Abstract
Adhesion to ECM is required for many cell functions including cytoskeletal organization, migration, and proliferation. We observed that when cells first adhere to extracellular matrix, they spread rapidly by extending filopodia-like projections and lamellipodia. These structures are similar to the Rac- and Cdc42-dependent structures observed in growth factor-stimulated cells. We therefore investigated the involvement of Rac and Cdc42 in adhesion and spreading on the ECM protein fibronectin. We found that integrin-dependent adhesion led to the rapid activation of p21-activated kinase, a downstream effector of Cdc42 and Rac, suggesting that integrins activate at least one of these GTPases. Dominant negative mutants of Rac and Cdc42 inhibit cell spreading in such a way as to suggest that integrins activate Cdc42, which leads to the subsequent activation of Rac; both GTPases then contribute to cell spreading. These results demonstrate that initial integrin-dependent activation of Rac and Cdc42 mediates cell spreading.
INTRODUCTION
Most cell types respond to surfaces coated with ECM proteins by adhering and then spreading out to acquire a flattened morphology. This process of cell adhesion and spreading is mediated by integrins and involves complex dynamic rearrangements of the actin cytoskeleton. These dynamics appear to be coordinated in space and time by intracellular signaling pathways involving tyrosine kinases, protein kinase C, arachidonic acid metabolism, and, in some cases, intracellular calcium (Chun and Jacobson, 1992, 1993; Pelletier et al., 1992; Auer et al., 1993; Vuori and Ruoslahti, 1993; Romer et al., 1994). How specific signaling pathways regulate the cytoskeleton is, however, poorly understood.
Cells spread by putting out extensions that contact the surface, form adhesions, and then exert tension to induce outward movement. This process is reminiscent of the extensions and adhesions induced by the small GTP-binding proteins Rac and Cdc42. These proteins are closely related members of the Ras superfamily of GTPases, which, like other Ras family members, act as guanine nucleotide-regulated switches. Cdc42 mediates formation of long, thin, actin-dependent extensions called filopodia, whereas Rac mediates formation of curtain-like extensions called lamellipodia and ruffles (Ridley et al., 1992; Nobes and Hall, 1995). Both can induce formation of small substrate adhesions called focal complexes.
Rac and Cdc42 interact with a number of effector proteins. The best characterized effectors are the p21-activated kinases (PAKs). Both Rac and Cdc42 in the GTP-bound state interact specifically with PAKs and strongly stimulate PAK kinase activity (Manser et al., 1994; Knaus et al., 1995; Martin et al., 1995). Mutants of Rac and Cdc42 that do not bind and activate PAK1 can still induce lamellipodia and filopodia, respectively (Joneson et al., 1996; Lamarche et al., 1996), however, activated PAK1 mutants themselves induce lamellipodia and cytoskeletal rearrangements (Sells et al., 1997). Thus, the role of PAK1 in mediating effects of small GTPases on the cytoskeleton is presently unclear. In addition to effectors that are common to Rac and Cdc42, there are molecules such as WASP, POR-1, and p120ACK that interact with one or the other specifically, but the functions of these proteins are even less well defined (for review, see Tapon and Hall, 1997).
The similarity between cell spreading and Rac-induced lamellipodia formation prompted us to investigate the role of these small GTPases in cell spreading. Our results indicate that integrins activate these proteins and that both Rac and Cdc42 contribute to cell spreading.
MATERIALS AND METHODS
Proteins and Plasmids
Fibronectin (Fn) was purified from human plasma by affinity chromatography on gelatin-Sepharose (Miekka et al., 1982). Fn 40-kDa α-chymotryptic fragment was purchased from Life Technologies (Gaithersburg, MD). The anti-β1-integrin antibody HMβ1-1 was purchased from PharMingen (San Diego, CA). Myelin basic protein (MBP) was purified from bovine spinal cord as described (Deibler et al., 1972). NP-40 and leupeptin were purchased from ICN Biomedicals (Aurora, OH). Other chemicals and reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated.
Rac mutants were in a pcDNA3 vector; Cdc42 mutants were in pCMV5 (Zhang et al., 1995); and PAK mutants were in pCMV6 (Sells et al., 1997). The GFP vector was from Clontech (Palo Alto, Ca).
Microscopy
Cells were made quiescent by maintaining them in DMEM containing 0.5% serum for 24 h. Quiescent cells were trypsinized, washed, resuspended in serum-free DMEM, and plated on Fn- or poly-l-lysine-coated coverslips. Microscope images were collected continuously on a Panasonic video recorder. For quantification of spreading, cells were fixed in 3.7% formaldehyde at each time point, and the proportion of spread cells was determined under light microscopy. To visualize membrane ruffles, fixed cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min, and actin filaments were stained with rhodamine–phalloidin (0.1 μg/ml) for 30 min. Coverslips were mounted in Fluoromount G and viewed by fluorescence microscopy.
Microinjection and Respreading
NIH 3T3 cells on coverslips were injected with cDNAs coding for various inhibitors of Cdc42 and Rac as described in the text. In some cases, DNA coding for GFP was included at 0.02 μg/ml to allow identification of injected cells. cDNAs were injected into the nucleus according to the method of Meredith et al. (1995). Dishes were returned to the incubator for 4 h to allow protein expression. Cells were incubated in trypsin-EDTA sufficiently to induce rounding without detachment, and then the trypsin was carefully aspirated, and fresh medium with 10% serum was added to stop the trypsin. Cells were returned to the incubator for 1 or 4 h as indicated and then fixed with 2% formaldehyde. They were stained for actin filaments with rhodamine–phalloidin (Molecular Probes, Eugene, OR) used at 0.1 μg/ml. Injected cells were identified either by GFP fluorescence or by staining for the myc-tagged dominant negative proteins with a monoclonal anti-myc antibody (9E10) and fluorescein-conjugated sheep anti-mouse secondary antibody. Both methods gave identical results.
Kinase Assays
To assay PAK kinase activity, 70% confluent NIH 3T3 fibroblasts were serum starved in DMEM with 0.5% bovine calf serum for 24 h. Where indicated, cells were trypsinized and suspended for three hours in serum-free DMEM containing 0.1% BSA (protease free) and 0.25 mg/ml soybean trypsin inhibitor. In some cases, cells were then pelleted by centrifugation, rinsed, and extracted in lysis buffer (20 mM Tris, pH 7.6, 0.5% NP-40, 250 mM NaCl, 5 mM EDTA, 3 mM EGTA, 20 mM sodium phosphate, 10 mM sodium pyrophosphate, 3 mM β-glycerophosphate, 10 μg/ml leupeptin, 1 mM sodium vanadate, 1 mM PMSF, 1 mM NaF). Alternatively, cells were transferred to dishes that had been coated with 25 μg/ml Fn, anti-β1 immunoglobulin G, or the 40-kDa fragment of Fn and then blocked with 1% heat-denatured BSA. Cells were allowed to adhere for the indicated period, rinsed two times with cold PBS, and extracted in lysis buffer.
Endogenous PAK was immunoprecipitated from 150–250 μg cell lysate with anti-PAK1 antibodies (polyclonal anti-PAK1 R626; Dharmawardhane et al., 1997) and dissolved in SDS-sample buffer. To estimate the amount of immunoprecipitated PAK, one-fifth of each sample was run on 10% SDS-polyacrylamide gels and proteins transferred to nitrocellulose (Hybond C; Amersham, Little Chalfont, United Kingdom). Membranes were probed with the anti-PAK antibody, and protein was detected by enhanced chemiluminescence. PAK kinase activity in the immunoprecipitates was determined using an in-gel kinase assay as described previously (Renshaw et al., 1996). Briefly, immunoprecipitates were run on 10% SDS-polyacrylamide gels containing 0.5 mg/ml MBP. Proteins in the gel were then renatured, and their kinase activity toward MBP was initiated by soaking the gels in kinase buffer containing 25 μCi/ml [γ-32P]ATP and 10 μM unlabeled ATP. Gels were then washed extensively and autoradiographed, and films were scanned by densitometry using an Alphaimager 2000 (Alpha Innotech, San Leandro, CA).
RESULTS
Adhesion to Fn Stimulates Spreading and Membrane Ruffling
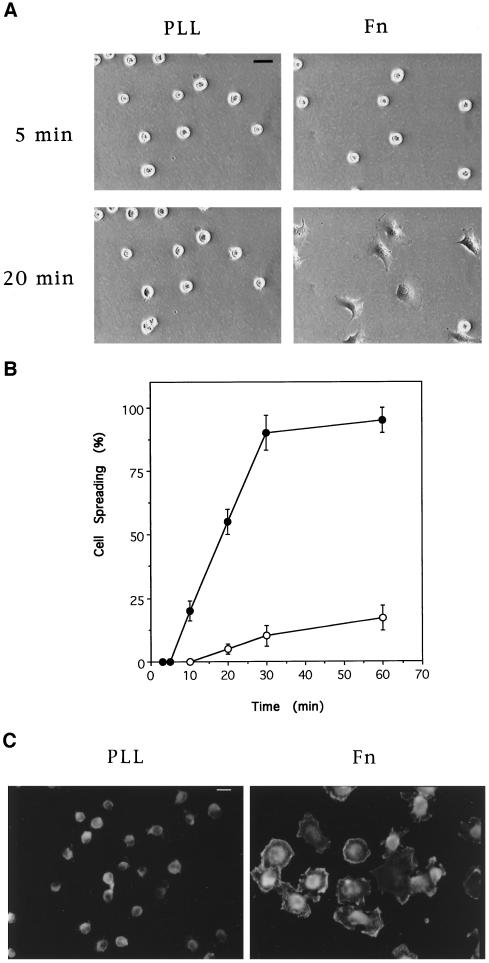
NIH 3T3 fibroblasts plated on Fn-coated surfaces adhere within ∼5 min and then spread over a period of ∼1 h (Figure 1, A and B). During this period, cells extend filopodia and lamellipodia and show extensive membrane ruffles (Figure 1C). Similar structures observed in growth factor-stimulated cells are mediated by the small GTPases Cdc42 and Rac, respectively (Ridley et al., 1992; Nobes and Hall, 1995). These results suggest that, in this cell type, Rac and or Cdc42 may be activated during cell spreading.
Figure 1.
Cell spreading on Fn and poly-l-lysine. Quiescent cells in suspension were plated on coverslips coated with Fn or poly-l-lysine (PLL). Cells were fixed at the indicated times after plating and visualized by phase contrast microscopy (A) and scored for the percentage of spread cells (B) (•, Fn; ○, poly-l-lysine). (C) Twenty minutes after plating, cells were fixed and permeabilized, and filamentous actin was labeled with rhodamine–phalloidin; cells were then visualized by fluorescence microscopy. Bar, 20 μm.
Integrin Activation of the Rac and Cdc42 Effector PAK
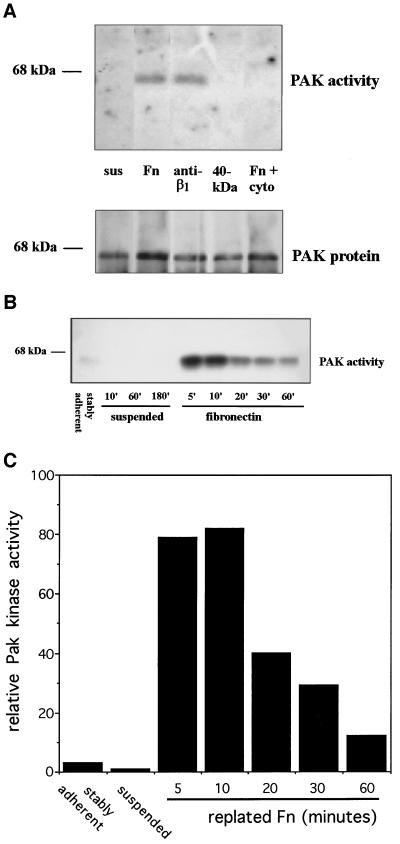
Direct assays of Rac or Cdc42 activation are technically difficult; therefore, to investigate possible integrin dependence of Rac and/or Cdc42 activity, we assayed the serine/threonine kinase PAK, which is a direct downstream target of these GTPases (Manser et al., 1994; Knaus et al., 1995). Cells were incubated for 24 h in 0.5% serum to minimize growth factor activation and then were detached and incubated in serum-free medium. After 3 h in suspension, cells had minimal PAK kinase activity, but plating on Fn-coated tissue culture dishes strongly stimulated PAK (Figure 2A). Activation was also observed when cells were plated on an antibody to β1-integrins but not on dishes coated with the 40-kDa tryptic fragment of Fn, to which cells adhere via heparin sulfate proteoglycans (Woods et al., 1993). Consistent with the presence of PAK kinase activity, cells placed on Fn or anti-β1 antibodies extended processes and spread, whereas cells plated on the Fn 40-kDa fragment remained completely rounded (our unpublished results). Cell spreading and PAK activation were also observed when cells were plated on vitronectin. Pretreatment with cytochalasin D before plating cells on Fn prevented activation of PAK (Figure 2A), indicating that organization of the actin cytoskeleton is essential for integrin-mediated activation of this pathway. Cytochalasin D treatment also blocked cell spreading. These results demonstrate that integrin-dependent adhesion specifically activates PAK and, by inference, Rac and/or Cdc42.
Figure 2.
PAK kinase activity is stimulated after integrin-mediated cell adhesion. Cells were trypsinized, placed in suspension, and then plated on tissue culture dishes coated with the indicated ligands. PAK protein was immunoprecipitated, and its kinase activity was assayed. (A) Cells in suspension for 3 h (sus), replated for 20 min on Fn, anti-β1-integrin antibody, 40-kDa Fn fragment, or plated on Fn after a 30-min pretreatment with cytochalasin D (1 μM). The bottom panel shows the amount of PAK protein in immunoprecipitates as determined by Western blotting. (B) Stably adherent cells or cells suspended and replated on Fn for the indicated periods. (C) Densitometric quantification of B showing induction of kinase activity relative to suspended cells.
Examination of the time course of PAK activation showed that kinase activity was nearly maximal at 5 min (the earliest time point measured), peaked at 10 min, and fell to a near-baseline level of activity after ∼1 h (Figure 2, B and C). Thus, activation of this pathway is an early response to cell adhesion that precedes cell spreading.
Effect of Inhibitors of Rac and Cdc42 on Cell Spreading
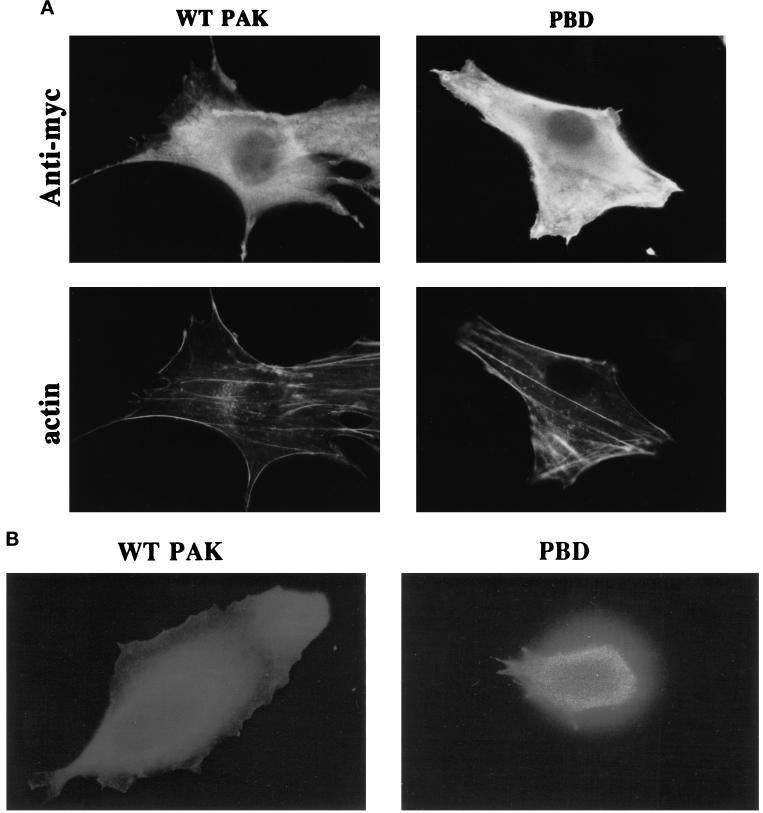
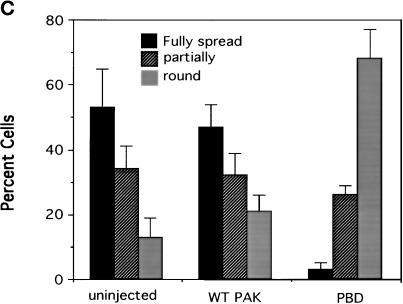
To investigate whether activation of Rac and/or Cdc42 is required for cell spreading, cells were microinjected with cDNAs encoding epitope-tagged dominant negative mutant proteins, which inhibit these GTPases. We first examined the Rac and Cdc42 binding domain of PAK (p21 binding domain [PBD]) that binds and sequesters both Rac and Cdc42 and prevents their interaction with downstream effectors (Sells et al., 1997). Expression of neither PBD nor wild-type PAK (which was used as a control) had any detectable effect on cell morphology or actin distribution in stably adherent cells after 4 h (Figure 3A). Expression of the proteins was confirmed by staining with an antibody against the epitope tag. To enable examination of spreading in microinjected cells, cells were induced to round up (but not detach) by brief trypsinization. The trypsin was stopped, and cells were allowed to respread for 1 h. Expression of wild-type PAK had no effect, but expression of PBD strongly inhibited respreading of cells in this assay (Figure 3, B and C). These results indicate that Rac and/or Cdc42 are required for cell spreading.
Figure 3.
The PBD domain of PAK inhibits cell spreading. cDNAs of myc-tagged wild-type PAK (0.5 μg/ml) or the PBD domain of PAK (0.2 μg/ml) were injected into the nuclei of cells. (A) Labeling of expressed myc-tagged proteins with anti-myc antibody and of filamentous actin with rhodamine–phalloidin in stably spread cells after 4 h. (B) Respreading of injected cells for 1 h after mild trypsinization; expression of myc-tagged proteins is demonstrated by anti-myc staining. (C, facing page) Quantification of cell spreading. Values are means ± SD from three experiments in which >25 cells were scored per condition.
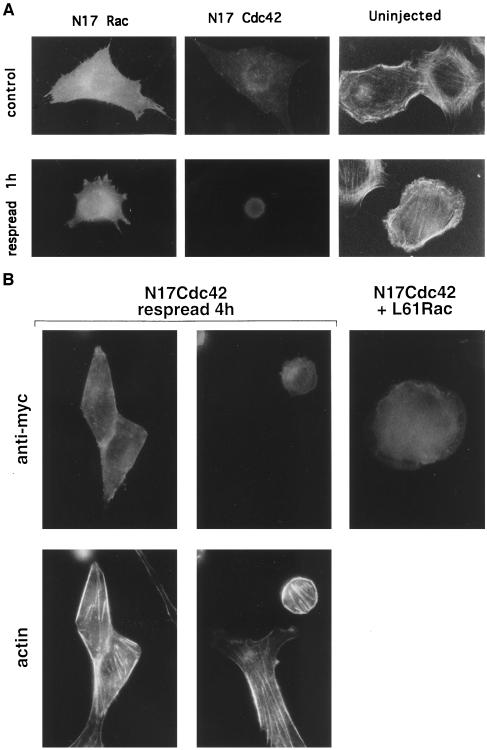
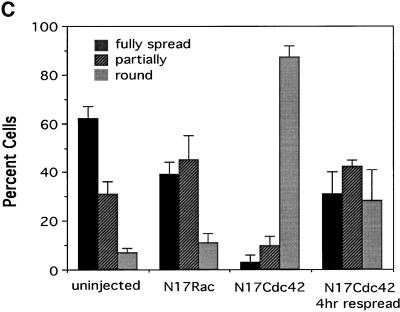
To determine to what extent Rac or Cdc42 or both mediate spreading, cells were injected with cDNAs encoding the dominant negative mutants N17Rac and N17Cdc42. Expression of these proteins in stably adherent cells did not cause gross changes in morphology or cytoskeletal structure, although N17Rac-expressing cells showed a loss of lamellipodia and an increase in filamentous projections, which were most likely either retraction fibers or filopodia (Figure 4A). Expression of N17Rac caused a partial inhibition of respreading of rounded cells (Figure 4, A and C). Notably, cells appeared to spread by means of narrow extensions instead of the usual broad lamellipodia. It should be noted that expression of fourfold lower levels of N17 Rac completely inhibited PDGF-induced ruffling, suggesting that the concentration of N17 Rac DNA used in these experiments should be effective. By contrast, N17Cdc42 profoundly inhibited respreading; after 1 h, almost all injected cells remained completely round.
Figure 4.
Effect of N17Rac and N17Cdc42 on cell spreading. N17Rac and N17Cdc42 cDNAs (0.2 μg/ml) were injected into the nuclei of cells. (A) Effect of expressed proteins on stably adherent cells (control) and cells allowed to respread for 1 h after mild trypsinization; anti-myc staining of injected cells and rhodamine–phalloidin staining of uninjected cells is shown. (B) Cells injected with N17Cdc42 and allowed to respread for 4 h or cells injected with N17Cdc42 plus constitutively active Rac (L61Rac) (0.5 μg/ml) and allowed to respread for 1 h. (C, facing page) Quantification of cell spreading. Values are means ± SD from four or five experiments in which >25 cells were scored per condition. The difference between N17 Rac and control cells is statistically significant (p < 0.005).
The inhibition by N17Cdc42 was so dramatic that additional controls were performed. To determine whether the inhibition of respreading by N17Cdc42 was reversible, cells were left to recover for longer periods. When cells were allowed to recover for 4 h, the majority of cells showed some degree of respreading (Figure 4, B and C). Interestingly, even those cells that failed to respread developed actin stress fibers (Figure 4B). Stress fibers have been shown to be a consequence of Rho activation, suggesting that Rho activation is independent of Cdc42. These results indicate that N17Cdc42-expressing cells remained viable and capable of assembling actin-based structures that are independent of Cdc42.
It has been demonstrated in some systems that Cdc42 can lead to activation of Rac (Nobes and Hall, 1995). Thus, the nearly complete inhibition of spreading by N17Cdc42 could be explained if initial activation of Cdc42 by integrins induced both Cdc42- and Rac-dependent events. To determine whether the inhibition of spreading by N17Cdc42-was due to the loss of Cdc42 activity alone or the additional loss of signaling to Rac, N17Cdc42 was expressed along with a constitutively activated mutant of Rac, L61Rac. Coexpression of activated Rac partially restored the ability of cells to respread after 1 h (Figure 4B). Quantification of these results showed that L61Rac increased the total number of spread cells (full plus partial) threefold from 15.2 ± 5.9 to 48.7 ± 18.1% (p < 0.005; n = 5). This result further demonstrates that N17Cdc42 is not toxic and suggests that Rac lies downstream of Cdc42 in this system.
DISCUSSION
Plating cells on Fn or antibodies to integrins leads to cell spreading and membrane ruffling. This result suggested that the small GTPase Rac was activated. We therefore investigated whether integrins activated Rac and whether this activation was involved in cell spreading.
The serine/threonine kinase PAK is activated directly by Rac and Cdc42 (Manser et al., 1994; Knaus et al., 1995; Martin et al., 1995). We therefore assayed PAK kinase activity as an indicator of GTPase activation. PAK kinase activity was rapidly induced upon plating cells on Fn or anti-β1-integrin immunoglobulin G, suggesting that Rac and/or Cdc42 was indeed activated. PAK was not activated when cells adhered to a control protein that does not bind integrins, demonstrating a specific requirement for integrins. The time course of activation was rapid, with almost maximal kinase activity at 5 min. At this early time point, cell spreading is either minimal or absent, suggesting that Rac and/or Cdc42 activation precedes cell spreading.
To test whether Rac and Cdc42 were required for integrin-mediated cell spreading, we expressed the PBD domain of PAK, which inhibits these GTPases (Sells et al., 1997). We found that the PBD domain profoundly inhibited cell spreading, demonstrating that Rac and/or Cdc42 GTPases are essential. To determine which of the two GTPases were required, we expressed dominant negative inhibitory forms of the proteins. We observed that dominant negative Rac retarded cell spreading, demonstrating a role for Rac. In the presence of N17Rac, cells spread by extending thin processes instead of lamellipodia, suggesting that spreading occurred via Cdc42. Dominant negative Cdc42 profoundly inhibited spreading, with nearly all cells appearing completely round at 1 h, demonstrating a pivotal role for this protein. The deficiency in spreading in cells expressing N17Cdc42 could be overcome with time. This may have occurred via a Cdc42-independent mechanism, perhaps by the independent activation of Rac, or may be a consequence of incomplete inhibition of Cdc42. Interestingly, even in those cells that did not significantly spread, stress fibers still formed, indicating that Rho function was not dependent on Cdc42 or Rac.
To determine whether integrins activated Cdc42 and Rac independently or whether activation of these proteins was linear, we coexpressed N17Cdc42 with activated Rac. Activated Rac partially reversed the inhibition caused by N17Cdc42. As might be expected, the cells that spread did so by extending broad, symmetrical lamellipodia indicative of Rac activation (Ridley et al., 1992). The incomplete recovery of spreading could be because Rac activation was not properly coordinated in space and time or because Cdc42 contributes something distinct from Rac that enhances spreading. Despite these uncertainties, the data suggest that integrin engagement leads to the activation of Cdc42, which results in the subsequent activation of Rac, and that both GTPases contribute to cell spreading.
Our results show that adhesion to ECM stimulates the activation of PAK. However, published data as to the role of PAK in Rac- and Cdc42-dependent control of cell architecture has been contradictory. PAK has been shown to colocalize with Rac and Cdc42 at focal adhesions and membrane ruffles (Harden et al., 1996; Dharmawardhane et al., 1997) and to induce filopodia and lamellipodia when injected into Swiss 3T3 cells (Sells et al., 1997). Other studies, however, showed that mutations in Rac and Cdc42 that abolish binding to PAK in vitro did not block the ability to induce the formation of lamellipodia and filopodia (Joneson et al., 1996; Lamarche et al., 1996). To determine whether PAK is a functionally important effector in this system, the activated mutant PAK 83/86 (Sells et al., 1997) was coexpressed with N17 Cdc42. No recovery of cell spreading after trypsinization was observed, indicating that PAK is not sufficient for spreading in the absence of other effectors. These experiments do not, however, exclude the possibility that PAK could influence events triggered by other effectors. Thus, the function of PAK in GTPase-regulated cytoskeletal regulation is still unclear.
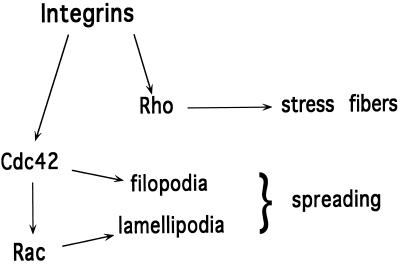
These results suggest a model for cell spreading whereby initial contact with ECM proteins activates Cdc42 and induces the extension of filopodial processes. Activation of Cdc42 leads to the subsequent activation of Rac and the formation of lamellipodia, which extend between the filopodia. Rho is activated independently to induce stress fibers and generate tension. This model is shown in Figure 5. It is notable that this model proposes a molecular mechanism for cell spreading that closely resembles cell migration, with the exception that instead of the unidirectional extension of filopodia and lamellipodia toward a chemotactic stimulus, processes extend isotropically to increase cell area.
Figure 5.
Schematic diagram showing integrin-dependent activation of Rho family GTPases. Engagement of integrins with ECM leads to the activation of Cdc42, which subsequently leads to the activation of Rac. Together, these GTPases mediate cell spreading. Activation of Rho can occur independently of activation of Cdc42 and leads to the formation of stress fibers.
We have shown here that integrins induce Rac- and Cdc42-mediated cell spreading in the absence of exogenous growth factors. By contrast, adherent serum-starved Swiss 3T3 cells do not possess stress fibers or membrane ruffles and require growth factors or addition of activated Rho and Rac to induce these structures (Ridley and Hall, 1992; Ridley et al., 1992). These differences may be due to basic differences in the signaling pathways between the two cell types. However, it has been shown that if the level of integrin occupancy in starved Swiss 3T3 cells is increased by replating cells on Fn or by adding the Fn cell-binding peptide GRGDS, cells do spread and form Rho-dependent stress fibers (Allen et al., 1997). These results suggest that it is primarily the extent of integrin occupancy required to induce activation of GTPases that differs between the two cell types.
Adhesion is a basic requirement for proliferation of most cell types. It has been proposed that constitutive activation of signaling pathways that are normally activated by integrins may overcome the adhesion requirement for cell growth (Schwartz, 1993; Schwartz et al., 1996). Recent results showing that activated Cdc42 confers anchorage-independent growth but that cells remain serum dependent (Qiu et al., 1997) therefore support our conclusion that integrins activate Cdc42. These results further suggest that integrin-mediated adhesion may therefore contribute to cell proliferation via the activation of Cdc42.
Evidence has also been presented that Rho is activated by integrins and that enhanced activation of Rho by overexpression of Rho nucleotide exchange factors can also confer anchorage independence (Chong et al., 1994; Schwartz et al., 1996). How integrins activate Cdc42 and Rho is not known, but the most obvious hypothesis is that they may activate nucleotide exchange factors for these GTPases. Although not yet identified in NIH 3T3 cells, exchange factors have been identified in other cell types that act on Cdc42 and Rho (Horii et al., 1994; Olson et al., 1996). Regulation of these factors by integrins may therefore be a useful direction for future investigations.
ACKNOWLEDGMENTS
This work was supported by grants from the United States Public Health Service, National Institutes of Health granst R01 GM47214 and P01 HL48728 to M.A.S. and R01 GM44428 to G.M.B., and US Army Medical Research and Material Command grant DAMD17-96-1-6104 to J.L.
REFERENCES
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Auer KL, Schmidt H, Jacobson BS. Signal transduction during HeLa cell adhesion to collagen substrate: role of β1 integrins in the regulation of second messengers. Mol Biol Cell. 1993;4:365a. (abstract). [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Chun J-S, Jacobson BS. Spreading of Hela cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachadonic acid released by collagen receptor clustering. Mol Biol Cell. 1992;3:481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J-S, Jacobson BS. Requirement for diacylglycerol and protein kinase C in Hela cell-substratum adhesion and their feedback amplification of arachidonic acid production for optimum cell spreading. Mol Biol Cell. 1993;4:271–281. doi: 10.1091/mbc.4.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler GE, Martenson RE, Kies MW. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2:139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-Activated Kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1–14. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N, Lee J, Loh HY, Ong YM, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–1908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Y, Beeler JF, Sakaguchi K, Tachibana M, Miki T. A novel oncogene, ost, encodes a guanine nucleotide exchange factor that potentially links Rho and Rac signaling pathways. EMBO J. 1994;13:4776–4786. doi: 10.1002/j.1460-2075.1994.tb06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein–coupled receptors. Science. 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by Rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JE, Takada Y, Fornaro M, Languino L, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science. 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Miekka SI, Ingham KC, Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982;27:1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Olson MF, Pasteris NG, Gorski JL, Hall A. Faciogenital dysplasia protein (FGD1) and Vav, two related proteins required for normal embryo development, are upstream regulators of Rho GTPases. Curr Biol. 1996;6:1628–1633. doi: 10.1016/s0960-9822(02)70786-0. [DOI] [PubMed] [Google Scholar]
- Pelletier AJ, Bodary SC, Levinson AD. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992;3:989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Toksoz D, Schwartz MA. Involvement of the small GTPase Rho in integrin-mediated activation of MAP kinase. J Biol Chem. 1996;271:21691–21694. doi: 10.1074/jbc.271.36.21691. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Romer LH, McLean N, Turner CE, Burridge K. Tyrosine kinase activity, cytoskeletal organization and motility in human vascular endothelial cells. Mol Biol Cell. 1994;5:349–361. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Signaling by integrins: implications for tumorigenesis. Cancer Res. 1993;53:1503–1506. [PubMed] [Google Scholar]
- Schwartz MA, Toksoz D, Khosravi-Far R. Transformation by Rho exchange factor oncogenes is mediated by activation of an integrin-dependent pathway. EMBO J. 1996;15:6525–6530. [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- Woods A, McCarthy JB, Furcht LT, Couchman JR. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993;4:605–613. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]