inset). Using this spectrum the J1″2″, J2″3″, J3″4″, J4″5″, and J5″6″ coupling constants were measured and are listed in Table 2. The coupling constant values were essentially identical to published values for the UDP-4-amino-sugar, hence the UDP-Bac2,4diNAc glucopyranose ring has the same stereochemistry. The 31P spectrum (supplemental information, Figure S-2 and S-3) and chemical shift assignments for uracil and ribose moiety protons (Table 2) matched published values for UDP-sugars (18, 29). On the basis of the results presented the product of PglD was identified as UDP-2,4-diacetamido-2,4,6-trideoxy-α-D-glucopyranose.
Figure 7.
1H NMR spectrum of enzymatically synthesized UDP-Bac. Proton chemical shift labels follow the scheme represented in Figure 2 and values in Table 2. Peaks designated by ‘x’ indicate signals from impurities and TEAB buffer. Peak surface and integration values are also indicated. Inset enlarges the view of proximally occurring chemical shifts from ribose and pyranose ring moieties.
Table 2.
1H and 31P NMR chemical shift and
| Moiety | δH (ppm) | J | [Hz] |
|---|---|---|---|
| Uracil | |||
| H-5 | 5.97 [d] | J5′,6′ | 8.1 |
| H-6 | 7.95 [d] | ||
| Ribose | |||
| H-1′ | 5.97 [d] | J1′,2′ | 4.5 |
| H-2′ | ~4.37 | ||
| H-3′ | ~4.37 | ||
| H-4′ | 4.28 [m] | ||
| H-5′a | 4.18 [ddd] | J5′a,P | 5.5 |
| J5′a,4′ | 3.0 | ||
| J5′a,5′b | 11.7 | ||
| H-5′b | 4.23 [ddd] | J5′b,P | 4.3 |
| J5′b,4′ | 2.6 | ||
| Pyranose | |||
| H-1″ | 5.48 [dd] | J1″,2″ | 3.2 |
| J1″,P | 6.9 | ||
| H-2″ | 4.02 [m] | ||
| H-3″ | 3.79 [at] | J2″,3″ | 10.2 |
| J3″,4″ | 10.2 | ||
| H-4″ | 3.69 [at] | J4″,5″ | 10.2 |
| H-5″ | 4.05 [m] | ||
| H-6″ | 1.19 [d] | J5″,6″ | 6.2 |
| Acetyl | |||
| CH3 | 2.06 [s] | ||
| CH3 | 2.04 [s] | ||
| Pyrophosphate | |||
| Pa | −10.84 [d] | ||
| Pb | −12.66 [d] | ||
Data collected at 25 °C in D2O, using 4.8 ppm for the solvent chemical shift.
[d], doublet; [dd], doublet of doublets; [ddd], doublet of doublets of doublets [m], multiplet; [at], apparent triplet, [s] singlet.
Kinetic analysis of PglF and PglD
GST-PglF and PglD appeared suitable for analysis by Michaelis-Menten kinetics since the reaction velocities were observed to plateau below 20 mM and 2 mM substrate concentration, respectively, in the presence of 200 μM NAD+ for GST-PglF and 2 mM AcCoA for PglD. Product formation velocities were averaged from two reactions run in parallel for each substrate concentration. Observed values were plotted and kinetic parameters derived with the software SigmaPlot (Systat Software, Inc). Apparent kinetic parameters for GST-PglF were Km = 7.0 mM ± 1.4 mM, kcat = 7.1 ± 0.53 min−1. Apparent kinetic parameters for PglD were Km = 0.41 mM ± 0.078 mM, kcat = 4.83 × 105 ± 0.30 × 105 min−1.
Coupled reaction with PglF, E, D, C, A, J, H, & I
We noted inhibition of GST-PglF130 dehydratase activity on UDP-GlcNAc in the presence of PglE (data not shown). This observation suggested a physical interaction between the distinct enzymes, as reported by Logan and coworkers using a PglF130 construct with a C-terminal His-tag only (18). We coupled the reaction of GST-PglF with PglE and analyzed the products by CE. A peak eluting ahead of the UDP-GlcNAc peak was observed, consistent with the CE migration of the UDP-4-amino sugar (supplementary information, Figure S-4). To confirm the UDP-4-amino sugar was indeed formed in the reaction, GST-PglF and PglE were combined with UDP-GlcNAc, PLP, and L-glu. The reaction mixture was incubated overnight and the reaction product purified by anion exchange chromatography. ESI-MS analysis of the purified material showed a prominent mass peak of 588.5 (Figure 5c). Because PglE specifically uses the UDP-4-keto-sugar as its primary substrate we conclude the product of GST-PglF is indeed the UDP-4-keto-sugar.
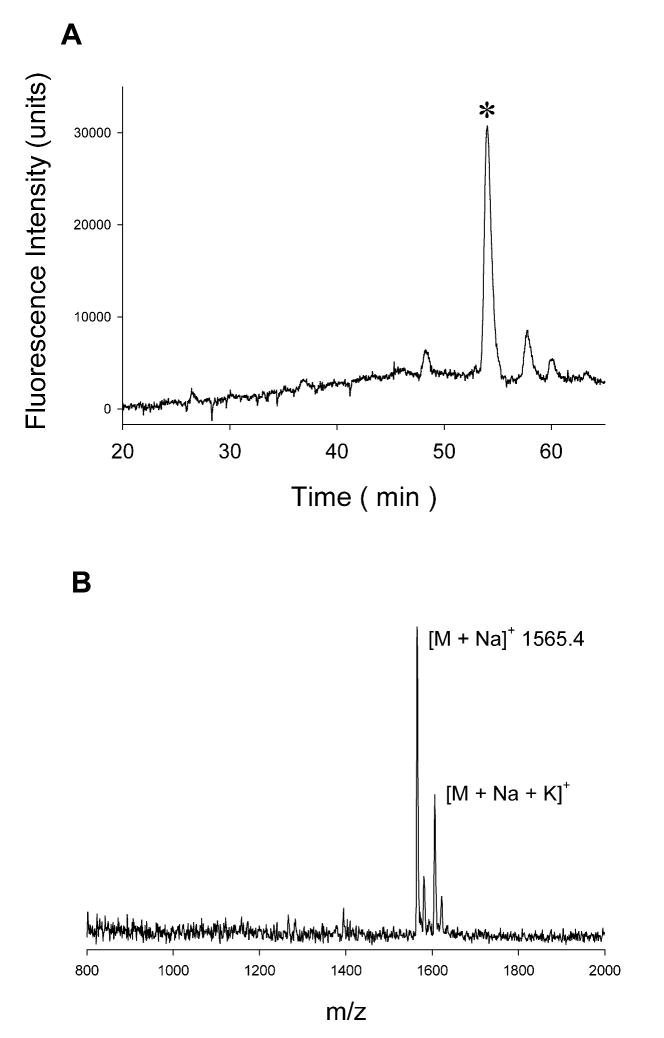
Using purified Pgl enzymes from C. jejuni NCTC 11168, our lab has investigated the in vitro transfer of N,N′-diacetylbacillosamine phosphate onto Und-P and the in vitro enzymatic synthesis of the heptasacccharide using a chemically synthesized undecaprenyl pyrophosphate-linked N,N′-diacetylbacillosamine (15, 17). Starting from UDP-GalNAc, UDP-Glc, Und-P and chemically synthesized UDP-Bac-2,4-NAc, the reactions of PglC, PglA, PglH, PglJ and PglI have been coupled in a single reaction vessel to synthesize the bacterial glycan heptasaccharide (15). We wanted to confirm the presence of GST-PglF, PglE and PglD in the same reaction vessel did not inhibit the downstream reactions. In the absence of chemically synthesized UDP-Bac-2,4-NAc we combined the required substrates and cofactors for the reaction of each specific enzyme to proceed. The polyisoprene pyrophosphate-linked glycan was extracted from the organic phase and subsequently released from the polyisoprene by trifluoroacetic acid treatment and labeled with the fluorophore 2-aminobenzamide (2AB) at the C1 position of the diacetamido bacillosamine residue through reductive amination. HPLC analysis yielded a peak consistent with the known elution profile of the heptasaccharide and the identity of the eluted material was confirmed by MALDI-MS (Figure 8).
Figure 8.
(A) Fluorescence trace from HPLC analysis of 2AB-labeled heptasaccharide isolated from a reaction that coupled PglF, E, D, C, A, J, H and cell envelope fraction of PglI in one reaction vessel. ‘*’ denotes the major saccharide peak. (B) MALDI-MS of eluted material.
DISCUSSION
UDP-GlcNAc is modified by PglF to form the UDP-4-keto-sugar substrate of PglE in the prokaryotic glycoprotein N-linked heptasaccharide biosynthesis pathway (18). In C. jejuni there are distinct metabolic pathways that share UDP-GlcNAc as a substrate, thus suggesting a rationale for the reaction kinetics observed in vitro. Pseudaminic acid, the major modification of flagellin, is synthesized via the UDP-GlcNAc dehydratase PseB pathway (30, 18). GalNAc, a major component in the biosynthesis of glycoprotein N-linked heptasaccharide, lipooligosaccharide (LOS), and capsule (7, 8, 10), is synthesized intracellularly by the C4 epimerase Gne (31). Table 3 lists the kinetic parameters of PseB and Gne derived from other studies, as well as GST-PglF from this study. Clearly Gne is the most efficient of all three enzymes with a kcat/Km greater than two orders of magnitude over the PseB and GST-PglF. This is to be expected since one or more copies of GalNAc/Gal or subsequent derivatives occur more frequently on cell surface structures (heptasaccharide, LOS, capsule) than GlcNAc/Glc related glycosides. The kcat/Km value of PseB indicates this enzyme is a moderately effective dehydratase, more so than GST-PglF by a factor of thirty. However, pgl gene knockout studies on C. jejuni NCTC 11168 showed a significant disparity between wild-type and mutant strain signatures of the high resolution-magic angle spinning NMR spectra and whole-cell lysate blotting with lectins (32). These results indicate the substrate turnover rate of PseB is insufficient to complement the wild-type consumption levels of the UDP-4-keto-sugar by the Pgl pathway in the absence of PglF. Comparison of the kinetics data, together with the knockout study results support the hypothesis of a channeling mechanism where there exists a sequential transfer of products between enzymes of the same biosynthetic pathway (33). Of the two dehydratases compared, PseB has the lowest Km, indicating a higher affinity for substrate. Studies on PseB knockouts in C. jejuni strain 81–176 showed the cells accumulated unglycosylated flagella intracellularly thus explaining a lack of motility (30). Arguably, when UDP-GlcNAc is in low abundance the pathogen would likely channel available stores toward the function of motility over host cell interactions.
Table 3.
Kinetic parameters for GST-PglF and PglD compared with published values of PglE and Gne.
| Enzyme | Substrate | Protein | Km(app) | kcat | kcat/Km |
|---|---|---|---|---|---|
| pmol/assay | MM | Min−1 | min−1x mM−1 | ||
|
|
|||||
| GST-PglFa | UDP-GlcNAc | 20.7 | 7.00 | 7.1 | 1.0 |
| PglDa | UDP-4-amino | 0.011 | 0.41 | 4.83 × 105 | 1.18 × 106 |
| PglEb | UDP-4-keto | 3.32 | 0.048 | 144 | 3000 |
| MalE-Gnec | UDP-GlcNAc | 0.083 | 1.087 | 4938 | 4543 |
| PseBd | UDP-GlcNAc | 25.8 | 0.05 | 1.5 | 30.6 |
This study, values are the average of two experiments, assays conducted at 37 ºC, pH 7.7.
From C. jejuni NCTC 11168, assays conducted at 37 ºC, pH 7.2 (18).
From C. jejuni NCTC 11168, assays conducted at 37 ºC, pH 8.0 (31).
From C. jejuni subspecies doylei ATCC49349, assays conducted at 42 ºC, pH 7.0 (33).
Using a combination of radiolabel transfer, NMR, and ESI-MS we have shown that PglD catalyzes the acetyl group transfer from AcCoA to the UDP-4-amino-sugar, resulting in the formation of UDP-N,N′-diacetylbacillosamine (Figure 2). The proton NMR spectrum of UDP-Bac2,4diNAc shows a significant chemical shift of the H4 proton to a more deshielded position, consistent with the conversion of a primary amine at the C4 position of the pyranose ring to an amide (Figure 7). The kcat/Km of PglD is 1.18 × 106 min−1 mM−1, indicating the enzyme is very efficient in catalyzing the acetyl group transfer to the UDP-4-amino sugar. An advantage to such a high degree of activity results in the rapid consumption of the UDP-4-amino-sugar, thereby driving the enzymatic conversion of UDP-GlcNAc by PglF to UDP-4-keto-sugar; the substrate of PglE. Comparing kinetic data of the Pgl enzymes in Table 3 indicates the rate-limiting step in UDP-Bac2,4diNAc biosynthesis is the formation of the UDP-4-keto-sugar.
In vitro synthesis of N,N′-diacetylbacillosamine and its precursors by enzymes of the Pgl pathway broadens the scope for potential applications. The C. jejuni oligosaccharyl transferase, PglB, was shown to prefer a diacetylated sugar linked to the polysioprene pyrophosphate for glycosylation of peptides when the transferred sugar was a disaccharide (34). As the structure/function relationship between Pgl enzymes and respective substrates become more defined, the potential to derive novel substrate analogues arises. Additionally, the capacity to synthesize milligram quantities of UDP-Bac2,4diNAc relatively inexpensively brings forth a new biologically relevant molecule that is suitable for modification by chemical means for probing biological processes. Because enzyme quantities were used in excess to ensure complete substrate turnover the in vitro reaction conditions may be optimized further to improve enzyme efficiency on a preparative scale. A more specific application is the use of purified UDP-Bac2,4diNAc to study protein modification pathways in other pathogens such as the Neisseria species, known to synthesize a diacetylated sugar for pilin glycosylation (35).
Successful coupling of reactions by PglE and GST-PglF suggests the involvement of the PglF transmembrane domain in preventing the inhibitory reaction observed between PglE and the soluble catalytic domain of PglF (18). Addition of the GST tag to the full-length PglF was critical to enhancing expression levels and simplifying the purification process. Reconstitution of heptasaccharide synthesis in vitro with full-length isolated proteins clearly demonstrates all eight pgl enzymes are able to perform their respective functions in a single reaction vessel. Because substrate turnover was not observed in a reaction coupling GST-PglF130 and PglE, we believe the presence of the GST tag fused to PglF was not responsible for disruption of inhibition in the coupled assay with full-length PglF and PglE. Experiments currently underway in our lab focus on the characterization of the presumed physical interaction between PglF and other enzymes of the Pgl pathway.
In summary, the evidence presented in this report identifies PglD as an acetyltransferase that catalyzes the last step in a three-step biosynthetic reaction of UDP-Bac2,4diNAc from UDP-GlcNAc. This enzyme proved to be very efficient and may act as the engine that drives the metabolic conversion of UDP-GlcNAc to UDP-Bac2,4diNAc. The in vitro enzymatic synthesis of UDP-Bac2,4diNAc provides a straightforward method for producing milligram quantities of this material. In turn, this allows for a shift of attention towards investigating the mechanism of N-linked protein glycosylation and further the development of a bacterial protein glycosylation system for applications in biotechnology. We also report that as a GST fusion protein, the dehydratase PglF functioned in a coupled reaction with enzymes of the pgl locus to synthesize the undecaprenylpyrophosphate heptasaccharide in vitro. This result suggests a greater role for the PglF transmembrane domain in the bacterial glycan biosynthesis other than to tether the catalytic domain of PglF to the lipid bilayer.
Supplementary Material
Figure S 1. Homonuclear decoupling spectra
Figure S 2. 31P NMR spectrum
Figure S 3. 1H COSY spectrum
Figure S 4. CE A262 elution profile of coupled reaction between PglF and PglE
Supporting Information Paragraph. Figures contained herein are various NMR spectra of UDP-Bac and CE elution profile of the coupled reaction between PglF and PglE:
Acknowledgments
We thank M. Sainlos and L. Martin for assistance in obtaining MALDI-MS data, H. Pan and R. Kennedy for assistance in NMR data collection, in addition to K. Glover and B. Sculimbrene for critical reading of the manuscript.
This work was supported by the NIH: Grant GM039334 to B.I., GM075415 postdoctoral fellowship to N.B.O., and a HHMI predoctoral fellowship to J.R.B.
ABBREVIATIONS
- 2AB
2-aminobenzamide
- AcCoA
acetyl Coenzyme A
- Bac2
4diNAc, 2,4-diacetamido-2,4,6-trideoxy-α-D-glucopyranose (also called N,N′-diacetylbacillosamine in this report)
- BCA
bicinchoninic acid
- CE
capillary electrophoresis
- C. jejuni
Campylobacter jejuni
- CoASH
Coenzyme A
- cv
column volume
- Da
Daltons
- E. coli
Escherichia coli
- ESI
electrospray ionization
- GS
glutathione Sepharose 4 Fast Flow resin
- GST
glutathione S-transferase
- IPTG
isopropyl _-D-1-thiogalactopyranoside
- LB
Luria-Bertani
- LOS
lipooligosaccharide
- MALDI
matrix-assisted laser desorption/ionization
- MWC
molecular weight cut-off
- NAD+
nicotinamide-adenine dinucleotide
- NADP+
nicotinamide-adenine dinucleotide phosphate
- Ni-NTA
nickel-nitrilotriacetic acid
- NMR
nuclear magnetic resonance
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate buffered saline
- Pgl
protein glycosylation
- PLP
pyridoxal-5′-phosphate
- P. aeruginosa
Pseudomonas aeruginosa
- RP-HPLC
reverse phase high performance liquid chromatography
- SDS
sodium dodecyl sulfate
- UDP
uridine-5′-diphosphate
- UDP-4-amino
UDP-2-acetamido-4-amino-2,4,6-trideoxy-α-D-glucopyranose
- UDP-4-keto
UDP-2-acetamido-2,6-dideoxy-α-D--xylo-4-hexulose
- UDP-GlcNAc
UDP-2-acetamido-α-D-glucopyranose
- UDP-GalNAc
UDP-2-acetamido-α-D-galactopyranose
- Und-P
undecaprenyl phosphate
- Glc
glucose
- Gal
galactose
Footnotes
This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni - an emerging foodborne pathogen. Emerg Infect Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butzler JP, Skirrow MB. Campylobacter enteritis. Clin Gastroenterol. 1979;8:737–765. [PubMed] [Google Scholar]
- 3.Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barré syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 4.Nita-Lazar M, Wacker M, Schegg B, Amber S, Aebi M. The N-X-S/T consensus sequence is required but not sufficient for bacterial N-linked protein glycosylation. Glycobiology. 2005;15:361–367. doi: 10.1093/glycob/cwi019. [DOI] [PubMed] [Google Scholar]
- 5.Szymanski CM, Burr DH, Guerry P. Campylobacter protein glycosylation affects host cell interactions. Infect Immun. 2002;70:2242–2244. doi: 10.1128/IAI.70.4.2242-2244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wacker M, Linton D, Hitchen PG, Nita-Lazar M, Haslam SM, North SJ, Panico M, Morris HR, Dell A, Wren BW, Aebi M. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 7.Linton D, Gilbert M, Hitchen PG, Dell A, Morris HR, Wakarchuk WW, Gregson NA, Wren BW. Phase variation of a β-1,3 galactosyltransferase involved in generation of the ganglioside Gm1-like lipo-oligosaccharide of Campylobacter jejuni. Mol Microbiol. 2000;37:501–514. doi: 10.1046/j.1365-2958.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- 8.St Michael F, Szymanski CM, Li J, Chan KH, Khieu NH, Larocque S, Wakarchuk WW, Brisson JR, Monteiro MA. The structures of the lipooligosaccharide and capsule polysaccharide of Campylobacter jejuni genome sequenced strain NCTC 11168. Eur J Biochem. 2002;269:5119–5136. doi: 10.1046/j.1432-1033.2002.03201.x. [DOI] [PubMed] [Google Scholar]
- 9.Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry P. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J Biol Chem. 2001;276:34862–34870. doi: 10.1074/jbc.M104529200. [DOI] [PubMed] [Google Scholar]
- 10.Young NM, Brisson JR, Kelly J, Watson DC, Tessier L, Lanthier PH, Jarrell HC, Cadotte N, St Michael F, Aberg E, Szymanski CM. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J Biol Chem. 2002;277:42530–42539. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 11.Karlyshev AV, Ketley JM, Wren BW. The Campylobacter jejuni glycome. FEMS Microbiol Rev. 2005;29:377–390. doi: 10.1016/j.femsre.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Szymanski CM, Logan SM, Linton D, Wren BW. Campylobacter - a tale of two protein glycosylation systems. Trends Microbiol. 2003;11:233–238. doi: 10.1016/s0966-842x(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 13.Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- 14.Linton D, Allan E, Karlyshev AV, Cronshaw AD, Wren BW. Identification of N-acetylgalactosamine-containing glycoproteins Peb3 and CgpA in Campylobacter jejuni. Mol Microbiol. 2002;43:497–508. doi: 10.1046/j.1365-2958.2002.02762.x. [DOI] [PubMed] [Google Scholar]
- 15.Glover KJ, Weerapana E, Chen MM, Imperiali B. Direct biochemical evidence for the utilization of UDP-bacillosamine by PglC, an essential glycosyl-1-phosphate transferase in the Campylobacter jejuni N-linked glycosylation pathway. Biochemistry. 2006;45:5343–5350. doi: 10.1021/bi0602056. [DOI] [PubMed] [Google Scholar]
- 16.Linton D, Dorrell N, Hitchen PG, Amber S, Karlyshev AV, Morris HR, Dell A, Valvano MA, Aebi M, Wren BW. Functional analysis of the Campylobacter jejuni N-linked protein glycosylation pathway. Mol Microbiol. 2005;55:1695–1703. doi: 10.1111/j.1365-2958.2005.04519.x. [DOI] [PubMed] [Google Scholar]
- 17.Glover KJ, Weerapana E, Imperiali B. In vitro assembly of the undecaprenylpyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation. Proc Natl Acad Sci U S A. 2005;102:14255–14259. doi: 10.1073/pnas.0507311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenhofen IC, McNally DJ, Vinogradov E, Whitfield D, Young NM, Dick S, Wakarchuk WW, Brisson JR, Logan SM. Functional characterization of dehydratase/aminotransferase pairs from Helicobacter and Campylobacter: Enzymes distinguishing the pseudaminic acid and bacillosamine biosynthetic pathways. J Biol Chem. 2006;281:723–732. doi: 10.1074/jbc.M511021200. [DOI] [PubMed] [Google Scholar]
- 19.Sharon N, Jeanloz RW. The diaminohexose component of a polysaccharide isolated from Bacillus subtilis. J Biol Chem. 1960;235:1–5. [PubMed] [Google Scholar]
- 20.Zehavi U, Sharon N. Structural studies of 4-acetamido-2-amino-2,4,6-trideoxy-D-glucose (N-acetylbacillosamine), the N-acetyldiamino sugar of Bacillus licheniformis. J Biol Chem. 1973;248:433–438. [PubMed] [Google Scholar]
- 21.Allard ST, Beis K, Giraud MF, Hegeman AD, Gross JW, Wilmouth RC, Whitfield C, Graninger M, Messner P, Allen AG, Maskell DJ, Naismith JH. Toward a structural understanding of the dehydratase mechanism. Structure (Camb) 2002;10:81–92. doi: 10.1016/s0969-2126(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 22.Creuzenet C, Lam JS. Topological and functional characterization of wbpm, an inner membrane UDP-GlcNAc C6 dehydratase essential for lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2001;41:1295–1310. doi: 10.1046/j.1365-2958.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- 23.Dawson RMC. Data for biochemical research. 3. Clarendon Press; Oxford: 1986. [Google Scholar]
- 24.Branch CL, Burton G, Moss SF. An expedient synthesis of allylic polyprenyl phosphates. Syn Commun. 1999;29:2639–2644. [Google Scholar]
- 25.Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. Better substrates for bacterial transglycosylases. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]
- 26.Hallis TM, Lei Y, Que NL, Liu H. Mechanistic studies of the biosynthesis of paratose: Purification and characterization of CDP-paratose synthase. Biochemistry. 1998;37:4935–4945. doi: 10.1021/bi9725529. [DOI] [PubMed] [Google Scholar]
- 27.Beaman TW, Sugantino M, Roderick SL. Structure of the hexapeptide xenobiotic acetyltransferase from Pseudomonas aeruginosa. Biochemistry. 1998;37:6689–6696. doi: 10.1021/bi980106v. [DOI] [PubMed] [Google Scholar]
- 28.Magnet S, Lambert T, Courvalin P, Blanchard JS. Kinetic and mutagenic characterization of the chromosomally encoded Salmonella enterica aac(6′)-iy aminoglycoside N-acetyltransferase. Biochemistry. 2001;40:3700–3709. doi: 10.1021/bi002736e. [DOI] [PubMed] [Google Scholar]
- 29.Sweet CR, Ribeiro AA, Raetz CR. Oxidation and transamination of the 3″-position of UDP-N-acetylglucosamine by enzymes from Acidithiobacillus ferrooxidans. Role in the formation of lipid A molecules with four amide-linked acyl chains. J Biol Chem. 2004;279:25400–25410. doi: 10.1074/jbc.M400596200. [DOI] [PubMed] [Google Scholar]
- 30.Goon S, Kelly JF, Logan SM, Ewing CP, Guerry P. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the cj1293 gene. Mol Microbiol. 2003;50:659–671. doi: 10.1046/j.1365-2958.2003.03725.x. [DOI] [PubMed] [Google Scholar]
- 31.Bernatchez S, Szymanski CM, Ishiyama N, Li J, Jarrell HC, Lau PC, Berghuis AM, Young NM, Wakarchuk WW. A single bifunctional UDP-GlcNAc/Glc 4-epimerase supports the synthesis of three cell surface glycoconjugates in Campylobacter jejuni. J Biol Chem. 2004;280:4792–4802. doi: 10.1074/jbc.M407767200. [DOI] [PubMed] [Google Scholar]
- 32.Kelly J, Jarrell H, Millar L, Tessier L, Fiori LM, Lau PC, Allan B, Szymanski CM. Biosynthesis of the N-linked glycan in Campylobacter jejuni and addition onto protein through block transfer. J Bacteriol. 2006;188:2427–2434. doi: 10.1128/JB.188.7.2427-2434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creuzenet C. Characterization of Cj1293, a new UDP-GlcNAc C6 dehydratase from Campylobacter jejuni. FEBS Lett. 2004;559:136–140. doi: 10.1016/S0014-5793(04)00057-2. [DOI] [PubMed] [Google Scholar]
- 34.Glover KJ, Weerapana E, Numao S, Imperiali B. Chemoenzymatic synthesis of glycopeptides with PglB, a bacterial oligosaccharyl transferase from Campylobacter jejuni. Chem Biol. 2005;12:1311–1315. doi: 10.1016/j.chembiol.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stimson E, Virji M, Makepeace K, Dell A, Morris HR, Payne G, Saunders JR, Jennings MP, Barker S, Panico M, et al. Meningococcal pilin: A glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S 1. Homonuclear decoupling spectra
Figure S 2. 31P NMR spectrum
Figure S 3. 1H COSY spectrum
Figure S 4. CE A262 elution profile of coupled reaction between PglF and PglE
Supporting Information Paragraph. Figures contained herein are various NMR spectra of UDP-Bac and CE elution profile of the coupled reaction between PglF and PglE: