Abstract
The bovine and human respiratory syncytial viruses cause severe lower respiratory tract infections. Effective vaccines against the respiratory syncytial viruses have been lacking since vaccine failures in the 1960s and 1970s. In this report, we describe a bovine respiratory syncytial virus (bRSV) challenge model in which both classical bRSV respiratory infection and vaccine-enhanced immune pathology were reproduced. The classical, formalin-inactivated (FI) bRSV vaccine that has been associated with vaccine failure was efficient in inducing high antibody titers and reducing viral loads but also primed calves for a far more serious enhanced respiratory disease after a bRSV challenge, thereby mimicking the enhanced clinical situation in FI human RSV (hRSV)-immunized and hRSV-infected infants in the 1960s. We show that immunization with FI-bRSV mainly primes a Th2-like inflammatory response that is characterized by a significant eosinophilic influx in the bronchial alveolar lung fluid and lung tissues and high levels of immunoglobulin E serum antibodies. The current model may be useful in the evaluation of new bRSV candidate vaccines for potency and safety.
The paramyxovirus bovine respiratory syncytial virus (bRSV) is, like its human counterpart human RSV (hRSV), a major cause of respiratory disease (23). Primary bRSV infection can result in severe lower respiratory tract disease in susceptible cattle, although asymptomatic infections also occur. The virus causes an acute interstitial pneumonia with alveolitis and bronchiololitis, especially in calves and yearlings (24).
bRSV causes a range of clinical symptoms. Mild respiratory disease is characterized by coughing, serous or mucopurulent nasal discharge, slight to moderately increased respiratory rates (RRs), and abnormal breath sounds. Tachypnea, harsh lung sounds, and profound coughing characterize moderately affected calves. The most severely affected calves may be dyspneic and may have subpleural and interstitial emphysema. Emphysematous bullae may be present between lung lobules. Generalized symptoms range from a slightly elevated rectal temperature, mild depression, and anorexia to a high fever, deep depression, and coma (2, 4, 14).
Vaccine development against hRSV and bRSV has been hampered by the dramatic hRSV vaccine failure in the 1960s: vaccination with formalin-inactivated (FI), alum-adjuvanted virus predisposed children to a far more serious, and sometimes lethal, form of RSV infection (13). Subsequently, it was found in the 1970s that a similarly inactivated bRSV vaccine could induce strikingly similar immunopathology in bRSV-infected calves (28). In fact, some inactivated veterinary vaccines were withdrawn from the market after safety problems were discovered (R. S. Schrijver, personal communication).
Studies with murine models of hRSV have demonstrated that alum-adjuvanted FI-hRSV is a strong inducer of Th2 cells, which proved to be the key mediators of immunological hypersensitivity reactions (20). In fact, immunopathogenesis in BALB/c mice can be attributed completely to an oligoclonal response of interleukin-5 (IL-5)-producing CD4 T cells that are specific for the viral attachment protein (G) (26). On the basis of these results, it is evident that further vaccine development depends on a better understanding of the immune mechanisms of this enhanced disease and that these parameters are defined in models that allow evaluation of the safety of candidate RSV vaccines, such as the bRSV model.
Experimental bRSV infection resulting in severe respiratory disease in cattle has been described in only a few reports (3, 5, 6). However, a potential drawback of these studies is that it was unclear in these studies whether other pathogenic microorganisms might also have been involved in pathogenesis. For instance, severe respiratory disease after bRSV infection was reported by Ciszewski et al. (6), but the calves used were not specific pathogen free (SPF) and pathogenic microorganisms were in fact cultured from several animals in the experiment. Evidently, for further study of the (immuno)pathogenesis of bRSV infection and for evaluation of vaccine safety and efficacy, development of a bRSV infection model is urgently needed. In the present study, we have developed such a bRSV challenge model. The impact of prior vaccination with FI or live virus on the outcome of subsequent bRSV infection was analyzed by using a panel of clinical and cellular parameters.
MATERIALS AND METHODS
Vaccine preparation.
bRSV, strain Lelystad, sixth passage, was grown in Earle's minimal essential medium (MEM; GIBCO) supplemented with 10% fetal bovine serum (FBS) and 0.5% antibiotic cocktail (ABC) on embryonic bovine trachea (EBTr) cells to a titer of 105.5 50% tissue culture infective doses (TCID50) per ml and harvested after 7 days. Supernatant (440 ml in total) was centrifuged (15 min, 1,000 × g) to remove cellular debris. The virus preparation was inactivated by addition of a 37% formaldehyde solution (1:4,000 at 37°C, 72 h), followed by ultracentrifugation (Beckman SW28, 110,000 × g, 60 min, 4°C). The virus pellet was resuspended in phosphate-buffered saline (PBS) and adjusted to a protein concentration of 0.75 mg/ml. A vaccine dose was prepared by mixing FI-bRSV (1:1) with 2% Al(OH)3 and incubating it for 20 min at room temperature.
Study design.
Three groups of six SPF calves obtained by caesarean section, deprived of colostrum, and reared in isolation were housed in separate isolation rooms. The calves were free of bovine viral diarrhea virus (BVDV), as shown by BVDV antigen enzyme-linked immunosorbent assay (ELISA) (SERELISA BVD/MD/BD Ag; SYNBIOTICS EUROPE). After the calves were found to be free of BVDV, they were loose housed in isolation units in groups of three. Starting at an age of 6 weeks, all of the calves received two intramuscular immunizations, with a 3-week interval, with either PBS, FI-bRSV adsorbed to Al(OH)3, or live bRSV (strain Lelystad, 104.3 TCID50/ml).
All calves were challenged approximately 5 months after the first immunization. bRSV field strain Odijk was used to prepare the challenge virus stock (25). This strain was obtained during an outbreak of acute respiratory disease in a herd of 3- to 5-month-old calves in December 1991 in Odijk, The Netherlands (25), and has never been passaged on cell culture. The virus stock contained 103.9 TCID50 of bRSV per ml and was found to be free of bacteria, mycoplasmas, bVDV, bovine herpesvirus 1, and parainfluenza 3 virus. For the challenge, all calves were inoculated intranasally with a 2-ml bRSV challenge inoculum with an air jet nebulizer and a jet stream of 0.2 mm, producing 10% droplets <26 μm in section, 50% droplets <50 μm in section, and 90% droplets <99 μm in section. Bronchoalveolar lung fluid (BALF), heparinized blood samples, and nasal swabs were obtained from all calves at 3 days prior to challenge and at 1, 4, 7, and 9 days after challenge. All calves were bled weekly and 3 days before challenge and daily thereafter for serology. All animal experiments were conducted in accordance with the Act on Experimental Animals of The Netherlands regulated by the Ethical Review Committee of ID-Lelystad.
Clinical examinations.
Calves were clinically examined once a day before challenge (from day −3 to day 0, the day of challenge infection) and then twice a day after challenge (from day 0 until day 9 postinfection). Calves were examined for signs of respiratory disease, ocular and nasal discharge, coughing, breathing, and lung sounds. Rectal temperatures and RRs were recorded. The mean of the prechallenge clinical scores was used as a baseline value (100%). Half-daily scores were expressed as a percentage of this baseline. Standardization was obtained by having all examinations performed by the same veterinarian. The severity of the clinical disease was expressed by allocating a weighting factor for each observation (Table 1).
TABLE 1.
Weighting factors for each clinical observationa
| Parameter | Clinical sign(s) of respiratory disease at rectal temp of:
|
||
|---|---|---|---|
| <39.5°C | 39.5-40.5°C | >40.5°C | |
| Ocular discharge or runny eyes | None | Serous or mucous | Purulent or hemorragic |
| Nasal discharge | None | Serous or mucous | Purulent |
| Coughing | None | Spontaneous | Spontaneous and productive |
| RR (breaths/min) | ≤60 | 60-100 | >100 |
| Respiratory distress | None | Dyspnea | |
| Costoabdominal | Exaggerated intercostal and/or abdominal effort | ||
| Regular breathing | Irregular breathing | ||
| Respiratory noise | None | Abnormal or intense respiratory sound (stridor) | |
| Trachea sensitivity | No induced cough after moderate pressure on trachea | Induced cough after moderate pressure on trachea at one particular place | Induced cough after moderate pressure on trachea on more than one place |
| Lung auscultation | No abnormalities | Abnormal left or right lungs | Abnormal both right and left lungs |
| Increased intensity | Vesicular lung sounds | ||
| Scoringb | 0 | 1 | 2 |
The severity of the clinical disease was expressed by allocating a weighting factor (0, 1, or 2) for each mentioned observation (first column). For example: if the rectal temperature is <39°C, the weighting factor is 0; if the rectal temperature is >39°C but ≤40.5°C, the weighting factor is 1; if the rectal temperature is >40.5°C, the weighting factor is 2.
Scoring values per observed observation. The total score was calculated by editing the weighting factors for each observation.
Bronchoalveolar lavages.
BALF samples were obtained as described by Fogarty et al. (8). Approximately 100 ml of BALF was obtained from each calf after instillation of approximately 120 ml of PBS. BALF samples were centrifuged (200 × g, 10 min, 4°C). Lavage cells were resuspended in fluorescence-activated cell sorter buffer (PBS containing 25% FBS, 0.5% bovine serum albumin, and 0.01% Na azide), counted, further diluted to 5 × 106 viable cells per ml, and used for further analysis. Droplets of resuspended cells were put on slides and air dried. A total of 100 cells were counted (Hema-Tek slide stainer), and the lymphocytes, monocytes, and neutrophils (mature and immature) were differentiated on the basis of morphology. Viral antigen was visualized by staining acetone-fixed cells with fluorescein isothiocyanate-conjugated monoclonal antibodies (MAbs) directed against the F protein of bRSV (30 min at 37°C). BALF cell supernatants were stored at −70°C for virus isolation, virus titration, and PCR analyses.
Virological examination.
Lavage fluid samples were centrifuged (200 × g, 10 min, 4°C), and supernatant was stored at −70°C until virus isolation was done. Virus isolations were performed in quadruplicate on EBTr cells as described previously (25).
Viral RNA was isolated with a viral RNA isolation kit (Qiagen, Valencia, Calif.). Reverse transcription (RT)-PCR to detect viral RNA was performed as described by Kuno (16). Briefly, an RT-PCR mixture was prepared that contained 0.5 U of RAV-2 polymerase (Amersham, Pharmacia Biotech, Roosendaal, The Netherlands), 1 U of Tth polymerase (Roche), 1× PCR buffer, 10 U of RNAguard (Amersham), primers, and a 3-μl RNA sample. The primers designed for bRSV-N and bRSV-P were N 5′ (GTTTAAACCATGGCTCTYAGCAAGGTC), N 3′ (CARTTCCACATCATTRTCTTT), P 5′ (GAAATTTCCATGGAAAAATTTGCACCTG), and P 3′ (GAAATCTTCAAGTGATAGATCATTG) (Y = C/T, R = A/G; degenerate because the Odijk sequence was unknown). Water was added to make the total volume 50 μl. RNA was reverse transcribed at 50°C for 45 min, followed by 12 cycles of touchdown PCR (94°C for 1 min; 40 to 34°C in 0.5°C increments for 1 min; 72°C for 2 min) and 30 cycles of 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min. Positive controls included plasmids containing the bRSV strain Odijk N and P genes, as well as cDNA prepared from bRSV strain Lelystad-infected cells. The N PCR generated a 1.1-kb product, and the P PCR generated a 0.7-kb product.
Lung tissue samples were stored at −70°C until virus isolation was performed. Tissues were homogenized in a mortar with sterile sea sand (Merck) in 5 ml of Dulbecco's MEM supplemented with 2% ABC and 5% FBS (PAN, 1302-P221701; Biotech GmbH). The tissue suspensions were centrifuged for 10 min at 2,000 × g, and the supernatant was collected. Virus isolation was performed on 24-well tissue culture plates. Each well contained a 200-μl tissue suspension and 2 ml of an EBTr cell suspension (± 60,000 cells) in Earle's MEM supplemented with 10% FBS and 0.5% ABC. After 7 days of incubation at 37°C in 5% CO2, an immunoperoxidase monolayer assay for bRSV was performed (25).
Humoral immune response.
A double-antibody sandwich (DAS) ELISA, as described by Westenbrink et al. (30), was used to determine bRSV-specific IgG antibody titers. This ELISA detects antibodies directed against the fusion protein of bRSV (F-ELISA, Ceditest ELISA for bRSV-Ab; Cedi Diagnostics, Lelystad, The Netherlands).
A blocking G peptide ELISA, as described by Langedijk et al. (17), was used to determine bRSV G-specific antibody titers and is based on blocking of the binding of a bRSV G-specific MAb (MAb 20) with the coated peptide by peptide-specific antibodies that may be present in the serum.
Neutralizing antibodies were determined in a virus neutralization assay (VNA). VNAs were carried out as previously described (17).
A sandwich ELISA, as described by Kooyman et al. (15), was used to detect total serum IgE responses. The value of the blank (PBS-GT) was set at 0. The corrected optical density (OD) value of the undiluted standard serum was set at 100%. On the basis of this standard, the OD values of the test sera were transformed to percentages, similar to the arbitrary units described by Kooyman et al. (15).
Haptoglobin assays.
Serum haptoglobin was determined (on days postimmunization [dpi] 139 and 146 and daily from dpi 146 to 156) with a haptoglobin assay from Tridelta Ltd. that is based on the preservation of hemoglobin peroxidase activity by haptoglobin in the serum samples. Haptoglobin concentrations were read from a standard curve.
Postmortem.
Calves were anaesthetized with a pentobarbital overdose and euthanized by exsanguination. The lungs were immediately removed, and dorsal and ventral photographs were taken, from which the extent of macroscopic lesions (consolidated lung areas) was rated as a percentage of the lung tissue area. The extent of consolidated lung tissue was scored on a scale of 0 to 5 as described by Viuff et al. (27) and used as an indication of the pneumonic lung tissue surface area.
Necropsy samples of 10 predetermined sites were stored in 10% neutral buffered formalin or snap-frozen in liquid nitrogen and/or frozen at −70°C. Formalinized samples were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin for histologic examination. Snap-frozen samples were examined for the presence of bRSV by immunohistochemistry. Long tissue samples stored at −70°C were also used for virus isolation.
Statistical analysis.
For statistical analyses, the average temperature, the average respiratory scores, and the average clinical scores of the calves before challenge (morning measurements on dpi 143, 144, 145, and 146) were taken as the reference (baseline) values. For each calf, the severity of the clinical symptoms was expressed as the area under the curve (AUC) for dpi 146 to 155 of the experiment. Significance of correlation was calculated with the Kruskal-Wallis nonparametric test and the Wilcoxon-Mann-Whitney test. Correlations and differences were considered significant when P was <0.05.
RESULTS
Development of a bRSV infection model.
The primary aim of our study was to evaluate the (immuno)pathogenesis of bRSV infection after prior immunization with an FI-bRSV vaccine. Therefore, it was essential to first set up a bRSV vaccination-challenge model. To achieve this, we used the bRSV field strain Odijk. We first sought to establish infection conditions that would produce clinical symptoms. After optimizing the inoculation method, we found that intranasal inoculation with an air jet nebulizer (104.2 TCID50) consistently resulted in productive bRSV infections and clinical symptoms (data not shown).
bRSV infection after vaccination with L-bRSV or FI-bRSV.
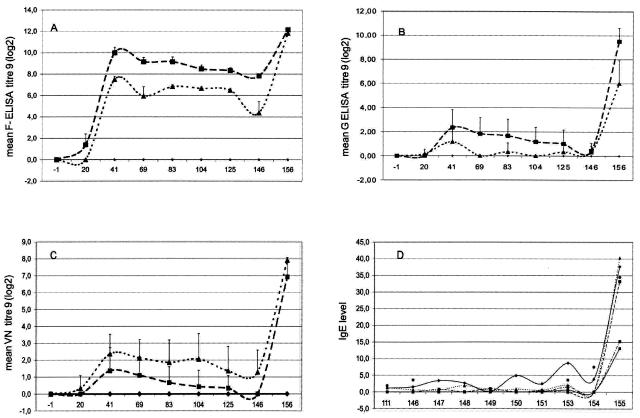
To assess the impact and efficacy of prior immunization with live attenuated bRSV (L-bRSV) or FI-bRSV, SPF calves were subjected to three different immunization regimens. Animals were immunized with FI-bRSV or L-bRSV or received a mock immunization with PBS. Humoral immune responses after vaccination were measured with commercially available F-ELISA kits, a G peptide ELISA, and the virus-neutralizing activity was tested in a neutralization assay. As expected, we found that none of the mock-immunized calves had bRSV-specific antibodies before inoculation. Immunization with either FI-bRSV or L-bRSV resulted in seroconversion in all animals. Figure 1 shows the average bRSV antibody titers for each group of calves. The first animal to test positive in the F-ELISA had received the FI-bRSV vaccine. F-specific antibodies were detected at 20 dpi. Three weeks later, all L-bRSV- and FI-bRSV-immunized calves were positive in the F-ELISA. Interestingly, a significantly greater antibody response (P = 0.0022) was observed in the FI-bRSV-immunized group. In contrast, the neutralizing antibody response was stronger in the L-bRSV-immunized group: Neutralizing antibodies were detected in three out of six FI-bRSV-immunized calves from dpi 41 onward, whereas all L-bRSV-immunized calves had neutralizing antibody responses starting from dpi 20 (Fig. 1).
FIG. 1.
Humoral immune responses. Average bRSV antibody titers, with standard deviations (A, B, and C), measured at 3-week intervals (dpi 0, 20, 41, 69, 83, 104, and 125), on the day of challenge administration (dpi 146), and at the end of the experiment (dpi 156). PBS group (♦), six mock-immunized calves; FI-bRSV group (▪), six calves immunized with FI bRSV; L-bRSV group (▴), six calves immunized with live bRSV. (D) IgE antibody responses detected in the IgE DAS ELISA. Corrected OD values were transformed to percentages of a standard positive serum. Only FI-bRSV-immunized calves were found to be IgE positive. Each line represents a calf immunized with FI-bRSV vaccine. VN, virus neutralizing.
All calves were challenged at dpi 146 by intranasal inoculation of 104.2 TCID50 of bRSV strain Odijk. The kinetics of viral replication in lung washes was analyzed by virus isolation and by RT-PCR. Unexpectedly, bRSV was detected by virus isolation in only two of the animals (animals 5539 and 5550, mock immunized and L-bRSV immunized, respectively) on days post challenge infection (dpci) 4 and 7, respectively. Next, the presence of viral RNA was measured by RT-PCR. Viral RNA was detected in BALF samples of four (out of six) calves at dpci 4 and in those of all six PBS-immunized calves at dpci 7. No viral RNA was detected in BALF samples from any of the FI-bRSV-immunized calves, whereas a weak RT-PCR signal was detected at dpci 7 in one L-bRSV-immunized animal (data not shown). Thus, both immunization protocols resulted in strongly decreased viral loads after challenge. Moreover, all FI-bRSV- and L-bRSV-immunized calves seroconverted in the F-ELISA, G-ELISA, and VNA after challenge. Interestingly, elevated levels of total IgE were detected in serum samples of all FI-bRSV-immunized calves at dpci 9 (Fig. 1D). None of the other animals showed any IgE responses.
In conclusion, both vaccines appear to be highly effective on the basis of the reduced viral loads and induction of and/or increases in (neutralizing) antibodies after challenge.
Clinical symptoms after challenge of FI-bRSV- or L-bRSV-immunized or mock-immunized animals.
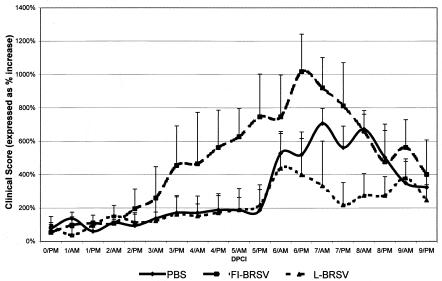
The clinical symptoms after challenge were expressed as the AUC. AUC values were calculated for 9 dpci. The parameters that were included in the AUC calculation were increased RR, increased body temperature, nasal discharge, coughing, sensitivity of the larynx and/or trachea, abnormal breathing, and lung sounds. Interestingly, clinical signs were first observed in the FI-bRSV-immunized group. Following this trend, we found the greatest increases in clinical scores after bRSV challenge administration in the FI-bRSV-immunized group (Fig. 2). Whereas some of the FI-bRSV-immunized calves presented severe clinical signs as early as 2 days after the challenge administration (>200% compared to the baseline), calves immunized with either L-bRSV or PBS displayed clinical signs no earlier than 5 days after challenge administration. More pronounced clinical signs were observed in the PBS-immunized group than in the L-bRSV-immunized group (Fig. 2). Differences in the average AUC values of the three different groups were significant (P = 0.0041). Compared to the AUC values of calves immunized with L-bRSV, both the PBS and FI-bRSV-immunized animals showed significantly more severe clinical symptoms (two-sided P values of 0.0216 and 0.0087, respectively, with the Wilcoxon-Mann-Whitney test).
FIG. 2.
Clinical observations. Average clinical scores per group, in percentages (with standard deviations) with regard to the baseline, determined twice daily (AM and PM) from the time of challenge (dpi 146) until the end of the study (dpi 155). PBS group, six mock-immunized calves; FI-bRSV group, six calves immunized with FI bRSV vaccine; L-bRSV group, six calves immunized with L-bRSV vaccine.
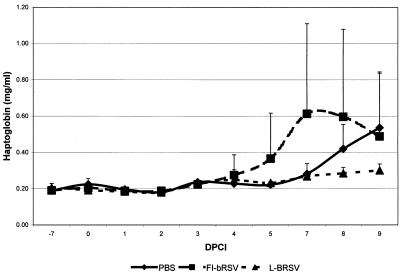
Our assessment of clinical symptoms was confirmed by the analysis of acute-phase responses (haptoglobin). Acute-phase responses were detected, and they peaked around 8 to 9 days after inoculation of bRSV (Fig. 3). The AUC was determined per calf and compared within groups. The mock-immunized group showed a significantly more severe response (P = 0.0476) than the L-bRSV-immunized group. The magnitude of the haptoglobin response correlated well with the severity of clinical signs and with the extent of lung consolidation in the PBS- and L-bRSV-immunized groups.
FIG. 3.
Haptoglobin analyses. Acute-phase responses were detected, and they peaked around 8 to 9 dpci. The AUC was determined per calf, and values were compared within groups. The mock-immunized group showed a significantly more severe response (P = 0.0476) than the L-bRSV-immunized group. The magnitude of the haptoglobin response correlated well with the severity of clinical signs and with the extent of lung consolidation in the PBS- and L-bRSV-immunized groups.
Thus, on the basis of clinical appearances, we have been able to model severe bRSV infection and, importantly, the enhanced disease after prior immunization with FI-bRSV. It is striking that enhanced disease in the FI-bRSV-vaccinated group coincided with a very strong reduction of viral loads compared to the PBS-immunized control group.
Cellular immune responses in the lungs.
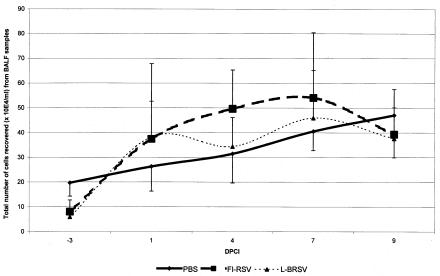
Respiratory infection is usually accompanied by an influx of lymphocytes and granulocytes into the lungs (Fig. 4). To obtain a better insight into the nature of the cell populations migrating into the infected lung, we performed phenotypic analyses of cells from BALF samples. BALF samples were collected at different time points before and after a bRSV challenge. Analysis of these samples revealed a strong eosinophil infiltration in FI-bRSV-immunized animals, starting at day 1 after challenge administration (Table 2). Four out of six FI-bRSV-immunized calves showed an eosinophilic infiltration (7% ± 10%) at day 4 after challenge administration. Eosinophil infiltration was observed in all calves at days 7 (19% ± 13%) and 9 (28% ± 19%) after challenge. The influx of eosinophils was slower in the L-bRSV-immunized group: only two BALF cell samples showed an eosinophilic infiltration at 7 days after challenge administration (5% ± 8%), and all animals showed eosinophil infiltration by day 9 after challenge administration (10% ± 7%). The eosinophilic infiltration observed at 7 days after the challenge infection was significantly greater (P = 0.0346) in the FI-bRSV group. No eosinophil infiltration was observed in the PBS-immunized group.
FIG. 4.
Total numbers of cells recovered from BALF samples (y axis) on particular days post challenge infection (x axis) per group. Significantly more bronchoalveolar cells were recovered from FI-bRSV-immunized calves (P = 0.0152) and L-bRSV-immunized calves (P = 0.0043) than from mock-immunized calves.
TABLE 2.
Total number of cells recovered in BALF samples and percentages of eosinophilic granulocytes (EO) before and after viral challenge
| Group | No. of bronchoalveolar cells (104/ml); % eosinophilic granules at:
|
||||
|---|---|---|---|---|---|
| −3 dpci | 1 dpci | 4 dpci | 7 dpci | 9 dpci | |
| Mock immunized | 13, 0 | 5, 0 | 48, 0 | 49, 0 | 11, 0 |
| 24, 0 | 29, 0 | 13, 0 | 39, 0 | 56, 0 | |
| 28, 0 | 31, 0 | 32, 0 | 26, 0 | 43, 0 | |
| 14, 0 | 27, 0 | 44, 0 | 37, 0 | 53, 0 | |
| 17, 0 | 36, 0 | 29, 0 | 48, 0 | 58, 0 | |
| 23, 0 | 30, 0 | 23, 0 | 44, 0 | 60, 0 | |
| FI-bRSV immunized | 13, 0 | 28, 1 | 49, 6 | 49, 18 | 36, 18 |
| 6, 0 | 41, 0 | 60, 27 | 31, 22 | 74, 54 | |
| 13, 0 | 29, 0 | 53, 7 | 38, 5 | 20, 4 | |
| 3, 0 | 52, 0 | 16, 3 | 107, 42 | 21, 14 | |
| 12, 0 | 59, 0 | 59, 0 | 33, 13 | 37, 35 | |
| 2, 0 | 15, 0 | 61, 0 | 67, 12 | 48, 40 | |
| L-bRSV immunized | 11, 0 | 18, 0 | 17, 0 | 80, 20 | 41, 18 |
| 5, 0 | 13, 0 | 44, 0 | 54, 0 | 48, 5 | |
| 9, 0 | 99, 1 | 31, 0 | 53, 8 | 56, 17 | |
| 0, 0 | 40, 0 | 53, 0 | 34, 0 | 23, 6 | |
| 5, 0 | 43, 0 | 34, 0 | 23, 0 | 37, 15 | |
| 7, 0 | 16, 0 | 27, 0 | 31, 0 | 21, 1 | |
Pathology.
All animals were euthanized at dpci 9 to allow a detailed analysis of virus- and/or immune response-induced pathology. At necropsy, the percentage of abnormal lung surface area ranged from 0.2 to 12.3% within the entire group of 18 animals. Mean percentages (± the standard error of the mean) in the PBS-, FI-bRSV-, and L-bRSV-immunized groups were 8.2% ± 2.6%, 3.6% ± 2.1%, and 0.8% ± 0.7%, respectively. When extended, consolidated lung tissue areas were scored as described by Viuff et al. (27), five out of six PBS-immunized calves and one FI-bRSV-immunized calf were scored 2 (5 to 15% consolidated lung tissue), whereas all of the others were scored 0 or 1. Differences between the PBS-immunized group and both of the other groups were significant (one-sided P values of 0.0390 for the PBS group versus the FI-bRSV group and 0.0076 for the PBS group versus the L-bRSV group).
Calves of all three groups had developed multifocal bronchointerstitial pneumonia, often with characteristic multinucleated syncytial cells, a marked proliferation of bronchiolar epithelium, alveolar epithelialization, alveolar edema, and hyaline membrane formation. The lumina of many bronchioles were narrow and were often filled with secretion. The superficial bronchial epithelial cells were swollen. In the bronchiolar walls, a variably dense lymphocytic infiltrate was observed. Lymphocytic aggregates and follicles were visible, especially in the calves that were immunized with PBS. The lung parenchyma showed atelectasis next to parts with alveolar emphysema.
The pathology data confirmed our findings on eosinophilic infiltrations in the lungs. In the calves immunized with FI-bRSV vaccine, we found marked eosinophilic tracheobronchitis and bronchiolitis characterized by diffuse infiltration of mainly eosinophils in the submucosa and in the interstitium surrounding the bronchi and bronchioles. In addition, a focal-to-diffuse eosinophilic periarteritis of the pulmonary arteries was observed. Quantification of eosinophilic densities in the respiratory tract showed no significant differences, although an indication of a difference (P = 0.0649) between the numbers of eosinophils in the FI-bRSV-primed animals compared to those in the live-bRSV-vaccinated calves was found. The highest numbers of eosinophils were found surrounding the walls of bronchi and bronchioles, and hardly any were found in the alveolar tissue and alveolar lumina. The calves immunized with PBS showed the most severe bronchointerstitial pneumonia but had no eosinophilic infiltration in the respiratory system. Finally, three predetermined site samples, chosen on the basis of gross pathology findings, were tested for bRSV by immunoperoxidase monolayer assay. In none of the samples tested was infectious bRSV detected.
DISCUSSION
In this report, we describe a bRSV challenge model in which both the classical bRSV respiratory infection and vaccine-enhanced immune pathology were reproduced. We have analyzed the kinetics of viral replication in the lungs, the humoral immune response, and the clinical symptoms in bRSV-infected calves that had been immunized with either L-bRSV or FI-bRSV or mock immunized with PBS.
One of the most striking findings in our study is the discrepancy between viral loads and humoral immune responses on the one hand and pathogenesis and clinical symptoms on the other hand. Three different patterns of infection and subsequent pathogenesis can be distinguished. First, in PBS-immunized and bRSV-infected animals, we have observed viral replication in the lungs and concurrent pathogenesis. In this situation, clearance of the viral infection coincided with a rise in (neutralizing) IgG antibody titers. Second, immunization with L-bRSV provides protective immunity: viral RNA was virtually undetectable, only mild clinical symptoms were observed, and (neutralizing) antibody titers increased rapidly after a challenge infection. The third, and most striking, pattern of infection was observed in the animals that had received the FI-bRSV vaccine. Immunization with FI-bRSV resulted in strong IgG antibody responses against F and G. Neutralizing antibodies could also be detected, but titers were lower than those of L-bRSV-immunized calves. Strikingly, these strong antibody responses did not prevent the early onset of severe clinical symptoms. Immune responses in these animals were characterized by high IgE antibody titers accompanied by marked eosinophilia. Preliminary data show that this IgE response is, at least in part, bRSV specific (unpublished results). Combined, these results are indicative of a type I hypersensitivity reaction. It should be noted, however, that challenge infection of L-bRSV-immunized calves was also associated with eosinophilia, albeit in a milder form (Table 2). Combined, we feel that our data are most consistent with a model in which pathogenesis in FI-bRSV-immunized calves has an immunological basis, whereas infection of mock-immunized animals leads to a more conventional virus-induced pathogenesis.
It is evident that accurate quantitation of clinical symptoms was essential for the interpretation of our data. The parameters that we have used include (i) the duration of fever, (ii) the extent of consolidated lung areas, and (iii) the analysis of acute-phase responses (haptoglobin). As shown by Heegaard and coworkers (10), the magnitude and duration of the haptoglobin response correlate with the severity of clinical signs (fever) and with the extent of lung consolidation. In our model, fever was recorded in PBS-immunized calves for 2 to 3 days and was associated with significant consolidated lung areas (and lack of eosinophilia). In contrast, less extensive acute-phase responses, shorter duration of fever, and less consolidated lung areas were found in L-bRSV-immunized group calves. Thus, viral loads, as well as clinical signs, were clearly reduced in L-bRSV-immunized calves. The situation was radically different in FI-bRSV-immunized animals: a pronounced acute-phase response was observed, peaking 6 to 7 days postchallenge, and fever was found in all six calves for 1 to 5 days. In contrast, pathological, consolidated lung areas were less extensive than in mock-immunized calves. Thus, for the FI-bRSV-immunized calves, we found no correlation between the haptoglobin response, eosinophilia, and clinical signs on the one hand and the extent of lung consolidation on the other hand. However, it should be noted that the calculated consolidated red lung areas, used as an indication of the pneumonic surface area, may not provide an accurate estimate of the total pneumonic involvement since we found many deep parenchymal lesions that did not have any surface involvement.
Our results obtained with FI-bRSV-immunized calves mimic the enhanced clinical situation in FI-hRSV-immunized and hRSV-infected infants, as reported by Kapikian et al. (12). So far, enhanced pathogenesis after immunization with FI virus has been difficult to reproduce in cattle. In one early study, similar adverse effects could be induced in calves after immunization with FI-bRSV vaccines (28), whereas West and coworkers only reported an earlier onset and resolution of clinical disease after immunization with FI-bRSV vaccine (29). In the field, Schreiber et al. reported high mortality rates associated with bRSV infection in Belgian Blue calves in the field previously vaccinated with (β-propiolactone-)inactivated bRSV vaccine (22). In contrast, Mohanty et al. observed both neutralizing antibodies and protection in calves after immunization with an FI-bRSV vaccine (18). Only Gershwin et al. have been able to demonstrate that immunization with an FI vaccine led to more severe clinical signs after a challenge compared to those of nonvaccinated control calves (9). However, in contrast to the findings of Gershwin et al., we found virus-neutralizing antibodies in FI-bRSV-immunized calves and found indications of a deregulated T-cell response (IgE), correlating with a hypersensitivity reaction (see below).
There has been extensive speculation on the nature of enhanced RSV pathogenesis after immunization (1, 19). So-called “sub-neutralizing” antibodies were the first potential culprits to be identified (1, 19). This hypothesis implies that the antibodies primed by immunization are insufficiently neutralizing, with the predicted result of higher virus titers after challenge. Consistent with this, Polack et al. (21) found that enhanced pathogenesis in the mouse model of RSV infection is associated with low neutralizing antibody titers, high viral titers, and the deposition of immune complexes. In contrast, we observed the opposite in our model: viral loads in FI-bRSV-immunized animals were strongly reduced, since no viral RNA was detected, and the FI-bRSV vaccine did induce virus-neutralizing antibody titers. Thus, our data are inconsistent with a model of enhancement that implies inadequate antibody function and an increased viral load.
The hypothesis that has attracted the most attention recently states that immunization with FI vaccines mainly primes a Th2-like inflammatory response (20). This vaccine-induced Th2-biased memory response would then set the stage for the expansion of Th2-polarized CD4 T cells and, indirectly, the enhanced pulmonary lesions found after a subsequent challenge. The Th2 cytokines IL-4, IL-13, and IL-5 would play major roles in this scheme. This model has received experimental support from the well-studied murine RSV infection model (11, 20, 26) and from an experimental RSV infection model involving macaques (7). Interestingly, these aberrant Th2-like responses are related to a hyperactive IgE response. Our findings of significant eosinophil infiltrations in BALF samples and high titers of IgE serum antibodies after a challenge infection in all FI-bRSV-immunized calves reflect an underlying Th2-biased CD4 T-cell response. It is of interest that a deregulated (Th2 biased) T-cell response has been hypothesized to play a key role in airway hypersensitivity reactions as asthma and airway hyperresponsiveness (11).
A deeper understanding of the role of vaccine-primed and T-cell-mediated immune dysregulation, as reflected by IgE responses, is a prerequisite for future rational vaccine design, and the bovine RSV infection model described in this study could be instrumental in understanding disease augmentation.
Acknowledgments
We thank Mieke Maris-Veldhuis, Klaas Weerdmeester, Tiny de Bruin, Eefke Weesendorp, Sjoerd Jobse, and Eline Verheij for technical support; Floor Bodet, Harry Rutgers, and their colleagues for biotechnical support; and A. Korevaar for performing the postmortem examinations. The IgE-specific antisera and the standard positive serum used for the indirect DAS ELISA were kindly provided by Frans Kooyman (Veterinary Faculty, Utrecht University, Utrecht, The Netherlands).
REFERENCES
- 1.Ananaba, G. A., and L. J. Anderson. 1991. Antibody enhancement of respiratory syncytial virus stimulation of leukotriene production by a macrophagelike cell line. J. Virol. 65:5052-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, J. C., R. E. Werdin, T. R. Ames, R. J. Markham, and V. L. Larson. 1986. Study on the etiologic role of bovine respiratory syncytial virus in pneumonia of dairy calves. J. Am. Vet. Med. Assoc. 189:66-70. [PubMed] [Google Scholar]
- 3.Belknap, E. B., J. C. Baker, J. S. Patterson, R. D. Walker, D. M. Haines, and E. G. Clark. 1991. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J. Infect. Dis. 163:470-476. [DOI] [PubMed] [Google Scholar]
- 4.Bryson, D. G., J. B. McFerran, H. J. Ball, and S. D. Neill. 1978. Observations on outbreaks of respiratory disease in housed calves. 2. Pathological and microbiological findings. Vet. Rec. 103:503-509. [DOI] [PubMed] [Google Scholar]
- 5.Bryson, D. G., M. S. McNulty, E. F. Logan, and P. F. Cush. 1983. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am. J. Vet. Res. 44:1648-1655. [PubMed] [Google Scholar]
- 6.Ciszewski, D. K., J. C. Baker, R. F. Slocombe, J. F. Reindel, D. M. Haines, and E. G. Clark. 1991. Experimental reproduction of respiratory tract disease with bovine respiratory syncytial virus. Vet. Microbiol. 28:39-60. [DOI] [PubMed] [Google Scholar]
- 7.De Swart, R. L., T. Kuiken, H. H. Timmerman, G. van Amerongen, B. G. van den Hoogen, H. W. Vos, H. J. Neijens, A. C. Andeweg, and A. D. M. E. Osterhaus. 2002. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J. Virol. 76:11561-11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogarty, U., P. J. Quinn, and J. Hannan. 1983. Bronchopulmonary lavage in the calf: a new technique. Irish Vet. J. 37:35-38.
- 9.Gershwin, L. J., E. S. Schelegle, R. A. Gunther, M. L. Anderson, A. R. Woolums, D. R. Larochelle, G. A. Boyle, K. E. Friebertshauser, and R. S. Singer. 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16:1225-1236. [DOI] [PubMed] [Google Scholar]
- 10.Heegaard, P. M., D. L. Godson, M. J. Toussaint, K. Tjornehoj, L. E. Larsen, B. Viuff, and L. Ronsholt. 2000. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 77:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, T. R., R. A. Parker, J. E. Johnson, and B. S. Graham. 2003. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 170:2037-2045. [DOI] [PubMed] [Google Scholar]
- 12.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shvedoff, and C. E. Stewart. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405-421. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 14.Kimman, T. G., G. M. Zimmer, F. Westenbrink, J. Mars, and E. van Leeuwen. 1988. Epidemiological study of bovine respiratory syncytial virus infections in calves: influence of maternal antibodies on the outcome of disease. Vet. Rec. 123:104-109. [DOI] [PubMed] [Google Scholar]
- 15.Kooyman, F. N., A. P. Yatsuda, H. W. Ploeger, and M. Eysker. 2002. Serum immunoglobulin E response in calves infected with the lungworm Dictyocaulus viviparus and its correlation with protection. Parasite Immunol. 24:47-56. [DOI] [PubMed] [Google Scholar]
- 16.Kuno, G. 1998. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 72:27-41. [DOI] [PubMed] [Google Scholar]
- 17.Langedijk, J. P., W. G. Middel, W. M. Schaaper, R. H. Meloen, J. A. Kramps, A. H. Brandenburg, and J. T. van Oirschot. 1996. Type-specific serologic diagnosis of respiratory syncytial virus infection, based on a synthetic peptide of the attachment protein G. J. Immunol. Methods 193:157-166. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty, S. B., D. D. Rockemann, J. P. Davidson, O. I. Sharabrin, and S. M. Forst. 1981. Effect of vaccinal serum antibodies on bovine respiratory syncytial viral infection in calves. Am. J. Vet. Res. 42:881-883. [PubMed] [Google Scholar]
- 19.Murphy, B. R., G. A. Prince, E. E. Walsh, H. W. Kim, R. H. Parrott, V. G. Hemming, W. J. Rodriguez, and R. M. Chanock. 1986. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J. Clin. Microbiol. 24:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Openshaw, P. J., F. J. Culley, and W. Olszewska. 2001. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20(Suppl. 1):S27-S31. [DOI] [PubMed] [Google Scholar]
- 21.Polack, F. P., M. N. Teng, P. L. Collins, G. A. Prince, M. Exner, H. Regele, D. D. Lirman, R. Rabold, S. J. Hoffman, C. L. Karp, S. R. Kleeberger, M. Wills-Karp, and R. A. Karron. 2002. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 196:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber, P., J. P. Matheise, F. Dessy, M. Heimann, J. J. Letesson, P. Coppe, and A. Collard. 2000. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J. Vet. Med. B Infect. Dis. Vet. Public Health 47:535-550. [DOI] [PubMed] [Google Scholar]
- 23.Stott, E. J., and G. Taylor. 1985. Respiratory syncytial virus. Brief review. Arch. Virol. 84:1-52. [DOI] [PubMed] [Google Scholar]
- 24.Van Den Ingh, T. S., J. Verhoeff, and A. P. Van Nieuwstadt. 1982. Clinical and pathological observations on spontaneous bovine respiratory syncytial virus infections in calves. Res. Vet. Sci. 33:152-158. [PubMed] [Google Scholar]
- 25.van der Poel, W. H., R. S. Schrijver, W. G. Middel, J. A. Kramps, A. Brand, and J. T. Van Oirschot. 1996. Experimental reproduction of respiratory disease in calves with non-cell-culture-passaged bovine respiratory syncytial virus. Vet. Q. 18:81-86. [DOI] [PubMed] [Google Scholar]
- 26.Varga, S. M., X. Wang, R. M. Welsh, and T. J. Braciale. 2001. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity 15:637-646. [DOI] [PubMed] [Google Scholar]
- 27.Viuff, B., K. Tjornehoj, L. E. Larsen, C. M. Rontved, A. Uttenthal, L. Ronsholt, and S. Alexandersen. 2002. Replication and clearance of respiratory syncytial virus: apoptosis is an important pathway of virus clearance after experimental infection with bovine respiratory syncytial virus. Am. J. Pathol. 161:2195-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wellemans, G., and J. Leunen. 1975. Le virus respiratoire syncytial et les troubles respiratoires des bovins. Ann. Med. Vet. 119:359-369. [Google Scholar]
- 29.West, K., L. Petrie, D. M. Haines, C. Konoby, E. G. Clark, K. Martin, and J. A. Ellis. 1999. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine 17:809-820. [DOI] [PubMed] [Google Scholar]
- 30.Westenbrink, F., J. M. Brinkhof, P. J. Straver, J. Quak, and P. W. De Leeuw. 1985. Comparison of a newly developed enzyme-linked immunosorbent assay with complement fixation and neutralisation tests for serology of bovine respiratory syncytial virus infections. Res. Vet. Sci. 38:334-340. [PubMed] [Google Scholar]