Abstract
Borna disease virus (BDV) is a nonsegmented negative-strand RNA virus with a tropism for neurons. Infection with BDV causes neurological diseases in a wide variety of animal species. Although it is known that the virus spreads from neuron to neuron, assembled viral particles have never been visualized in the brains of infected animals. This has led to the hypothesis that BDV spreads as nonenveloped ribonucleoproteins (RNP) rather than as enveloped viral particles. We assessed whether the viral envelope glycoprotein (GP) is required for neuronal dissemination of BDV by using primary cultures of rat hippocampal neurons. We show that upon in vitro infection, BDV replicated and spread efficiently in this system. Despite rapid virus dissemination, very few infectious viral particles were detectable in the culture. However, neutralizing antibodies directed against BDV-GP inhibited BDV spread. In addition, interference with BDV-GP processing by inhibiting furin-mediated cleavage of the glycoprotein blocked virus spread. Finally, antisense treatment with peptide nucleic acids directed against BDV-GP mRNA inhibited BDV dissemination, marking BDV-GP as an attractive target for antiviral therapy against BDV. Together, our results demonstrate that the expression and correct processing of BDV-GP are necessary for BDV dissemination in primary cultures of rat hippocampal neurons, arguing against the hypothesis that the virus spreads from neuron to neuron in the form of nonenveloped RNP.
Borna disease virus (BDV) is the etiological agent of Borna disease, a neurological disorder of horses, sheep, and other farm animals (27). Recent evidence indicates that the natural host range, the geographic distribution, and the prevalence of BDV are much broader than previously thought (21, 40, 45). The spectrum of clinical diseases due to BDV infection ranges from subtle behavioral abnormalities (e.g., impairment of learning and memory) to progressive, immune-mediated meningoencephalitis (17, 38). There is considerable evidence that BDV also infects humans (5, 9, 36). Although it has been linked to certain neuropsychiatric disorders, the epidemiology and the clinical consequences of human infection with BDV remain controversial (2, 3, 5, 26, 41).
BDV is a nonsegmented negative-strand RNA virus (8, 43) that has the property, unique among members of the order Mononegavirales that infect animals, of transcribing and replicating its genome in the nucleus (7). The BDV genome consists of an 8.9-kb RNA, which includes at least six open reading frames (ORFs) (23). ORF I codes for the nucleoprotein (N) p40, ORF II codes for the phosphoprotein (P) p24, ORF III codes for the matrix protein p16, ORF IV codes for the glycoprotein (GP) p56, ORF V codes for the polymerase protein p180/190, and an ORF overlapping with the P gene codes for protein p10. Characterization of amino acid sequences of several BDV strains and isolates from various parts of the world revealed that BDV is a remarkably conserved virus with 84 to 95% amino acid identities among all gene products (37).
Sensitivities to detergents and organic solvents indicate that BDV is an enveloped virus (31), a suggestion that has been supported by electron microscopy data of cell-released BDV infectious particles (15, 24, 49). Viral GPs that are present at the surfaces of enveloped viruses have important functions (42). They mediate virus attachment to the cell surface receptors, thus serving as primary determinants of viral tropism, and they facilitate penetration by triggering fusion of the viral envelope with cell membranes.
GPs of nonsegmented negative-strand RNA viruses, comprising the families Paramyxoviridae, Filoviridae, and Rhabdoviridae, have been extensively studied (12). The Paramyxoviridae possess two integral membrane proteins with separate functions. Hemagglutinin is involved in cell attachment, and the fusion protein is responsible for fusion of the viral envelope with the cell membrane. By contrast, the Filoviridae and Rhabdoviridae have a single GP present at the viral envelope. Among the proteins encoded in the BDV genome, ORF IV encodes the single GP of BDV (15, 44). In BDV-infected cells, BDV-GP can be detected as two glycosylated proteins of 84 and 43 kDa (GP-84 and GP-43, respectively) (15). GP-84 corresponds to the glycosylated full-length product encoded by ORF IV, whereas GP-43 is derived from the C terminus of GP-84 after cleavage by the cellular protease furin (14, 39). Subcellular-fractionation studies and colocalization experiments have demonstrated that GP-84 accumulates in the endoplasmic reticulum, whereas GP-43 reaches the cell surface (15). Both products appear to be associated with infectious virions: GP-84 is thought to be involved in attachment of the virus to its cell surface receptor, and GP-43 is thought to be involved in pH-dependent fusion after internalization of the virion by endocytosis (14, 35).
BDV infection is noncytolytic in all cell systems examined so far (20) and requires cell-cell contact for its spread. Little or no infectious virus is released from BDV-infected cells (6), and virus yields are low even after enhancement of virus release by treatment of infected cells with osmotic shock or n-butyrate (15, 24). In addition, assembled virus is very rarely observed in infected cells and assembled viral particles have never been visualized in the brains of BDV-infected animals. BDV antigen distribution in the central nervous systems (CNS) of infected animals strongly suggests that BDV disseminates by cell-cell contact, possibly using the synaptic contacts between neurons (17). These observations, combined with the notion that ribonucleoproteins (RNP) of BDV are infectious after transfection to susceptible cells (7), have led to the hypothesis that dissemination of BDV in the CNS takes place as nonenveloped RNP rather than as enveloped viral particles (16). This would imply that after initial entry of BDV into its target cell, BDV-GP is dispensable for BDV dissemination.
In this study, we assessed whether BDV-GP is required for neuronal dissemination of BDV. We show that BDV replicates and spreads efficiently in in vitro-infected primary cultures of rat hippocampal neurons. During virus dissemination, very few infectious viral particles could be detected in the culture. However, neutralizing antibodies directed against BDV-GP completely inhibited BDV spread. In addition, interference with BDV-GP processing in infected neurons blocked virus spread. These results demonstrate the necessity of BDV-GP for BDV dissemination in neurons. Furthermore, antisense treatment with peptide nucleic acids that were directed against BDV-GP mRNA inhibited BDV dissemination in neurons, marking BDV-GP as an attractive target for antiviral therapy for BDV.
MATERIALS AND METHODS
Cells and virus.
Vero cells (ATCC CCL-81) were grown in Dulbecco's modified Eagle medium with Glutamax-1 (Gibco) supplemented with 10% fetal calf serum (FCS) and 1× Bufferall (Sigma). Primary hippocampal neurons were isolated from 1-day-old Sprague-Dawley rats (Janvier) (1). Briefly, the hippocampi were dissected and dissociated by mechanical trituration and digestion with phosphate-buffered saline (PBS) plus 0.3% Aspergillus protease (Sigma) for 10 min at room temperature. DNase 1 (Sigma) was then added to a final concentration of 1 mg/ml, and the mixture was incubated for 10 min at room temperature. After the addition of 35% FCS, the suspension was passed over a 70-μm-pore-size cell strainer and centrifuged at 400 × g for 10 min. After resuspension in Neurobasal medium (Gibco), the cell suspension was centrifuged at 400 × g through a 4% bovine serum albumin (BSA) cushion for 10 min. Then, the cells were seeded on poly(dl-ornithine) (Sigma)-laminin (Roche)-coated glass coverslips and grown in Neurobasal medium supplemented with 25 mM β-mercaptoethanol, 0.5 mM glutamine, 2% B-27 supplement (Gibco), and 2% FCS. After 24 h in culture, the mitotic inhibitors 5-fluoro-2′-deoxyuridine (10 mg/ml; Sigma) and uridine (25 mg/ml; Sigma) were added to limit the growth of glial cell contaminants. BDV infection was performed by adding cell-released virus (CRV) to the culture medium. We used the BDV laboratory strain He/80.
Reagents and antibodies.
We used decanoyl-Arg-Val-Lys-Arg-chloromethylketone (decRVKRcmk) (Furin Inhibitor I; Calbiochem) as a 1-mg/ml stock concentration in methanol. As primary antibodies, we used anti-microtubule-associated protein 2 (anti-MAP-2) (Sigma) and anti-N (clone 38/17C1) (46) mouse monoclonal antibodies; anti-GP (clone H12) rat monoclonal antibody; and anti-N, anti-P, and anti-GP rabbit polyclonal antibodies. As secondary antibodies, we used fluorescein isothiocyanate (FITC)-labeled anti-rabbit immunoglobulin G (IgG) and Cy3-labeled anti-mouse IgG (Interchim), biotinylated goat anti-rabbit IgG, horseradish peroxidase-labeled streptavidin, and R-phycoerythrin-labeled streptavidin (Southern Biotechnology Associates).
Preparation of cell-free BDV from Vero cells persistently infected with BDV (Vero-BV cells) and titration.
Cells were treated with 10 mM HEPES (pH 7.2 to 7.5) plus 250 mM MgCl2 for 90 min at 37°C to enhance virus release (15). The supernatants were collected and clarified, and the virus was pelleted by ultracentrifugation (220,000 × g for 135 min) through a 20% sucrose cushion in 10 mM HEPES- 75 mM NaCl- 2 mM EDTA (pH 7.2). Titration of cell-free BDV (in focus-forming units [FFU] per milliliter) was performed using an immunofocus assay as described previously (15).
Virus neutralization assay.
Undiluted normal rat serum (NRS), as well as H12 antibodies, was incubated at 56°C for 30 min in order to heat inactivate the complement. Then, antibody dilutions were mixed with a standard quantity of virus (100 FFU) and incubated for 90 min at 37°C. Subsequently, dilutions were plated on top of Vero cells and incubated for 4 days, after which the cells were fixed and processed for immunofluorescence assay for BDV antigens.
Immunofluorescence.
Cells grown on glass coverslips were fixed for 30 min at room temperature with 4% paraformaldehyde, followed by a 10-min fixation at room temperature with methanol-acetone (1:1). Then, the cells were rinsed with PBS and blocked overnight at 4°C with PBS plus 2% normal goat serum and 2% normal horse serum. Incubation for 1 h at room temperature with primary antibodies was followed by a 1-h incubation at room temperature with secondary antibodies or biotinylated antibodies, which was (if necessary) followed by a 1-h incubation at room temperature with labeled streptavidin. Anti-GP immunostaining was amplified using the TSA Fluorescein System (Tyramide signal amplification; NEN Life Science Products) according to the manufacturer's protocol. After extensive washes, the coverslips were mounted using Vectashield (Vector Laboratories).
PNA construction and synthesis.
Eighteenmer peptide nucleic acids (PNAs), directed against the BDV-GP mRNA (PNAG) or generated at random (PNAran), were synthesized. The PNAG sequence was N terminus-GCA TTG CCA GAC TAC CCC-C terminus; the PNAran sequence was N terminus-CAT ACT TGA CTC GTT ATC-C terminus.
To promote cellular import, the designed PNA was coupled to 16 amino acid residues (RQIKIWFQNRRMKWKK) derived from the third helix of the antennapedia homodomain (pAntp) of Drosophila (10). In addition, the PNA construct was linked covalently to the nuclear localization signal (NLS) of the simian virus 40 T antigen (PKKKRKV) (see Fig. 5). The solid-phase synthesis of peptides, as well as that of the PNA, was performed in a fully automated synthesizer (Syro II; MultiSyn Tech, Heidelberg, Germany) using the N-(9-fluoenyl)methoxycarbonyl strategy (29). At the N terminus of the PNA, a spacer of two lysines was introduced to prevent spatial binding hindrance of the subsequent NLS sequence, and one cysteine was introduced to enable linkage with the pAntp (4). All products were precipitated in ether and purified by preparative high-performance liquid chromatography (HPLC) (LC-8A; Shimadzu, Kyoto, Japan) on a YMC ODS-A 7A S-7-μm reverse-phase column (20 by 250 mm). The fractions corresponding to the purified conjugate were lyophilized. Sequences were characterized by analytical HPLC (Shimadzu LC-10) and laser desorption mass spectrometry (Vision 2000; Finnigan). The cysteines of the pAntp peptide and the PNA-NLS construct were oxidized at the range of 2 mg/ml in a 20% dimethyl sulfoxide water solution. Five hours later, the reaction was completed and the progress of oxidation was monitored by analytical C18 reverse-phase HPLC (Shimadzu LC-10).
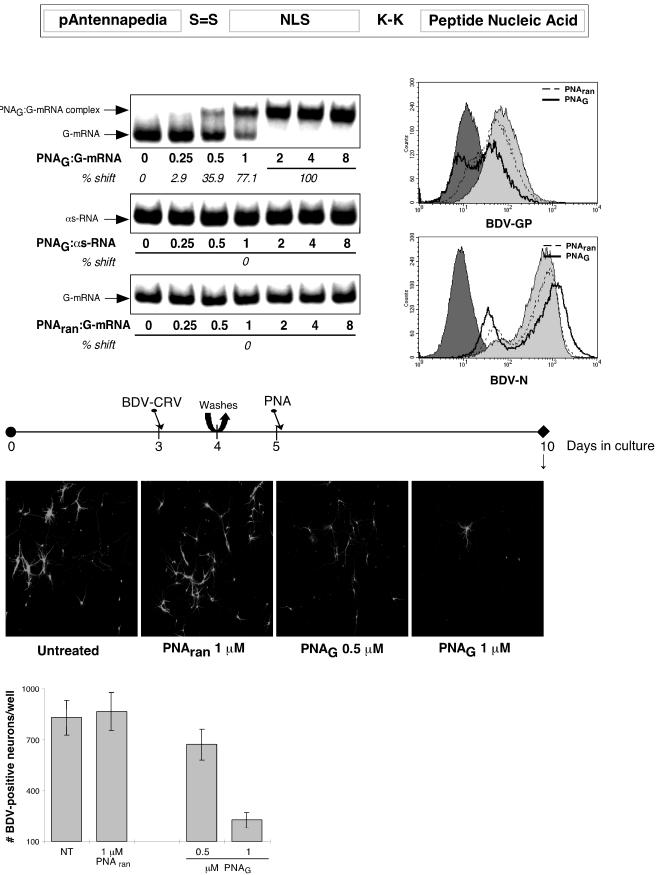
FIG. 5.
PNAs directed against BDV-GP mRNA block BDV dissemination in primary hippocampal neurons. (Top) Schematic representation of the constructs. To promote cellular import, the designed PNA was coupled to 16 amino acid residues derived from the third helix of the pAntp of Drosophila. Additionally, the PNA construct was linked covalently to an NLS sequence. At the N terminus of the PNA, a spacer of two lysines (K-K) was introduced to prevent spatial binding hindrance of the following NLS sequence, and one cysteine was introduced to enable linkage with the pAntp (4). On the left below the schematic is shown an electrophoretic mobility shift assay of PNAs with a random sequence (PNAran) or with a sequence directed against BDV-GP mRNA (PNAG) incubated with GP-mRNA or with GP-antisense (as) RNA. The arrows indicate the positions of uncomplexed RNAs and that of the PNAG- GP-mRNA complex. On the right below the schematic are histograms of the flow cytometric analyses of the expression of BDV-GP in Vero and Vero-BV cells. Vero-BV cells were treated with 1 μM PNAG or PNAran for 12 days or left untreated. The dark shading indicates background staining for BDV-GP in (uninfected) Vero cells; the light shading indicates expression levels of BDV-GP in untreated Vero-BV cells. In the middle of the figure is the experimental setup. Primary hippocampal neurons were infected with 250 FFU of BDV-CRV/well on day 3 of culture. On day 4, the cultures were washed twice with fresh medium, after which new medium was added. On day 5 of culture, PNAs were added. On day 10, samples were taken for immunofluorescence analysis and quantification. Below the timeline, immunofluorescence analysis is shown. BDV-N was detected with a FITC-labeled secondary antibody. Original magnification, ×100. (Bottom) Quantification of BDV-N-positive neurons per well on day 10 of culture. NT, not treated. Results are given for one representative experiment selected from a series of three independent experiments. The error bars indicate standard deviations.
Electrophoretic mobility shift assay.
Six micrograms of a plasmid containing the BDV-GP mRNA sequence was linearized by suitable restriction enzymes and in vitro transcribed (using T7 polymerase and T3 polymerase for the mRNA and antisense RNA, respectively) in the presence of [32P]UTP (10 mCi/ml) for 60 min at room temperature, followed by 60 min at 37°C. After in vitro transcription termination, radiolabeled probes were gel purified on a 5% denaturing polyacrylamide gel, and 20 × 103 cpm of probe was incubated with various molar concentrations of PNAG or PNAran in binding buffer (50 mM Tris-HCl [pH 7.8], 60 mM KCl, 5 mM MgCl2,10 mM dithiothreitol, 10% glycerol, 0.01% BSA, 0.01% NP-40 [Igepal; Sigma]) plus 500 ng of poly(rI-rC) per reaction for 2 h at 37°C. Then, samples were subjected to gel retardation analysis on native 6% polyacrylamide gels (Invitrogen) in Tris-borate-EDTA buffer at 100 to 150 V for 45 min at 4°C. Subsequently, the gels were dried and visualized using a PhosphorImager and quantified using Image-Quant software (Molecular Dynamics).
PNA treatment and flow cytometric analysis of Vero-BV cells.
Vero-BV cells (5 × 104) were incubated with PNAs for 4 h at 37°C in 100 μl of medium without FCS. Then, the cells and PNAs were diluted (final concentration of PNAs, 1 μM) in 2.4 ml of medium and plated in six-well plates. After 6 days of incubation, the cells were harvested by trypsin dissociation, and the exact procedure was repeated. After another 6 days of incubation, the cells were dissociated with trypsin, harvested, and fixed in 2% paraformaldehyde for 15 min at room temperature. After fixation, the cells were incubated twice for 10 min each time in reaction buffer (PBS- 1% BSA- 0.5% saponin) at room temperature and blocked for 45 min in 7% normal goat serum in reaction buffer at room temperature. The cells were incubated for 1 h with anti-GP or anti-N polyclonal antibody in reaction buffer on ice, washed extensively in reaction buffer, incubated for 30 min with biotinylated goat anti-rabbit IgG on ice, washed extensively, and incubated with R-phycoerythrin-labeled streptavidin in reaction buffer on ice. After being extensively washed in reaction buffer and PBS, the cells were analyzed using a FACScalibur (Becton-Dickinson).
RESULTS
BDV replicates and spreads efficiently in primary hippocampal neurons, while very few infectious viral particles are detectable in culture.
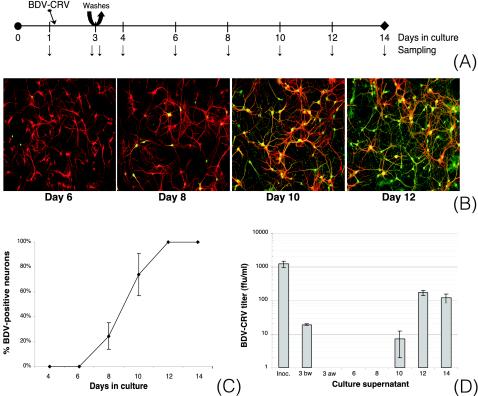
In vivo, BDV replicates and persists predominantly in neurons of the limbic system. Recently, it was shown in vitro that rat hippocampal neurons are highly susceptible to infection with cell-free BDV (1). In order to assess whether BDV-GP is required for neuronal dissemination of BDV, we used primary cultures of rat hippocampal neurons. Cells were infected with 500 FFU of BDV-CRV/well, corresponding to a multiplicity of infection (MOI) of ∼0.025 FFU/cell. The use of a low MOI allowed us to study viral dissemination occurring after primary infection. On days 1, 3 (before and after repeated changes of the culture medium), 4, 6, 8, 10, 12, and 14, samples were collected (Fig. 1A) and analyzed by immunofluorescence microscopy, and virus spread was quantified. BDV infection was first detectable on days 4 to 6, when <5% of all neurons were positive for BDV-N. Between day 6 and day 10, BDV spread rapidly, and by day 12, 100% of the neurons were infected (Fig. 1B and C). The distribution pattern of BDV-positive neurons strongly suggested that cell-cell contact was required for virus spread. On days 4 to 6, infected neurons were found isolated in the culture, as expected after primary infection with BDV-CRV at a low MOI. During virus dissemination, infected neurons were found either in foci or in direct contact with other infected neurons by means of neurites. BDV infection persisted for up to 22 days (the last time that was analyzed) and was noncytolytic (as assessed by trypan blue staining; data not shown).
FIG. 1.
BDV replicates and spreads efficiently in primary hippocampal neurons. (A) Experimental setup. Primary hippocampal neurons were infected with 500 FFU of BDV-CRV/well on day 1 of culture. On day 3, the cultures were washed twice with fresh medium, after which new medium was added. After various periods of incubation (arrows), samples were analyzed in different ways. (B) Double immunofluorescence analyses on different days of culture. BDV-N was detected with a rabbit polyclonal antibody, followed by a FITC-labeled secondary antibody (green), while the neuronal marker MAP-2 was detected with a mouse monoclonal antibody followed by a Cy3-labeled secondary antibody (red). Original magnification, ×100. (C) Quantification of virus spread. On different days of culture, percentages of BDV-N-positive neurons relative to MAP-2-positive neurons were assessed. (D) Analysis of BDV titers in culture supernatants. On different days of culture, viral titers in culture supernatants were determined using an immunofocus assay. BDV titers are given as FFU per milliliter of isolate. Results are given for one representative experiment selected from a series of three independent experiments. Inoc, inoculum; 3 bw, day 3 before washes; 3 aw, day 3 after washes. The error bars indicate standard deviations.
In addition to analyzing viral dissemination kinetics, we assessed BDV titers in culture supernatants (Fig. 1D). We confirmed that after the cultures were washed on day 3, no CRV originating from the inoculum was detectable in the culture. During the period of maximal virus dissemination (between days 6 and 10), viral titers in culture supernatants were negligible. When infection had reached 100% (from day 12 on), we were able to detect ∼100 FFU/ml in culture supernatants (Fig. 1D), corresponding to the production of ∼1 FFU/400 neurons. Analysis of viral titers after β-bungarotoxin-induced apoptosis (19) or total cell lysis on day 14 of culture resulted in titers of 500 to 1,000 FFU/ml (data not shown).
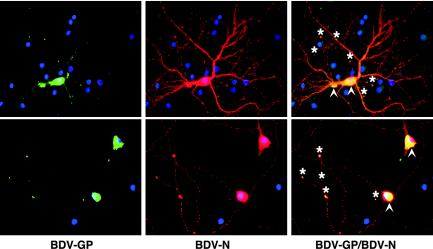
In infected neurons, BDV-GP is localized perinuclearly and at the termini of neurites.
To characterize the subcellular localization of BDV-GP in infected neurons, as well as to compare the expression of BDV-GP to that of BDV-N, we used immunofluorescence microscopy (Fig. 2). BDV-GP was detected mainly in the perinuclear region. This is in accordance with earlier studies that localized BDV-GP expression to the endoplasmic reticulum (15). In addition, BDV-GP expression was detected at the termini of neurites or at contact points of neurites. BDV-N was expressed in the nucleus, in the cytosol, and along neurites. Staining for BDV-N in neurites was punctate and colocalized with staining for BDV phosphoprotein (data not shown), consistent with transport of assembled RNP along neurites. BDV-N and BDV-GP colocalization was restricted to the perinuclear area and to those neuritic termini or contact points that were positive for BDV-GP. The lack of colocalization of BDV-N with BDV-GP along neurites suggests that nonenveloped RNP are transported separately from BDV-GP to neuronal termini. However, the possibility that the lack of colocalization may also be due to the known differences in the overall expressed amounts of BDV-N and BDV-GP cannot be excluded.
FIG. 2.
Subcellular localization of BDV-GP in infected neurons. (Left) BDV-GP was detected with a rabbit polyclonal antibody followed by a biotinylated secondary antibody, peroxidase-labeled streptavidin, and Tyramide signal amplification (green). (Middle) Note the punctate staining for BDV-N in neurites, detected with a mouse monoclonal antibody followed by a Cy3-labeled secondary antibody (red). (Right) Double immunofluorescence staining for BDV-GP (green) and BDV-N (red). Cell nuclei were counterstained with propidium iodide (blue). BDV-GP was mainly expressed perinuclearly (arrowheads), as well as at termini (asterisks) or contact points of neurites. Original magnification, ×400.
Neutralizing antibodies directed against BDV-GP block BDV dissemination in neurons.
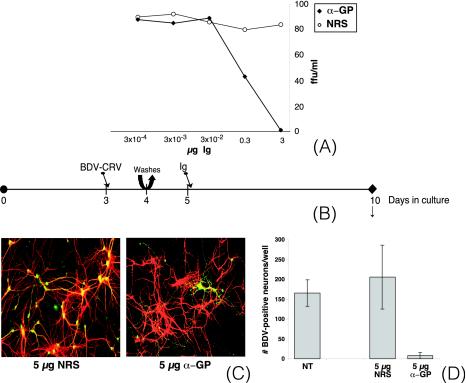
Monoclonal antibodies directed against BDV-GP were obtained after fusion of a mouse myeloma cell line with spleen cells of a BDV-infected rat that had been boosted with vaccinia virus expressing BDV-GP. The resulting hybridoma produces the monoclonal antibody H12, which has been shown to possess neutralizing capacity against BDV-CRV (13). In order to quantify the neutralizing capacities of H12 antibodies, we performed BDV-CRV neutralization assays using both H12 antibodies and NRS. Whereas NRS did not possess any neutralizing activity against BDV-CRV, the neutralizing capacity of H12 was determined to be 100 FFU of BDV-CRV/3 μg of Ig (Fig. 3A).
FIG. 3.
Neutralizing antibodies directed against BDV-GP block BDV dissemination in primary hippocampal neurons. (A) Seroneutralization assays. Aliquots (100 FFU) of purified BDV-CRV were incubated for 1 h at 37°C with serial dilutions of antisera (standardized for Ig content) and assayed for infectivity by immunofocus assay of Vero cells. α-GP, anti-GP antibodies. (B) Experimental setup. Primary hippocampal neurons were infected with 100 FFU of BDV-CRV/well on day 3 of culture. On day 4, the cultures were washed twice with fresh medium, after which new medium was added. On day 5 of culture, antibodies (standardized for Ig content) were added. On day 10, samples were taken for immunofluorescence analysis (C) and quantification (D). (C) Double immunofluorescence analysis. BDV-N was detected with a rabbit polyclonal antibody followed by a FITC-labeled secondary antibody (green), while MAP-2 was detected with a mouse monoclonal antibody followed by a Cy3-labeled secondary antibody (red). Original magnification, ×200. (D) Quantification of BDV-N-positive neurons per well on day 10 of culture. NT, not treated. The results are given for one representative experiment selected from a series of three independent experiments. The error bars indicate standard deviations.
Subsequently, we used H12 antibodies to study the role of the BDV-GP in BDV dissemination in neuronal cultures. One day after initial infection with BDV-CRV, the neurons were thoroughly washed, and 1 day later, controlled amounts of antibodies were added to the culture medium (Fig. 3B). Because of the modest neutralizing capacities of H12 antibodies, we used 100 FFU/well for primary infection instead of the 500 FFU/well described earlier (this resulted in delayed but comparable kinetics of infection; data not shown). Immunofluorescence analysis performed on day 10 revealed comparable levels of virus spread in untreated cultures and in cultures that were treated with NRS. Virus spread was characterized by foci of infected neurons (Fig. 3C). By contrast, BDV dissemination was totally inhibited in the presence of H12 antibodies. The few BDV-N-positive neurons detectable were found dispersed over the coverslip and were surrounded by uninfected neurons, suggesting that BDV positivity was due to primary infection rather than to virus dissemination. Quantification of BDV-N-positive neurons per well on day 10 of culture confirmed the inhibiting effect of the anti-GP antibody on BDV dissemination (Fig. 3D).
Inhibition of BDV-GP processing blocks viral dissemination in primary hippocampal neurons.
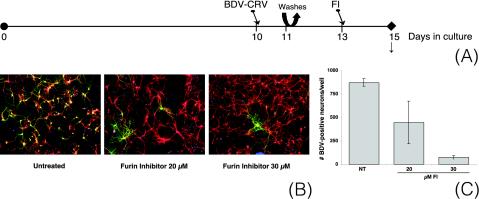
For full biological activity of the BDV-GP, proteolytic cleavage of the GP-84 precursor protein by the cellular protease furin is necessary (39). It was recently shown that treatment of Vero-BV cells with a furin inhibitor (FI) significantly decreased the production of infectious BDV (39). Since BDV-GP is the only BDV protein that possesses a furin cleavage site, this decrease was most likely due to interference with BDV-GP processing.
To study whether correct BDV-GP processing is necessary for virus dissemination in neurons, we used the FI decRVKRcmk. decRVKRcmk penetrates freely into cells, where it inhibits furin activity by mimicking the sequence motif of the furin cleavage site. To avoid possible effects of furin inhibition on the correct formation of synapses in the culture (48), we infected cultures on day 10 and started treatment with FI on day 13 (Fig. 4A).
FIG. 4.
The FI decRVKRcmk blocks BDV dissemination in primary hippocampal neurons. (A) Experimental setup. Primary hippocampal neurons were infected with 100 FFU of BDV-CRV/well on day 10 of culture. On day 11, the cultures were washed twice with fresh medium, after which new medium was added. On day 13 of culture, FI was added. On day 15, samples were taken for immunofluorescence analysis (B) and quantification (C). (B) Double immunofluorescence analysis. BDV-N was detected with a rabbit polyclonal antibody followed by a FITC-labeled secondary antibody (green), while MAP-2 was detected with a mouse monoclonal antibody followed by a Cy3-labeled secondary antibody (red). Cell nuclei were counterstained with propidium iodide (blue). Original magnification, ×100. (C) Quantification of BDV-N-positive neurons per well on day 15 of culture. NT, not treated. Results are given for one representative experiment selected from a series of three independent experiments. The error bars indicate standard deviations.
Immunofluorescence analysis on day 15 revealed productive virus spread in the absence of FI, characterized by foci of infected neurons (Fig. 4B). By contrast, BDV dissemination was totally inhibited by treatment with FI. As after treatment with neutralizing antibodies (see above), the distribution pattern of the few BDV-positive neurons suggested that they became infected as a result of the primary infection with BDV-CRV rather than as a result of virus dissemination. Quantification of BDV-N-positive neurons per well on day 15 of culture confirmed the inhibiting effect of FI on BDV dissemination and revealed that this effect was dose dependent (Fig. 4C).
Antisense treatment directed against BDV-GP mRNA blocks virus dissemination.
Since BDV-GP expression appeared to be crucial for BDV dissemination in neurons, it represented an attractive target for specific antiviral intervention. We chose to evaluate an antisense strategy that was directed against a sequence in the mRNA of BDV-GP (bp 934 to 951). As antisense agents, we made use of PNA constructs. PNAs are nucleic acid analogs with an achiral polyamide backbone consisting of N-(2-aminoethyl)glycine units (33). Those units bear the nucleobases that are attached through methylenecarbonyl linkers. PNAs are resistant to proteases and nucleases and thus are much more stable in cells than DNA or RNA. They bind to cRNA or DNA in a parallel or antiparallel orientation following the Watson-Crick base-pairing rules. The uncharged nature of the PNA oligomers even enhances the stability of the hybrid PNA-RNA (DNA) duplexes compared to natural homoduplexes (32). However, PNAs are poorly transported across cell membranes, which is a major obstacle to the application of PNAs as an antisense strategy. In order to promote cellular import, the designed PNA was coupled to 16 amino acid residues derived from the third helix of the pAntp of Drosophila, which has been reported to cross biological membranes by an energy-independent mechanism (10). Additionally, the PNA construct was linked covalently to an NLS sequence (Fig. 5) (4).
To characterize the binding specificities of the PNA constructs, we performed electrophoretic mobility shift assays (Fig. 5). These assays proved that the PNA construct that contained a sequence directed against BDV-GP mRNA (PNAG) bound specifically to the in vitro-produced target RNA sequence and not to the antisense RNA. In addition, we showed that the PNA with a random sequence (PNAran) did not recognize the BDV-GP target sequence.
Subsequently, we analyzed whether PNA treatment would lead to decreased expression levels of BDV-GP in infected cells. In order to be able to analyze the expression levels of BDV-GP by flow cytometry, we treated Vero-BV cells with PNA constructs (Fig. 5). Due to the persistent nature of BDV infection in Vero-BV cells, the cells contained considerable levels of BDV-GP at the start of treatment. Since the half-life of BDV-GP is unknown, we chose to treat Vero-BV cells for a period of 12 days before analysis. The analysis demonstrated that upon treatment with PNAG, BDV-GP expression levels in Vero-BV cells were decreased, whereas treatment with PNAran had no detectable effect. In addition, treatment with PNAG also slightly decreased the levels of BDV-N and BDV-P in Vero-BV cells (data not shown).
Encouraged by the effects of PNAs on persistently infected cells, we tested the potential of PNA treatment to inhibit BDV dissemination in acutely infected neurons. One day after initial infection with BDV-CRV, neurons were thoroughly washed, and 1 day later, PNAs were added to the culture medium (Fig. 5). Immunofluorescence analysis on day 10 revealed productive virus spread when cells were treated with PNAran, comparable to the amount of virus dissemination seen in untreated cells (Fig. 5). In contrast, BDV dissemination was progressively inhibited with increasing concentrations of PNAG, as could also be confirmed by quantitation of the BDV-N-positive neurons per well (Fig. 5). As after treatment with neutralizing antibodies and FI (see above), the distribution pattern of the few BDV-positive neurons suggested that infection of these neurons was due to primary infection rather than to virus dissemination.
DISCUSSION
In enveloped viruses like BDV, viral surface GPs are key factors in the initial infection of specific cell types via receptor recognition, as well as in the late steps of the replication cycle, namely, the assembly and release of infectious virions. Although there is consensus on the role of the BDV-GP in initial infection via receptor-mediated endocytosis, the role of the GP in BDV dissemination in the CNS is controversial (16). For example, assembled viral particles have never been visualized in the brains of BDV-infected animals, and little or no infectious virus is released from BDV-infected cells. In addition, BDV is noncytolytic in all cell systems examined so far and requires cell-cell contact for its spread. These observations, combined with the notion that RNP of BDV are infectious after transfection in susceptible cells (7), have led to the hypothesis that dissemination of BDV in the CNS takes place as nonenveloped complexes of RNP rather than as enveloped viruses. This would imply that once BDV gains entry into its initial target cells within the CNS, the BDV-GP is dispensable for its further dissemination.
Here, we analyzed whether BDV-GP is necessary for neuronal dissemination of BDV by using an in vitro approach. Since in vivo, BDV replicates and persists predominantly in neurons of the limbic system, we used primary cultures of rat hippocampal neurons. After in vitro infection with BDV-CRV, BDV efficiently replicated in cultured neurons, confirming previous observations (1). To selectively study the process of virus dissemination and to separate this process from the primary infection with BDV-CRV, we performed the initial infection with BDV-CRV at a low MOI (0.025 FFU/cell). In addition, we repeatedly changed the culture medium 1 to 2 days after the initial infection, thereby reducing the chances of infection by residual BDV-CRV from the original inoculum. Indeed, no residual BDV-CRV could be detected in the culture medium after the repeated washes had been performed (Fig. 1D). In the absence of residual BDV-CRV, BDV progressively disseminated in our cultured neurons to attain 100% infection within a 12-day period. The distribution pattern of infected neurons during BDV dissemination strongly suggested that the spread took place via cell-cell contacts, as is thought to occur in the CNS of infected animals. In addition, BDV persisted in culture without causing neuronal cell death or overt cytopathic effects. Taken together, these observations validate primary cultures of hippocampal neurons as an in vitro system to study BDV neuronal dissemination.
We report here that during BDV dissemination in neurons, very small amounts of viral particles were detectable in the supernatant (Fig. 1D). Even after cell lysis or β-bungarotoxin-induced apoptosis, the total number of cell-associated BDV viral particles obtained remained extremely low (on the order of 1 particle/50 to 100 cells), suggesting that the virus might indeed spread as nonenveloped RNP rather than as enveloped viral particles.
To our surprise, we found that neutralizing antibodies directed against BDV-GP completely inhibited BDV spread. Since antibody binding to viral GPs of Sindbis virus that are expressed on the cellular surface can inhibit viral replication (18), we examined whether anti-GP antibodies could have affected BDV replication in a similar way. We did not observe any inhibitory effects of antibody treatment on BDV replication, as analyzed by measuring the expression levels of BDV-N, either in Vero-BV cells or in infected neurons (data not shown). Also, when infected cultures that had been incubated with neutralizing antibodies for several days were washed, virus dissemination immediately followed antibody removal (data not shown). These results strongly suggest that binding of neutralizing antibodies to BDV-GP does not affect BDV replication efficacy but rather interferes directly with the role(s) of BDV-GP in the dissemination process.
A direct role for BDV-GP in BDV dissemination was confirmed by experiments in which BDV-GP processing in infected neurons was inhibited by treatment with an FI. The cellular protease furin cleaves BDV-GP during its maturation. Cell lysates of BDV-infected embryonic rabbit brain cells that had been treated with FI contained 2 log units fewer infectious viral particles than untreated cells. Full infectivity of the cell lysates could be restored by in vitro furin treatment, demonstrating that the correct processing of BDV-GP is important for the infectivity of viral particles (39). Using our dissemination assay in neurons, we showed that inhibition of BDV-GP processing also results in inhibition of BDV dissemination (Fig. 4). Taken together, these results demonstrate the necessity of correctly processed BDV-GP for BDV dissemination in neurons.
The requirement for BDV-GP in BDV neuronal dissemination is inconsistent with the hypothesis that BDV spreads as nonenveloped RNP. However, it also appears to be inconsistent with the small numbers of viral particles detectable in culture supernatants and in cell lysates during dissemination. A possible explanation is that expression of correctly processed BDV-GP on the surfaces of infected cells might induce local fusion of the infected neuron with its neighboring neuron, allowing passage of nonenveloped RNP. Fusion of infected neurons with uninfected neurons would then most likely take place at the synaptic termini, consistent with the subcellular staining pattern that was found for BDV-GP (Fig. 2). However, BDV-GP-mediated membrane fusion is strictly pH dependent and occurs only in an acidic environment, like intracellular endocytic vesicles (14). Since in the synaptic cleft, such low pH values are not encountered, we consider this possibility highly unlikely, although we cannot formally rule it out.
We rather consider the requirement for BDV-GP in BDV dissemination in neurons indicative of a crucial role for enveloped viral particles in this process. The small numbers of viral particles in culture supernatants, as well as in cell lysates, could be explained by the short half-life that BDV particles might have in vitro. The apparent lack of colocalization of BDV-GP with BDV-N along neurites and the accumulation of BDV-GP at the termini of neurites (Fig. 2) suggest that nonenveloped RNP may be transported separately from BDV-GP to neuronal termini, where local assembly of viral particles might take place. Such a mechanism has already been described for other neurotropic viruses, like herpes simplex virus type I and pseudorabies virus (30, 34, 47). After local viral-particle assembly and budding, a new cell will be infected by receptor-mediated endocytosis, and the envelope of the BDV particle will fuse with the membrane of the endocytic vesicle. The existence of viral particles in this process is temporary, and their half-lives after assembly will be highly dependent on the intercellular distance that needs to be crossed. If viral-particle assembly indeed takes place at neuritic termini, the distance to the closest cell will be very small (the synaptic cleft), resulting in short half-lives for BDV viral particles. This in turn would lead to low yields of viral particles, both in culture supernatants and in cell lysates, and it would also be consistent with the notorious difficulties that researchers have in visualizing intracellular BDV particles. It is clear that additional studies will be needed to better understand the mechanism of BDV dissemination in neurons.
As for all members of the order Mononegavirales, the expression levels of BDV proteins are relative to the positions of their ORFs in the genome. The closer the ORF is positioned to the 3′ end of the genome, the higher the expression level of the encoded protein. BDV-GP is encoded by ORF IV, and its expression levels are indeed known to be low compared to that of BDV-N (ORF I) or BDV-P (ORF II). With its low levels of expression and its crucial role in viral dissemination in neurons, BDV-GP represents an attractive target for antiviral therapy by antisense strategies. We chose to use cell-penetrating constructs of PNA (Fig. 5) that were directed against a sequence in the mRNA of BDV-GP. We demonstrated that PNA binds specifically to its target (Fig. 5) and that it effectively decreases expression levels of BDV-GP in Vero-BV cells (Fig. 5). The slightly decreased expression levels of BDV-N and BDV-P (data not shown) in those cells might be explained by binding of the PNA to its target sequence in the viral antigenome. Since the BDV antigenome is used as a template for replication of the BDV genomic RNA, PNA binding to the antigenome could in theory inhibit replication efficacy. To what extent such a mechanism contributes to the inhibitory effects of PNA treatment on BDV dissemination in neurons (Fig. 5) is unknown. PNA interference with human immunodeficiency virus infection has already proved to effectively inhibit viral replication in vitro (22, 25, 28), and this study underlines the therapeutic promise of antisense therapy by the use of cell-penetrating agents.
Recently, it has been shown that expression of the rabies virus GP is necessary for its dissemination in neurons, ending a debate on the possible transsynaptic transfer of rabies virus as nonenveloped RNP (11). In this paper, we demonstrate that the GP of BDV is required for BDV dissemination in primary cultures of rat hippocampal neurons, strongly suggesting that enveloped viral particles are crucial in this process and arguing against the idea that BDV spreads from neuron to neuron in the form of nonenveloped RNP.
Acknowledgments
This work was supported by grants from the Institut Pasteur, the Pasteur-Weizmann Council, the CNRS, and INSERM Avenir. J.J.B. is the recipient of a Marie Curie fellowship of the European Community program “Improving Human Research Potential and the Socioeconomic Knowledge Base” under contract number HPMF-CT-2000-01088. S.M. is the recipient of a DAAD Doktorandenstipendiums im Rahmen des gemeinsamen Hochschulsonderprogramms III von Bund und Länder.
We thank L. Stitz and O. Planz for generously making available the neutralizing monoclonal antibodies directed against BDV-GP and J. Debus and K. Braun for valuable advice and support with PNA synthesis. We thank J.-F. Bureau, S. B. Geutskens, A. Hans, L. Sartorius, and R. Volmer for critically reading the manuscript.
REFERENCES
- 1.Bajramovic, J. J., S. Syan, M. Brahic, J. C. De La Torre, and D. Gonzalez-Dunia. 2002. 1-β-d-Arabinofuranosylcytosine inhibits Borna disease virus replication and spread. J. Virol. 76:6268-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bode, L., P. Reckwald, W. E. Severus, R. Stoyloff, R. Ferszt, D. E. Dietrich, and H. Ludwig. 2001. Borna disease virus-specific circulating immune complexes, antigenemia, and free antibodies—the key marker triplet determining infection and prevailing in severe mood disorders. Mol. Psychiatry 6:481-491. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L., W. Zimmermann, R. Ferszt, F. Steinbach, and H. Ludwig. 1995. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat. Med. 1:232-236. [DOI] [PubMed] [Google Scholar]
- 4.Braun, K., P. Peschke, R. Pipkorn, S. Lampel, M. Wachsmuth, W. Waldeck, E. Friedrich, and J. Debus. 2002. A biological transporter for the delivery of peptide nucleic acids (PNAs) to the nuclear compartment of living cells. J. Mol. Biol. 318:237-243. [DOI] [PubMed] [Google Scholar]
- 5.Carbone, K. M. 2001. Borna disease virus and human disease. Clin. Microbiol. Rev. 14:513-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone, K. M., S. A. Rubin, A. M. Sierra-Honigmann, and H. M. Lederman. 1993. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J. Virol. 67:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Torre, J. C. 1994. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J. Virol. 68:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Torre, J. C., D. Gonzalez-Dunia, B. Cubitt, M. Mallory, N. Mueller-Lantzsch, F. A. Grässer, L. A. Hansen, and E. Masliah. 1996. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology 223:272-282. [DOI] [PubMed] [Google Scholar]
- 10.Derossi, D., S. Calvet, A. Trembleau, A. Brunissen, G. Chassaing, and A. Prochiantz. 1996. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271:18188-18193. [DOI] [PubMed] [Google Scholar]
- 11.Etessami, R., K. K. Conzelmann, B. Fadai-Ghotbi, B. Natelson, H. Tsiang, and P. E. Ceccaldi. 2000. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J. Gen. Virol. 81:2147-2153. [DOI] [PubMed] [Google Scholar]
- 12.Fields, B. N., D. M. Knipe, P. M. Howley, D. E. Griffin M. A. Martin, R. A. Lamb, B. Roizman, and S. E. Straus. 2001. Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 13.Furrer, E., T. Bilzer, L. Stitz, and O. Planz. 2001. Neutralizing antibodies in persistent Borna disease virus infection: prophylactic effect of gp94-specific monoclonal antibodies in preventing encephalitis. J. Virol. 75:943-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Dunia, D., B. Cubitt, and J. C. de la Torre. 1998. Mechanism of Borna disease virus entry into cells. J. Virol. 72:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Dunia, D., B. Cubitt, F. A. Grässer, and J. C. de la Torre. 1997. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J. Virol. 71:3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosztonyi, G., B. Dietzschold, M. Kao, C. E. Rupprecht, H. Ludwig, and H. Koprowski. 1993. Rabies and Borna disease. A comparative pathogenetic study of two neurovirulent agents. Lab. Investig. 68:285-295. [PubMed] [Google Scholar]
- 17.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis, p. 39-73. In H. Koprowski and I. Lipkin (ed.), Borna disease. Springer-Verlag KG, Berlin, Germany. [PubMed]
- 18.Griffin, D. E., S. Ubol, P. Despres, T. Kimura, and A. Byrnes. 2001. Role of antibodies in controlling alphavirus infection of neurons. Curr. Top. Microbiol. Immunol. 260:191-200. [DOI] [PubMed] [Google Scholar]
- 19.Herkert, M., O. Shakhman, E. Schweins, and C. M. Becker. 2001. Beta-bungarotoxin is a potent inducer of apoptosis in cultured rat neurons by receptor-mediated internalization. Eur. J. Neurosci. 14:821-828. [DOI] [PubMed] [Google Scholar]
- 20.Herzog, S., and R. Rott. 1980. Replication of Borna disease virus in cell cultures. Med. Microbiol. Immunol. 168:153-158. [DOI] [PubMed] [Google Scholar]
- 21.Ikuta, K., K. Hagiwara, H. Taniyama, and N. Nowotny. 2002. Epidemiology and infection of natural animal hosts, p. 87-124. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 22.Kaushik, N., A. Basu, P. Palumbo, R. L. Myers, and V. N. Pandey. 2002. Anti-TAR polyamide nucleotide analog conjugated with a membrane-permeating peptide inhibits human immunodeficiency virus type 1 production. J. Virol. 76:3881-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishi, M., K. Tomonaga, P. K. Lai, and J. C. de la Torre. 2002. Borna disease virus molecular virology, p. 23-44. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 24.Kohno, T., T. Goto, T. Takasaki, C. Morita, T. Nakaya, K. Ikuta, I. Kurane, K. Sano, and M. Nakai. 1999. Fine structure and morphogenesis of Borna disease virus. J. Virol. 73:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koppelhus, U., V. Zachar, P. E. Nielsen, X. Liu, J. Eugen-Olsen, and P. Ebbesen. 1997. Efficient in vitro inhibition of HIV-1 gag reverse transcription by peptide nucleic acid (PNA) at minimal ratios of PNA/RNA. Nucleic Acids Res. 25:2167-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipkin, W. I., M. Hornig, and T. Briese. 2001. Borna disease virus and neuropsychiatric disease—a reappraisal. Trends Microbiol. 9:295-298. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 28.Mayhood, T., N. Kaushik, P. K. Pandey, F. Kashanchi, L. Deng, and V. N. Pandey. 2000. Inhibition of Tat-mediated transactivation of HIV-1 LTR transcription by polyamide nucleic acid targeted to TAR hairpin element. Biochemistry 39:11532-11539. [DOI] [PubMed] [Google Scholar]
- 29.Merrifield, R. B. 1963. Merrifield solid-phase peptide synthesis (SPPS). J. Am. Chem. Soc. 85:2149-2152. [Google Scholar]
- 30.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolau, S., and I. A. Galloway. 1928. Borna disease and enzootic encephalomyelitis of sheep and cattle. Special Report Series, Med. Res. Council 121:7-90. [Google Scholar]
- 32.Nielsen, P. E. 2000. Antisense peptide nucleic acids. Curr. Opin. Mol. Ther. 2:282-287. [PubMed] [Google Scholar]
- 33.Nielsen, P. E., M. Egholm, R. H. Berg, and O. Buchardt. 1991. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254:1497-1500. [DOI] [PubMed] [Google Scholar]
- 34.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de La Torre. 2001. N-terminal domain of Borna disease virus g (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Planz, O., K. Bechter, and M. Schwemmle. 2002. Human Borna disease virus infection, p. 179-226. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 37.Pleschka, S., P. Staeheli, J. Kolodziejek, J. A. Richt, N. Nowotny, and M. Schwemmle. 2001. Conservation of coding potential and terminal sequences in four different isolates of Borna disease virus. J. Gen. Virol. 82:2681-2690. [DOI] [PubMed] [Google Scholar]
- 38.Pletnikov, M., D. Gonzalez-Dunia, and L. Stitz. 2002. Experimental infection: pathogenesis of neurobehavioral disease, p. 125-178. In K. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, D.C.
- 39.Richt, J. A., T. Furbringer, A. Koch, I. Pfeuffer, C. Herden, I. Bause-Niedrig, and W. Garten. 1998. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals, p. 17-30. In H. Koprowski and I. Lipkin (ed.), Borna disease. Springer-Verlag KG, Berlin, Germany. [DOI] [PubMed]
- 41.Rott, R., S. Herzog, B. Fleischer, A. Winokur, J. Amsterdam, W. Dyson, and H. Koprowski. 1985. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 228:755-756. [DOI] [PubMed] [Google Scholar]
- 42.Schlesinger, M. J., and S. Schlesinger. 1987. Domains of virus glycoproteins. Adv. Virus Res. 33:1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Schneider, P. A., C. G. Hatalski, A. J. Lewis, and W. I. Lipkin. 1997. Biochemical and functional analysis of Borna disease virus G protein. J. Virol. 71:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 46.Thiedemann, N., P. Presek, R. Rott, and L. Stitz. 1992. Antigenic relationship and further characterization of two major Borna disease virus-specific proteins. J. Gen. Virol. 73:1057-1064. [DOI] [PubMed] [Google Scholar]
- 47.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 48.Verderio, C., S. Coco, E. Pravettoni, A. Bacci, and M. Matteoli. 1999. Synaptogenesis in hippocampal cultures. Cell. Mol. Life Sci. 55:1448-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmermann, W., H. Breter, M. Rudolph, and H. Ludwig. 1994. Borna disease virus: immunoelectron microscopic characterization of cell-free virus and further information about the genome. J. Virol. 68:6755-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]