Abstract
The Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) functions to maintain latency in EBV-infected B lymphocytes. Although LMP2A is nonessential for the immortalization of B lymphocytes by EBV, its expression in B lymphocytes prevents viral reactivation by blocking B-cell receptor activation and signaling. LMP2A also provides an antiapoptotic signal in transgenic mice that express LMP2A in B lymphocytes. LMP2A activates phosphatidylinositol 3-kinase (PI3K) and the serine/threonine kinase Akt in lymphocytes and epithelial cells. Here we show that EBV LMP2A activates the PI3K and β-catenin signaling pathways in telomerase-immortalized human foreskin keratinocytes (HFK). LMP2A activated Akt in a PI3K-dependent manner, and the downstream Akt targets glycogen synthase kinase 3β (GSK3β) and the Forkhead transcription factor FKHR were phosphorylated and inactivated in LMP2A-expressing HFK cells. GSK3β is a negative regulator of the Wnt signaling pathway, and inactivation of GSK3β by LMP2A signaling led to stabilization of β-catenin, the central oncoprotein of Wnt signaling. In LMP2A-expressing cells, β-catenin accumulated in the cytoplasm and translocated into the nucleus via a two-step mechanism. The cytoplasmic accumulation of β-catenin downstream of LMP2A was independent of PI3K signaling, whereas its nuclear translocation was dependent on PI3K signaling. In the nucleus, β-catenin activated a reporter responsive to T-cell factor, and this activation was augmented by LMP2A coexpression. The Wnt pathway is inappropriately activated in 90% of colon cancers and is dysregulated in several other cancers, and these data suggest that activation of this pathway by LMP2A may contribute to the generation of EBV-associated cancers.
Epstein-Barr Virus (EBV) is an important human tumor virus that is causally associated with several human malignancies and can efficiently immortalize primary B lymphocytes after infection in vitro (24, 27, 52). Cancers linked to EBV include lymphomas that develop in the immunocompromised, a subset of Hodgkin's disease, endemic Burkitt's lymphoma, and the epithelial malignancy nasopharyngeal carcinoma (NPC) (44). EBV is strongly associated with NPC, where it is consistently detected in samples from areas of high and low incidence. EBV infection in NPC is latent, and the tumor cells contain the episomal form of the viral genome (42, 43). Specific viral gene products are consistently expressed in NPC, including EBNA1, latent membrane protein 1 (LMP1) and LMP2, and a series of transcripts of the BamHI-A region of the genome. Expression of the EBV oncoprotein, LMP1, is essential for the immortalization of B lymphocytes in vitro, and LMP1 can transform rodent fibroblasts (27). LMP1 is often coordinately expressed with LMP2A, and transcription of both genes is consistently detected in NPC (3, 4). In addition, NPC patients have high titers of antibodies against LMP2A, which suggests its expression at the protein level (30).
Although LMP2A is not essential for the immortalization of B lymphocytes by EBV, its expression in B cells plays a major role in the maintenance of latent infection (31, 34). LMP2A blocks B-cell receptor signaling, thereby preventing activation of the resting lymphocyte that leads to reactivation of the virus' lytic cycle (34, 35). In addition, LMP2A induces constitutive activation of survival pathways involving phosphatidylinositol 3-kinase (PI3K) and the serine/threonine kinase Akt, and activation of these pathways contributes to the survival of LMP2A-expressing B lymphocytes (53). In LMP2A transgenic mice, B lymphocytes that lack a functional B-cell receptor are able to bypass normal checkpoints in B-cell development, escape apoptosis, and survive in the periphery (5).
In order to determine the possible contribution of LMP2A to the development of epithelial malignancies, analysis of the effects of LMP2A expression and signaling in epithelial cells is needed. In the human keratinocyte cell line HaCaT, LMP2A expression induced activation of PI3K with subsequent phosphorylation and activation of Akt (47). Epithelial differentiation was inhibited when the LMP2A-expressing HaCaT cells were grown in organotypic raft cultures. The cells also displayed anchorage-independent growth, which was inhibited by treatment with PI3K inhibitors and, therefore, was dependent upon PI3K signaling. When injected into nude mice, LMP2A-expressing HaCaT cells gave rise to aggressive, poorly differentiated, and highly metastatic tumors (47). These tumors abundantly expressed LMP2A and contained high levels of the phosphorylated, activated form of Akt. These data suggest that LMP2A may similarly affect this PI3K/Akt pathway in the development of malignancies associated with EBV, such as NPC, in which the LMP2A transcript is present in abundance.
In both B lymphocytes and HaCaT cells, LMP2A signaling leads to Akt activation (47, 53). Akt is fully active when phosphorylated at Thr308 and Ser473, and this occurs after recruitment of Akt to the plasma membrane via interaction of its pleckstrin homology domain with 3′-phosphoinositides generated by activated PI3K (2). Targets of activated Akt include several proapoptotic proteins such as the Forkhead family of transciption factors, Bad, and procaspase 9 (2). These and other proapoptotic proteins are inactivated upon phosphorylation by Akt, indicating a prominent role for Akt in cell survival. In addition, Akt functions in glucose metabolism. Activated Akt phosphorylates and activates 6-phosphofructo-2-kinase, an enzyme involved in the regulation of glycolysis, and Akt phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β), a serine/threonine kinase that contributes to the regulation of glycogen metabolism (8, 11).
In addition to its role in metabolism, GSK3β functions in the Wnt signaling pathway, which is dysregulated in over 90% of human colon cancers (40, 57). GSK3β exists in a cytoplasmic complex with the tumor suppressors adenomatous polyposis coli (APC) and axin and the proto-oncogene β-catenin. In the absence of a Wnt signal, GSK3β constitutively phosphorylates β-catenin, leading to its ubiquitination and proteasome-mediated degradation (38). In the presence of a Wnt signal, the Disheveled (Dvl) protein is activated, and Dvl signaling leads to a block in the ability of GSK3β to associate with and phosphorylate β-catenin. As a result, β-catenin is stabilized, accumulates in the cytoplasm, and translocates into the nucleus. In the nucleus, β-catenin interacts with members of the T-cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors to activate expression of target genes such as CCND1 (cyclin D1) and c-myc (22, 50, 55). Constitutive nuclear β-catenin and expression of TCF/LEF target genes are hallmarks of the vast majority of colon cancers that have inappropriate activation of the Wnt pathway. APC and axin are considered tumor suppressor genes due to their inhibitory effects on this pathway, whereas the Wnt ligand protein and β-catenin have been shown to have oncogenic capacity (28, 40). Mutations affecting the GSK3β phosphorylation sites on β-catenin or mutations in APC, which also affect β-catenin turnover, have frequently been identified in colon, prostate, and skin cancers, as well as in medulloblastomas and hepatocellular carcinomas (38, 40). In carcinomas that lack mutations in APC or β-catenin, mutations in axin have frequently been identified, indicating that constitutive activation of this pathway can occur through multiple genetic events (1, 9, 45, 54).
A link between Akt activation and its effects on GSK3β and β-catenin activation has been demonstrated in some cell systems (12, 20, 21, 37, 46, 49). In some fibroblast cell lines, inactivation of GSK3β induces translocation of β-catenin to the nucleus with activation of TCF/LEF mediated transcription (51). In contrast, in NIH 3T3 cells or T lymphocytes, inactivation of GSK3β, by treatment with lithium chloride or by phosphorylation induced by constitutively activated Akt, did not affect β-catenin localization or TCF/LEF transcription (51, 59).
To further characterize the effects of LMP2A expression in epithelial cells on Akt activation and potential effects on β-catenin, LMP2A was expressed in a normal epithelial cell line derived from foreskin epithelial cells immortalized with the catalytic subunit of telomerase, denoted human foreskin keratinocytes (HFK) (15). In these cells, LMP2A expression induced phosphorylation and activation of Akt via PI3K signaling. The phosphorylated Akt was functional, as demonstrated by the phosphorylation of Akt targets GSK3β and the Forkhead family member FKHR. To determine whether the LMP2A-mediated effects on PI3K, Akt, and GSK3β affected the regulation of β-catenin and Wnt signaling in epithelial cells, this pathway was analyzed in LMP2A-expressing HFK cells. LMP2A expression resulted in dramatically increased levels of β-catenin in the cytoplasm and increased translocation of β-catenin to the nucleus. The translocated β-catenin was functional and, in reporter assays with a TCF-responsive reporter, LMP2A increased activation of the reporter by β-catenin. These data reveal that a human tumor virus protein activates the Wnt/β-catenin pathway through effects on PI3K, Akt, and GSK3β. The activation of the PI3K and β-catenin pathways by LMP2A suggests that activation of these pathways may be an important factor in the generation of EBV-associated epithelial cancers.
MATERIALS AND METHODS
Cell culture and retrovirus.
HFK cells (15) were maintained in keratinocyte serum-free medium (K-SFM) supplemented with 0.2 ng of epidermal growth factor (EGF)/ml, 30 μg of bovine pituitary extract (BPE)/ml, and 1% antibiotic-antimycotic solution (Gibco). HEK 293 cells were maintained in Dulbecco modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic solution. HFK and 293 cells were grown in a humidified incubator at 37°C with 5% CO2. To generate stable cell lines, recombinant retroviruses expressing either vector pBabe or pBabe subcloned with hemagglutinin (HA)-tagged LMP2A were generated as previously described and used to transduce HFK cells (47). A stable pool of HFK cells expressing either pBabe or pBabe-LMP2A was selected in the presence of 0.5 μg of puromycin (Sigma)/ml.
Plasmids, preparation of lysates, and fractionations.
Plasmids used for transient transfections included pSG5 (Stratagene), pcDNA3-Hyg (Invitrogen), pLMP2A-HA encoding HA-tagged LMP2A (16), XE28 encoding β-catenin and XE69 encoding green fluorescent protein (GFP)-tagged β-catenin (gifts from Randall T. Moon [36]), pSV-β-galactosidase (Promega), and pTOPFlash (pGL3-OT) and pFOPFlash (pGL3-OF) containing the luciferase gene under the control of a promoter with four wild-type or mutant TCF-responsive sites, respectively (gifts from B. Vogelstein [22]). Transfections were performed with FuGENE 6 (54) according to the manufacturer's instructions. Whole-cell lysates were prepared 48 h posttransfection by using Nonidet P-40 (NP-40) lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% NP-40, 0.4 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium vanadate (Na3VO4), and protease and phosphatase inhibitor cocktails (Sigma). Where indicated, cells were incubated with the PI3K inhibitor LY294002 (25 to 50 μM; Calbiochem) or dimethyl sulfoxide (DMSO; Sigma) vehicle control for 24 h prior to harvesting. Fractionations were performed by using OptiPrep (Gibco), as adapted from the manufacturer's protocol. Briefly, cells were resuspended in buffer A (20 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM PMSF, 1 mM Na3VO4, protease and inhibitor cocktails at 1:100) with 1% NP-40. Crude nuclei were pelleted at low speed, and the cytosolic fractions were extracted. Nuclei were purified over an OptiPrep gradient with 25, 30, and 35% layers and then lysed with hypotonic NE buffer (20 mM Tris-HCl, 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 0.5 mM PMSF, 1 mM Na3VO4, and protease and phosphatase inhibitor cocktails at 1:100).
Western blot analysis and antibodies.
Protein concentrations were determined with the Bio-Rad DC protein assay system (Bio-Rad). Lysates were boiled in sodium dodecyl sulfate (SDS) buffer for 5 min and subjected to SDS-7.5% polyacrylamide gel electrophoresis. Proteins were transferred to Optitran nitrocellulose membrane (Schleicher & Schuell) in a Bio-Rad transfer unit, and Western blot analysis was performed. The antibodies used included anti-HA, anti-actin, anti-GRP78, and anti-TCF-4 from Santa Cruz; anti-Akt, anti-phospho-Akt Ser473, anti-phospho-FKHR Ser256, and anti-phospho-GSK3β Ser9 from Cell Signaling; anti-β-catenin (Transduction Laboratories); anti-GFP (Living Colors); and anti-GSK3 (Upstate Biotechnology). Horseradish peroxidase-tagged secondary antibodies (Amersham Biosciences and Dako) and the Pierce SuperSignal West Pico System (Pierce) were used to detect antibody-bound proteins. Densitometry was performed by using Image J software (National Institutes of Health [NIH]), and values were normalized to actin levels.
Immunofluorescence.
HFK cells were plated onto chamber slides and transfected with vector, vector with XE69 (GFP-tagged β-catenin), or pLMP2A-HA with XE69. Three hours prior to fixation, a subset of the cells was stimulated with 10 mM LiCl, an inhibitor of GSK3β. Cells were rinsed with phosphate-buffered saline (PBS) and fixed in ice-cold methanol-acetone for 5 min at −20°C. Cells were blocked in 10% normal goat serum (NGS) in PBS and then incubated with anti-HA antibody (1:50; Santa Cruz) in 5% NGS in PBS for 1 h. After three 5-min washes in PBS, secondary antibody conjugated to Texas red (Jackson Laboratories) was used at a dilution of 1:100 in 5% NGS in PBS for 30 min. Staining with DAPI (4′,6′-diamidino-2-phenylindole) was performed at a concentration of 100 nM in PBS for 5 min to visualize nuclei. Slides were mounted by using Vectashield (Vector Laboratories) and viewed via fluorescent microscopy on a Zeiss Axioskop. Confocal microscopy was performed by using a Zeiss confocal laser scanning microscope.
Luciferase assays.
HEK 293 cells were transfected with TOPFlash or FOPFlash reporter plasmids (22), pSG5, XE28 (β-catenin), pLMP2A-HA, and pSV-β-galactosidase by using FuGENE 6 (54). Cells were lysed in GLO lysis buffer (Promega) 48 h posttransfection, and luciferase assays were performed by using the Steady-Glo luciferase assay system according to the manufacturer's instructions. Each condition was performed in triplicate in two separate experiments, and luciferase activity was normalized to β-galactosidase activity to control for transfection efficiency. TOPFlash activities were normalized to FOPFlash values for each condition, and the data were graphed as the fold activation.
RESULTS
LMP2A signaling in telomerase-immortalized HFK cells leads to activation of the PI3K/Akt signaling pathway.
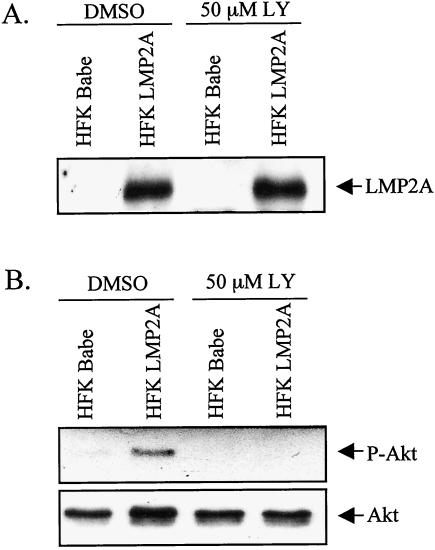
As previously shown, LMP2A expression and signaling in B lymphocytes and HaCaT cells induces activation of Akt in a PI3K-dependent manner (47, 53). To determine whether LMP2A consistently activates Akt in epithelial cells, LMP2A was expressed in the normal HFK cell line (15). Stable HFK cell lines expressing HA-tagged LMP2A in the pBabe vector or vector alone were established, and homogeneous pools were generated after selection. Immunoblot analysis confirmed LMP2A expression in the stable cell lines (Fig. 1A). To investigate the activation status of Akt in these cells, the stable cells were grown to 90 to 95% confluency and then treated with 50 μM LY294002 (LY), a PI3K inhibitor, or DMSO vehicle control for 24 h prior to harvest. Western blot analysis using a phosphospecific antibody against Ser473 was performed to detect activated Akt (Fig. 1B). Akt is activated in LMP2A-expressing cells but not in control cells. The activation of Akt was inhibited by treatment with the PI3K inhibitor LY. These data indicate that Akt is activated in these cells by LMP2A in a PI3K-dependent manner.
FIG. 1.
LMP2A activates Akt in a PI3K-dependent manner. HFK cells with stable expression of HA-tagged LMP2A or vector control (Babe) were cultured for 24 h in the presence of the PI3K inhibitor 50 μM LY or DMSO vehicle control. Western blot analyses of cell lysates were performed with anti-HA antibody to detect LMP2A expression (A) and antibodies that recognize phosphorylated Ser473 of activated Akt (B, top panel) or total Akt (B, bottom panel).
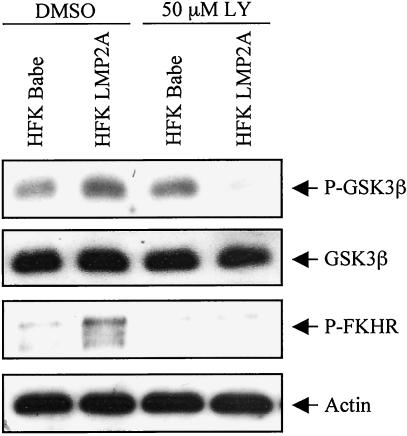
To identify downstream effects of LMP2A-induced Akt activation in HFK cells, the phosphorylation status of known Akt targets was analyzed. Akt phosphorylates and inactivates several different proteins, including the Forkhead family member FKHR and GSK3β (2). When Ser256 of FKHR is phosphorylated, it is sequestered in an inactive complex in the cytoplasm. GSK3β is also inactivated by phosphorylation on Ser9 by Akt. Using phospho-specific antibodies, immunoblot analyses revealed increased phosphorylation of the Akt targets GSK3β and FKHR in LMP2A-expressing cells relative to vector control cells that was sensitive to treatment with LY (Fig. 2). Interestingly, in the vector control cells, the baseline level of GSK3β phosphorylation was unaffected by LY treatment. This finding is consistent with the reported existence of additional kinases that can phosphorylate GSK3β independently of PI3K signaling. It is noteworthy, however, that the phosphorylation of GSK3β in LMP2A-expressing cells was sensitive to the PI3K inhibitor, indicating that PI3K signaling was responsible for the majority of GSK3β phosphorylation observed in these cells (Fig. 2). In summary, these data show that LMP2A expression in HFK cells leads to activation of Akt and phosphorylation of the Akt targets FKHR and GSK3β in a PI3K-dependent manner.
FIG. 2.
Akt targets are phosphorylated and inactivated in LMP2A-expressing cells. HFK cells with stable expression of LMP2A or vector control were cultured for 24 h in the presence of LY (50 μM) or DMSO. Western blot analyses of cell lysates were performed with antibodies to GSK3β phosphorylated at Ser9, FKHR phosphorylated at Ser256, and actin. The phospho-GSK3β immunoblot was reprobed with an antibody that recognizes total GSK3.
LMP2A signaling results in increased expression and nuclear accumulation of β-catenin.
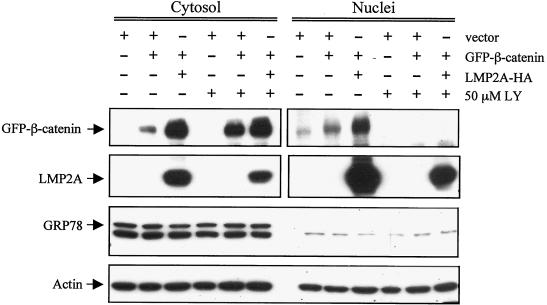
It is known that GSK3β binds to the axin complex, and it is thought that within this complex GSK3β phosphorylates β-catenin and targets it for ubiquitination and degradation (39). Phosphorylation of GSK3β by Akt has been shown to inactivate GSK3β kinase activity (8). However, GSK3β inactivation is not always linked to effects on β-catenin. For example, in CHO cells expressing insulin receptor (CHOIR) and 293 cells, GSK3β phosphorylation and inactivation by insulin signaling was not sufficient to induce β-catenin accumulation and activation of TCF/LEF-regulated transcription, whereas activation of Wnt signaling did not induce phosphorylation of GSK3β but did induce β-catenin accumulation and transcriptional activation (13). To determine whether the effects of LMP2A on Akt and GSK3β in HFK cells affect the β-catenin/Wnt pathway, the status of β-catenin in HFK cells was investigated. HFK cells were transfected with vector, vector plus GFP-tagged β-catenin, or LMP2A plus GFP-β-catenin. A subset of the cells was treated with the PI3K inhibitor LY (50 μM) for 24 h, and nuclear and cytosolic extracts were analyzed for GFP-β-catenin expression by immunoblot analysis with antibodies against GFP (Fig. 3). Increased levels of GFP-β-catenin expression were detected in the cytosol in the presence of LMP2A compared to the vector-transfected cells. Surprisingly, this increase in expression was not blocked by the PI3K inhibitor LY. To ensure that this increase in expression of the transfected GFP-β-catenin was not due to effects of LMP2A on the heterologous human cytomegalovirus promoter that regulates expression of the transfected GFP-β-catenin, reporter assays with β-galactosidase under the control of the cytomegalovirus promoter were performed. Cotransfected LMP2A did not affect expression levels or activity of the reporter, thereby confirming that LMP2A did not increase GFP-β-catenin levels via transcriptional effects on the expression plasmid (data not shown).
FIG. 3.
LMP2A induces increased cytosolic and nuclear accumulation of β-catenin. HFK cells were transfected with vector alone, vector plus GFP-tagged β-catenin, or HA-tagged LMP2A plus GFP-β-catenin. Cells were incubated with 50 μM LY for 24 h prior to harvest, and media supplements (EGF and BPE) were withheld from all cells during this time. Cells were fractionated into their cytosolic and nuclear components and subjected to Western blot analysis with antibodies to GFP, HA, GRP78 (an ER marker to assess the purity of the nuclear extract preparation), and actin (loading control).
Nuclear levels of GFP-β-catenin were also greatly enhanced by expression of LMP2A, and this translocation was inhibited by LY treatment (Fig. 3). These data indicate that LMP2A increases total levels of β-catenin independently of its effects on PI3K, whereas the LMP2A-induced increase in nuclear localization of β-catenin is dependent on PI3K activation. Although LMP2A is usually detected in the plasma membrane, we and others have detected LMP2A in the perinuclear region, and in these transfected cells, LMP2A was abundant in the nuclear fraction (Fig. 3) (10, 32, 48). Immunoblot analysis with antibodies against GRP78, a protein localized to the endoplasmic reticulum (ER), was performed to attest to the purity of the nuclear preparations. A nuclear protein with a slightly smaller molecular weight was detected in the vector control of the anti-GFP immunoblot and in all channels upon longer exposure.
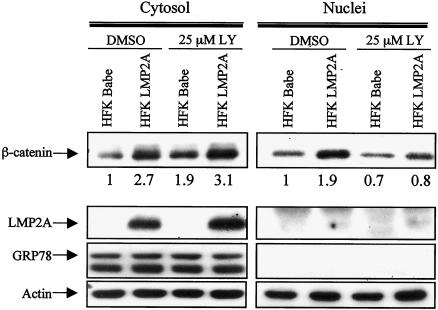
To determine whether LMP2A signaling also affects endogenous β-catenin levels, stable cell lines expressing pBabe or pBabe-LMP2A were treated with 25 μM LY or vehicle control for 24 h and then fractionated. Western blot analysis with an antibody against β-catenin revealed increased cytosolic levels of β-catenin in LMP2A-expressing cells compared to control cells, and this increase was not blocked by LY treatment (Fig. 4). Similar to the above data, the nuclei of the LMP2A-expressing cells also contained increased amounts of β-catenin relative to controls, and this increase in nuclear accumulation was inhibited by LY treatment. Densitometry was performed to quantify these effects, and levels of β-catenin were normalized to the corresponding actin levels. The fold activation relative to HFK Babe treated with DMSO was determined, and the nuclei of LMP2A-expressing cells contained a twofold increase in β-catenin levels relative to HFK Babe. After treatment with LY, the LMP2A-expressing and control cells contained approximately equal amounts of nuclear β-catenin, which was lower than the baseline levels of nuclear β-catenin of HFK Babe plus DMSO. These results indicate a PI3K signaling requirement for nuclear translocation of β-catenin. In addition, in these stable cells, the level of LMP2A expression was much lower than that observed in transient transfections, and LMP2A protein was not detected in the nuclear fraction of these cells. To confirm that LMP2A affected the levels of β-catenin protein and did not induce transcription of the β-catenin gene, RNase protection assays were performed with a probe specific for β-catenin mRNA. These assays revealed that LMP2A did not affect the levels of β-catenin mRNA (data not shown) and that the increase in β-catenin protein occurs posttranscriptionally.
FIG. 4.
Stable LMP2A expression results in increased cytosolic and nuclear accumulation of endogenous β-catenin. HFK cells stably expressing HA-tagged LMP2A or Babe vector were deprived of supplements (EGF and BPE) and incubated with 25 μM LY or DMSO control for 24 h prior to harvest and fractionation into cytosolic and nuclear components. Western blot analysis with an antibody that recognizes endogenous β-catenin was performed. Anti-HA, anti-GRP78, and anti-actin Western blots were included to show the corresponding LMP2A levels, attest to the purity of the fractionation procedure, and serve as a loading control, respectively. Densitometry with the Image J software was performed to normalize β-catenin levels to the corresponding actin levels. Fold increase (or decrease) of the normalized β-catenin values relative to the HFK Babe plus DMSO controls are indicated below the β-catenin immunoblot.
LMP2A enhances expression and nuclear localization of GFP-β-catenin.
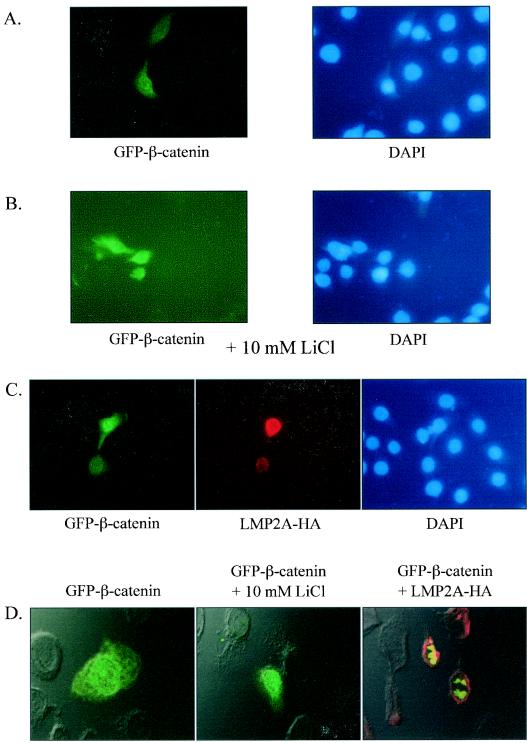
To determine whether β-catenin nuclear translocation could be induced in HFK cells by inactivation of GSK3β and to confirm the immunoblot analyses, cells were transfected with GFP-β-catenin and treated with lithium chloride (LiCl), a known inhibitor of GSK3β, or were cotransfected with LMP2A (23). GFP expression was visualized by fluorescent microscopy, and nuclei were identified by DAPI staining. Fluorescent microscopy revealed GFP-β-catenin expression throughout the cytoplasm of the cell (Fig. 5A, left panel). Pretreatment of the cells for 3 h with LiCl (10 mM) induced nuclear GFP-β-catenin expression (Fig. 5B, left panel). These data reveal that in these cells there is a direct link between GSK3β inactivation and nuclear translocation of β-catenin. Similarly, in the LMP2A-expressing cells, there was an increase both in the nuclear accumulation of GFP-β-catenin and intensity of expression of the protein (Fig. 5C, left panel). LMP2A expression was in the perinuclear distribution pattern (Fig. 5C, middle panel) that was observed in the previous analysis of transient-transfection and fractionation data (Fig. 3) and has been previously described in epithelial cells (10, 32, 48). The intensity of GFP-β-catenin expression and nuclear accumulation was proportional to the degree of LMP2A expression in the same cell. Analysis of the samples with confocal microscopy corroborated these findings and detected GFP-β-catenin throughout the cell in vector cotransfected cells (Fig. 5D, left panel) and in the nucleus in LiCl-treated and LMP2A-cotransfected cells (Fig. 5D, middle and right panels). These confocal analyses also confirmed that LMP2A was restricted to the perinuclear region and was not in the nucleus, whereas β-catenin was clearly localized within the nucleus. These data confirmed the fractionation and immunoblot data and indicate that LMP2A expression and the resultant inactivation of GSK3β directly affect β-catenin localization.
FIG. 5.
LMP2A enhances expression and induces nuclear localization of GFP-β-catenin. (A) HFK cells were cotransfected with GFP-β-catenin and vector control and examined via fluorescent microscopy (magnification, ×100). The corresponding DAPI-stained nuclei are also shown (right panel). (B) HFK cells transfected with GFP-β-catenin and vector control were treated for 3 h with the GSK3β inhibitor LiCl (10 mM) and then viewed by fluorescent microscopy (magnification, ×100). (C) HFK cells were cotransfected with GFP-β-catenin and HA-tagged LMP2A. LMP2A expression was revealed by immunofluorescence with a rabbit polyclonal antibody against HA and a secondary antibody conjugated to Texas red. (D) Confocal merges of fluorescence and phase contrast images of A to C are shown. The left panel depicts HFK cells cotransfected with GFP-β-catenin and vector, the middle panel shows HFK cells cotransfected with GFP-β-catenin and vector plus treatment with 10 mM LiCl, and the right panel displays HFK cells transfected with GFP-β-catenin and LMP2A-HA.
LMP2A coexpression enhances activation of a TCF-sensitive reporter by β-catenin.
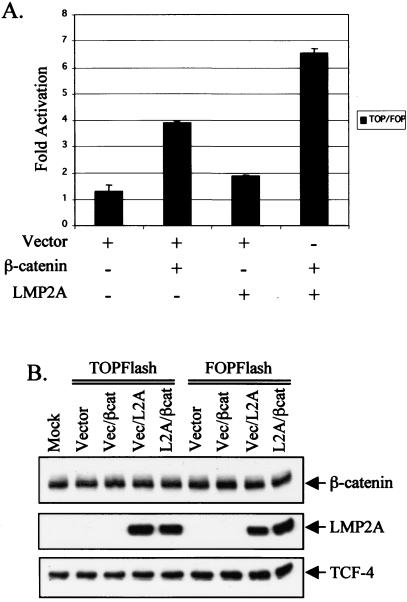
In the cell nucleus, β-catenin interacts with members of the TCF/LEF transcription factor family to activate expression of target genes such as c-myc and cyclin D1 (39). To determine whether the increased nuclear β-catenin in LMP2A-expressing cells activates gene transcription, assays with a reporter plasmid that contains four TCF4-responsive sites upstream of the luciferase gene (TOPFlash) and the corresponding negative control plasmid with mutated TCF sites (FOPFlash) were used for luciferase assays in the readily transfectable HEK 293 cell line (22). HEK 293 cells are frequently used for luciferase assays to assess activation of the Wnt pathway (6, 13, 22). The reporters were cotransfected with vector, vector plus β-catenin, vector plus pLMP2A-HA, or pLMP2A-HA plus β-catenin. A plasmid encoding SV-β-galactosidase was also included to normalize each assay for transfection efficiency. As expected, the negative control FOPFlash was not activated in any of the conditions, and TOPFlash values were normalized to the corresponding FOPFlash activity. Cotransfection with β-catenin activated the TOPFlash reporter approximately three to fourfold over the vector control, whereas cotransfection with LMP2A led to an approximate twofold increase over the vector control (Fig. 6A). Consistent with the effects of LMP2A on nuclear transport of β-catenin, cells cotransfected with LMP2A and β-catenin exhibited an approximate sixfold increase in TOPFlash luciferase activity (Fig. 6A). Immunoblot analysis confirmed the presence of transfected LMP2A and revealed equal levels of the TCF-4 transcription factor (Fig. 6B). The addition of transfected β-catenin was less discernible due to the high endogenous levels of the protein in 293 cells (Fig. 6B). These data reveal that LMP2A not only increases β-catenin expression levels and nuclear accumulation but also leads to enhanced activation of TCF-mediated transcription by β-catenin.
FIG. 6.
LMP2A augments activation of the TOPFlash luciferase reporter by β-catenin. (A) 293 cells were transfected with SV-β-galactosidase and either TOPFlash or FOPFlash plus vector, β-catenin, LMP2A, or β-catenin with LMP2A. TOPFlash contains four consensus TCF-4 binding sites in a promoter driving expression of the luciferase gene, and FOPFlash is an analogous construct with the four TCF-4 sites mutated. Transfections and assays were performed in triplicate in two separate experiments, and luciferase activity was normalized to β-galactosidase activity to control for transfection efficiency. The data are presented graphically as TOPFlash activity normalized to FOPFlash activity for each of the indicated conditions. (B) Immunoblot analyses were performed on the lysates used in the luciferase assay described above. Antibodies against HA confirm the presence of transfected HA-tagged LMP2A. Transfected and endogenous β-catenin levels were revealed with an anti-β-catenin antibody, and immunoblot with an antibody against TCF-4 attested to equal levels of this transcription factor in the transfected 293 cells.
DISCUSSION
This study reveals that the EBV protein LMP2A activates PI3K/Akt signaling and the Wnt/β-catenin signaling pathways in normal keratinocytes (HFK). Expression of LMP2A in HFK cells led to the PI3K-dependent phosphorylation and activation of Akt and the subsequent phosphorylation and inactivation of the Akt targets GSK3β and FKHR. Upon inactivation of the negative Wnt signaling regulator GSK3β, β-catenin accumulated in the cytoplasm, translocated into the nucleus, and activated a TCF-responsive reporter.
Interestingly, the increase in cytosolic levels of β-catenin and its translocation into the nucleus occur via a two-step process downstream of LMP2A signaling. The increase in cytosolic β-catenin in LMP2A-expressing HFK cells is not dependent on PI3K signaling, since it is not inhibited by the PI3K inhibitor LY, and yet the nuclear translocation step does require PI3K signaling (Fig. 3 and 4). In prototypical Wnt signaling, inhibition of GSK3β activity is followed by an increase in cytoplasmic β-catenin levels. This effect, however, is not dependent on the phosphorylation of Ser9 of GSK3β in all contexts. Ding et al. showed that both Wnt and insulin signaling in C57MG mammary epithelial cells and CHOIR cells led to inhibition of GSK3β kinase activity, but only insulin triggered increased phosphorylation at Ser9 (13). Therefore, Wnt-mediated inhibition of GSK3β kinase activity is possibly due to disruption of protein-protein interactions. These data suggest the possible existence of two different pools of GSK3β that can be differentially regulated by various signaling pathways. Our finding that LY treatment of the control cells did not block phosphorylation of endogenous GSK3β and yet did block nuclear translocation of β-catenin also suggests the existence of two pools of GSK3β with different targets. Fukumoto et al. reported that Dvl, a member of the Wnt signaling pathway, stimulated Ser9 phosphorylation of GSK3β associated with the axin complex, likely through enhancing the association of activated Akt with the complex (18). Furthermore, Gas6 signaling through the Axl receptor tyrosine kinase in C57MG cells led to increased phosphorylation of both Akt and GSK3β (at Ser9), and this was accompanied by an increase in cytosolic β-catenin levels (20). These studies and others suggest that a high level of complexity exists within the Wnt pathway and that other signaling pathways cross-regulate it in particular cellular contexts or in response to specific stimuli. GSK3β seems a likely candidate at which signaling pathways such as the Wnt and PI3K pathways might converge in some circumstances.
Although the cytosolic increase in β-catenin levels downstream of LMP2A signaling in HFK cells was not dependent on PI3K signaling, the nuclear translocation of β-catenin required PI3K activation and signaling, as has also been suggested by several previous studies. In murine B cells, cytoplasmic and nuclear β-catenin levels increased upon B-cell receptor engagement via a PKC-dependent mechanism, and inhibition of PI3K signaling by treatment with LY or wortmannin inhibited nuclear β-catenin accumulation (7). In addition, treatment of human alveolar macrophages with lipopolysaccharide led to nuclear accumulation and transcriptional activity of β-catenin, and these effects were inhibited by LY (37). These reports, in addition to the experiments presented here, indicate that PI3K signaling is essential for nuclear import of β-catenin. It is unclear, however, at what stage of nuclear translocation this regulation occurs and what downstream PI3K signaling mediators are required for the cytoplasmic-to-nuclear translocation of β-catenin. As shown in Fig. 5, lithium-mediated inactivation of GSK3β led to nuclear translocation of β-catenin in HFK cells, thereby implicating GSK3β inhibition in this process.
This is the first report that a human tumor virus protein, LMP2A, activates Wnt signaling through effects on PI3K, Akt, and GSK3β. Simian virus 40 large and small T antigens and polyomavirus middle T antigen have been shown to activate Akt (33, 58, 60), and the large T antigen of JC virus (JCV), a human polyomavirus, has been shown to interact with β-catenin in cotransfected HCT116 colon cancer cells and activate a c-myc promoter (14). Furthermore, there were increased β-catenin and LEF-1 levels in the nuclei of T-antigen-positive cells of mouse medulloblastomas that developed in transgenic mice expressing the early genome of JCV, and a corresponding increase in the mRNA and protein levels of the β-catenin/LEF-1 target c-myc was evident (19). It is likely, therefore, that JCV large T antigen also activates β-catenin signaling, possibly via an intranuclear interaction directly with β-catenin/LEF-1 transcription complexes. In a recent report, the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen (LANA) was shown to interact with GSK3β and sequester it in the nucleus, thereby allowing β-catenin to accumulate and signal in primary effusion lymphoma cells (17). Therefore, the Wnt pathway is targeted by two different oncogenic herpesvirus proteins, LMP2A and LANA, via distinct mechanisms, indicating the importance of this pathway in the genesis of herpesvirus-associated tumors.
These data reveal that LMP2A has at least two distinct effects on the process of β-catenin activation and signaling: cytoplasmic accumulation and nuclear translocation. Interestingly, LMP2A has been shown to interact with the Nedd4 family of ubiquitin ligases (25, 26, 56). These interactions allow LMP2A to manipulate turnover of itself and other proteins, such as the Lyn kinase, in B lymphocytes. It is possible, therefore, that LMP2A might interact with ubiquitin ligases involved in turnover of β-catenin, and the best-characterized ligase in this role is the F-box protein β-TrCP (29). Alternatively, LMP2A might affect the turnover of other proteins involved in the Wnt signaling pathway or other pathways that might indirectly regulate β-catenin signaling. LMP2A has recently been shown to alter expression levels of LEF/TCF family members. In bone marrow B lymphocytes of LMP2A transgenic mice, there was a 3.2-fold decrease in LEF-1 expression and a 2.6-fold increase in TCF-1 expression as determined by microarray analysis (41). The effects on expression of factors involved in LMP2A-mediated signaling may also affect β-catenin-regulated transcription.
In summary, the data presented here show activation of the PI3K/Akt pathway by LMP2A in epithelial cells. Activation of the PI3K/Akt pathway by LMP2A induced the inactivation of GSK3β, increased cytoplasmic levels of β-catenin, and stimulated nuclear translocation of β-catenin. The increase in nuclear β-catenin expression induced by LMP2A signaling was followed by upregulation of β-catenin-induced transcriptional activity. Importantly, this is a report of a human tumor virus protein that activates the Wnt/β-catenin signaling pathway through effects on PI3K, Akt, and GSK3β, and these data highlight the potential importance of LMP2A and the PI3K/β-catenin pathway in the development of EBV-associated malignancies.
Acknowledgments
This work was supported by NIH grant CA32979.
We thank David Everly for performing the RNase protection assays and Greg Hong for assistance in generating the stable cell lines. We also thank Shannon Kenney and Blossom Damania for critical reading of the manuscript.
REFERENCES
- 1.Baeza, N., J. Masuoka, P. Kleihues, and H. Ohgaki. 2003. AXIN1 mutations but not deletions in cerebellar medulloblastomas. Oncogene 22:632-636. [DOI] [PubMed] [Google Scholar]
- 2.Brazil, D. P., and B. A. Hemmings. 2001. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 26:657-664. [DOI] [PubMed] [Google Scholar]
- 3.Brooks, L., Q. Y. Yao, A. B. Rickinson, and L. S. Young. 1992. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 66:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busson, P., R. McCoy, R. Sadler, K. Gilligan, T. Tursz, and N. Raab-Traub. 1992. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J. Virol. 66:3257-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B-cell development and survival in the absence of normal B-cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 6.Chen, R. H., W. V. Ding, and F. McCormick. 2000. Wnt signaling to β-catenin involves two interactive components: glycogen synthase kinase-3β inhibition and activation of protein kinase C. J. Biol. Chem. 275:17894-17899. [DOI] [PubMed] [Google Scholar]
- 7.Christian, S. L., P. V. Sims, and M. R. Gold. 2002. The B-cell antigen receptor regulates the transcriptional activator β-catenin via protein kinase C-mediated inhibition of glycogen synthase kinase-3. J. Immunol. 169:758-769. [DOI] [PubMed] [Google Scholar]
- 8.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 9.Dahmen, R. P., A. Koch, D. Denkhaus, J. C. Tonn, N. Sorensen, F. Berthold, J. Behrens, W. Birchmeier, O. D. Wiestler, and T. Pietsch. 2001. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 61:7039-7043. [PubMed] [Google Scholar]
- 10.Dawson, C. W., J. H. George, S. M. Blake, R. Longnecker, and L. S. Young. 2001. The Epstein-Barr virus encoded latent membrane protein 2A augments signaling from latent membrane protein 1. Virology 289:192-207. [DOI] [PubMed] [Google Scholar]
- 11.Deprez, J., D. Vertommen, D. R. Alessi, L. Hue, and M. H. Rider. 1997. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem. 272:17269-17275. [DOI] [PubMed] [Google Scholar]
- 12.Desbois-Mouthon, C., A. Cadoret, M. J. Blivet-Van Eggelpoel, F. Bertrand, G. Cherqui, C. Perret, and J. Capeau. 2001. Insulin and IGF-1 stimulate the β-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene 20:252-259. [DOI] [PubMed] [Google Scholar]
- 13.Ding, V. W., R. H. Chen, and F. McCormick. 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275:32475-32481. [DOI] [PubMed] [Google Scholar]
- 14.Enam, S., L. Del Valle, C. Lara, D. D. Gan, C. Ortiz-Hidalgo, J. P. Palazzo, and K. Khalili. 2002. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and β-catenin. Cancer Res. 62: 7093-7101. [PubMed]
- 15.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fruehling, S., R. Swart, K. M. Dolwick, E. Kremmer, and R. Longnecker. 1998. Tyrosine 112 of latent membrane protein 2A is essential for protein tyrosine kinase loading and regulation of Epstein-Barr virus latency. J. Virol. 72:7796-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of β-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 18:18. [DOI] [PubMed] [Google Scholar]
- 18.Fukumoto, S., C. M. Hsieh, K. Maemura, M. D. Layne, S. F. Yet, K. H. Lee, T. Matsui, A. Rosenzweig, W. G. Taylor, J. S. Rubin, M. A. Perrella, and M. E. Lee. 2001. Akt participation in the Wnt signaling pathway through Disheveled. J. Biol. Chem. 276:17479-17483. [DOI] [PubMed] [Google Scholar]
- 19.Gan, D. D., K. Reiss, T. Carrill, L. Del Valle, S. Croul, A. Giordano, P. Fishman, and K. Khalili. 2001. Involvement of Wnt signaling pathway in murine medulloblastoma induced by human neurotropic JC virus. Oncogene 20:4864-4870. [DOI] [PubMed] [Google Scholar]
- 20.Goruppi, S., C. Chiaruttini, M. E. Ruaro, B. Varnum, and C. Schneider. 2001. Gas6 induces growth, β-catenin stabilization, and T-cell factor transcriptional activation in contact-inhibited C57 mammary cells. Mol. Cell. Biol. 21:902-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto, M., Y. Sagara, D. Langford, I. P. Everall, M. Mallory, A. Everson, M. Digicaylioglu, and E. Masliah. 2002. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3β pathway. Implications for neuroprotection. J. Biol. Chem. 277:32985-32991. [DOI] [PubMed] [Google Scholar]
- 22.He, T. C., A. B. Sparks, C. Rago, H. Hermeking, L. Zawel, L. T. da Costa, P. J. Morin, B. Vogelstein, and K. W. Kinzler. 1998. Identification of c-MYC as a target of the APC pathway. Science 281:1509-1512. [DOI] [PubMed] [Google Scholar]
- 23.Hedgepeth, C. M., L. J. Conrad, J. Zhang, H. C. Huang, V. M. Lee, and P. S. Klein. 1997. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 185:82-91. [DOI] [PubMed] [Google Scholar]
- 24.Henderson, E., G. Miller, J. Robinson, and L. Heston. 1977. Efficiency of transformation of lymphocytes by Epstein-Barr virus. Virology 76:152-163. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda, M., A. Ikeda, L. C. Longan, and R. Longnecker. 2000. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology 268:178-191. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda, M., A. Ikeda, and R. Longnecker. 2001. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J. Virol. 75:5711-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr Virus and Its Replication, p. 2511-2573. In D. M. Knipe (ed.), Field's Virology, Fourth ed., vol. 2. Lippincott/Williams & Wilkins Publishers, Philadelphia, Pa.
- 28.Korinek, V., N. Barker, P. J. Morin, D. van Wichen, R. de Weger, K. W. Kinzler, B. Vogelstein, and H. Clevers. 1997. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784-1787. [DOI] [PubMed] [Google Scholar]
- 29.Latres, E., D. S. Chiaur, and M. Pagano. 1999. The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18:849-854. [DOI] [PubMed] [Google Scholar]
- 30.Lennette, E. T., G. Winberg, M. Yadav, G. Enblad, and G. Klein. 1995. Antibodies to LMP2A/2B in EBV-carrying malignancies. Eur. J. Cancer 31A:1875-1878. [DOI] [PubMed] [Google Scholar]
- 31.Longnecker, R., C. L. Miller, X. Q. Miao, A. Marchini, and E. Kieff. 1992. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro; LMP2A is therefore nonessential. J. Virol. 66:6461-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch, D. T., J. S. Zimmerman, and D. T. Rowe. 2002. Epstein-Barr virus latent membrane protein 2B (LMP2B) colocalizes with LMP2A in perinuclear regions in transiently transfected cells. J. Gen. Virol. 83:1025-1035. [DOI] [PubMed] [Google Scholar]
- 33.Meili, R., P. Cron, B. A. Hemmings, and K. Ballmer-Hofer. 1998. Protein kinase B/Akt is activated by polyomavirus middle-T antigen via a phosphatidylinositol 3-kinase-dependent mechanism. Oncogene 16:903-907. [DOI] [PubMed] [Google Scholar]
- 34.Miller, C. L., J. H. Lee, E. Kieff, and R. Longnecker. 1994. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. USA 91:772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, C. L., R. Longnecker, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J. Virol. 67:3087-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. R., and R. T. Moon. 1997. Analysis of the signaling activities of localization mutants of β-catenin during axis specification in Xenopus. J. Cell Biol. 139:229-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monick, M. M., A. B. Carter, P. K. Robeff, D. M. Flaherty, M. W. Peterson, and G. W. Hunninghake. 2001. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J. Immunol. 166:4713-4720. [DOI] [PubMed] [Google Scholar]
- 38.Morin, P. J. 1999. β-Catenin signaling and cancer. Bioessays 21:1021-1030. [DOI] [PubMed] [Google Scholar]
- 39.Novak, A., and S. Dedhar. 1999. Signaling through β-catenin and Lef/Tcf. Cell Mol. Life Sci. 56:523-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 41.Portis, T., and R. Longnecker. 2003. Epstein-Barr virus LMP2A interferes with global transcription factor regulation when expressed during B-lymphocyte development. J. Virol. 77:105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 43.Raab-Traub, N., R. Hood, C. S. Yang, B. Henry III, and J. S. Pagano. 1983. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J. Virol. 48:580-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe et al. (ed.), Fields virology, 4th ed., vol. 2. Lippincott/Williams & Wilkins Publishers, Philadelphia, Pa.
- 45.Satoh, S., Y. Daigo, Y. Furukawa, T. Kato, N. Miwa, T. Nishiwaki, T. Kawasoe, H. Ishiguro, M. Fujita, T. Tokino, Y. Sasaki, S. Imaoka, M. Murata, T. Shimano, Y. Yamaoka, and Y. Nakamura. 2000. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat. Genet. 24:245-250. [DOI] [PubMed] [Google Scholar]
- 46.Satyamoorthy, K., G. Li, B. Vaidya, D. Patel, and M. Herlyn. 2001. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 61:7318-7324. [PubMed] [Google Scholar]
- 47.Scholle, F., K. M. Bendt, and N. Raab-Traub. 2000. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 74:10681-10689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholle, F., R. Longnecker, and N. Raab-Traub. 2001. Analysis of the phosphorylation status of Epstein-Barr virus LMP2A in epithelial cells. Virology 291:208-214. [DOI] [PubMed] [Google Scholar]
- 49.Sharma, M., W. W. Chuang, and Z. Sun. 2002. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear beta-catenin accumulation. J. Biol. Chem. 277:30935-30941. [DOI] [PubMed] [Google Scholar]
- 50.Shtutman, M., J. Zhurinsky, I. Simcha, C. Albanese, M. D'Amico, R. Pestell, and A. Ben-Ze'ev. 1999. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96:5522-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staal, F. J., B. M. Burgering, M. van de Wetering, and H. C. Clevers. 1999. Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int. Immunol. 11:317-323. [DOI] [PubMed] [Google Scholar]
- 52.Sugden, B., and W. Mark. 1977. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J. Virol. 23:503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swart, R., I. K. Ruf, J. Sample, and R. Longnecker. 2000. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 74:10838-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taniguchi, K., L. R. Roberts, I. N. Aderca, X. Dong, C. Qian, L. M. Murphy, D. M. Nagorney, L. J. Burgart, P. C. Roche, D. I. Smith, J. A. Ross, and W. Liu. 2002. Mutational spectrum of β-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 21:4863-4871. [DOI] [PubMed] [Google Scholar]
- 55.Tetsu, O., and F. McCormick. 1999. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 56.Winberg, G., L. Matskova, F. Chen, P. Plant, D. Rotin, G. Gish, R. Ingham, I. Ernberg, and T. Pawson. 2000. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol. Cell. Biol. 20:8526-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong, N. A., and M. Pignatelli. 2002. β-Catenin: a linchpin in colorectal carcinogenesis? Am. J. Pathol. 160:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 76:3731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan, H., J. Mao, L. Li, and D. Wu. 1999. Suppression of glycogen synthase kinase activity is not sufficient for leukemia enhancer factor-1 activation. J. Biol. Chem. 274:30419-30423. [DOI] [PubMed] [Google Scholar]
- 60.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 76:10685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]