Abstract
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 are highly related viruses that differ in disease manifestation. HTLV-1 is the etiologic agent of adult T-cell leukemia and lymphoma, an aggressive clonal malignancy of human CD4-bearing T lymphocytes. Infection with HTLV-2 has not been conclusively linked to lymphoproliferative disorders. We previously showed that human hematopoietic progenitor (CD34+) cells can be infected by HTLV-1 and that proviral sequences were maintained after differentiation of infected CD34+ cells in vitro and in vivo. To investigate the role of the Tax oncoprotein of HTLV on hematopoiesis, bicistronic lentiviral vectors were constructed encoding the HTLV-1 or HTLV-2 tax genes (Tax1 and Tax2, respectively) and the green fluorescent protein marker gene. Human hematopoietic progenitor (CD34+) cells were infected with lentivirus vectors, and transduced cells were cultured in a semisolid medium permissive for the development of erythroid, myeloid, and primitive progenitor colonies. Tax1-transduced CD34+ cells displayed a two- to fivefold reduction in the total number of hematopoietic clonogenic colonies that arose in vitro, in contrast to Tax2-transduced cells, which showed no perturbation of hematopoiesis. The ratio of colony types that developed from Tax1-transduced CD34+ cells remained unaffected, suggesting that Tax1 inhibited the maturation of relatively early, uncommitted hematopoietic stem cells. Since previous reports have linked Tax1 expression with initiation of apoptosis, lentiviral vector-mediated transduction of Tax1 or Tax2 was investigated in CEM and Jurkat T-cell lines. Ectopic expression of either Tax1 or Tax2 failed to induce apoptosis in T-cell lines. These data demonstrate that Tax1 expression perturbs development and maturation of pluripotent hematopoietic progenitor cells, an activity that is not displayed by Tax2, and that the suppression of hematopoiesis is not attributable to induction of apoptosis. Since hematopoietic progenitor cells may serve as a latently infected reservoir for HTLV infection in vivo, the different abilities of HTLV-1 and -2 Tax to suppress hematopoiesis may play a role in the respective clinical outcomes after infection with HTLV-1 or -2.
Human T-cell leukemia virus type 1 (HTLV-1) infection is associated with development of adult T-cell leukemia and lymphoma (ATL/ATLL), an aggressive clonal malignancy of HTLV-infected CD4-bearing T lymphocytes which manifests decades after the initial infection event (99, 100). Although deletion of HTLV proviral sequences is relatively common in ATL cells, the HTLV-1 tax gene is always retained in tumor cells, suggesting that it plays a key role in leukemogenesis (57). Previous investigations have failed to demonstrate conclusively an etiologic association of HTLV-2 infection with lymphoproliferative disorders (35, 46, 47, 69). Studies with infectious HTLV-1 and HTLV-2 molecular clones have directly shown that Tax expression is essential for cellular transformation (82, 85). Tax functions to transactivate viral transcription by interaction with the 5′ long terminal repeat (LTR) (11, 17, 30, 83). Tax also modulates the expression of a variety of cellular genes that impact cell cycle regulatory pathways, including the stimulation of expression of cytokines and interleukins. Cellular genes upregulated by Tax include interleukin-2 (IL-2), IL-2 receptor α, IL-1, IL-3, IL-6, IL-8, IL-15, tumor necrosis factors alpha and beta, lymphotoxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte-colony-stimulating factor (G-CSF) (6, 13, 25, 26, 37, 45, 62, 72, 73, 76). HTLV-1 Tax (Tax1) inhibits DNA repair by repression of DNA β-polymerase expression and nucleotide base excision repair and also downmodulates the activity of tumor suppressor proteins p53, hDLG, and p16INK4a (2, 12, 28, 48, 53, 58, 95, 96). Tax1 has also been shown to induce expression of the cyclin-dependent kinase inhibitor p21Cip1/Waf1, a critical regulator of cell cycle progression (28). Reduction of genomic stability and mediation of cell cycle progression by Tax1 presumably play a role in the induction of cellular transformation and in the development of ATL (67, 71, 88). Some reports have also linked Tax expression with initiation of apoptosis in transformed cell lines, while other investigators have demonstrated that Tax1 inhibits apoptosis (21-23, 44, 50, 51, 54, 64, 75, 81, 98).
Tax1 exhibits more than 77% amino acid homology with HTLV-2 Tax (Tax2), and expression of either Tax1 or Tax2 is sufficient for immortalization of T lymphocytes for growth in culture (41, 84). Distinct biological activities of Tax1 and Tax2 have, however, been reported. Tax1 can induce micronuclei in simian cell cultures, in contrast to Tax2, which lacks this function (67, 89). Tax1 is more effective at inhibiting cellular p53 activity and displays elevated transformation activity in Rat-1 cells in comparison to Tax2 (29, 43, 66). HTLV-1-transformed T-cell lines also displayed a higher tumorigenic potential when inoculated into severe combined immunodeficient (SCID) mice in comparison to HTLV-2-transformed lymphoid cells (32). Although differences in phenotype exhibited by Tax1 and Tax2 have been demonstrated, the specific regions of Tax accounting for these behaviors in cells have not been well characterized.
Human hematopoietic progenitor cells bearing the CD34 antigen are capable of differentiation into mature endstage cells, and abnormalities in the developmental program can result in the development of blood cell diseases including leukemia. We previously reported that HTLV-1 was capable of infecting human hematopoietic progenitor (CD34+) cells (31). HTLV-1 proviral sequences were detected in clonogenic colonies of all hematopoietic lineages arising from infected CD34+ cells. In addition, reconstitution of T lymphopoiesis with HTLV-1-infected CD34+ cells in SCID-hu mice resulted in the development of HTLV-infected T cells and an altered display of thymocyte subsets, suggesting that HTLV infection and Tax1 expression in hematopoietic progenitor cells may have a profound effect on the perturbation of hematopoiesis.
Here we describe the generation and characterization of lentivirus-based vectors capable of coexpressing HTLV-1 and -2 tax and the green fluorescent protein (GFP) marker gene from a bicistronic mRNA. These lentivirus vectors allow for the analysis and characterization of Tax activity on the differentiation of hematopoietic progenitor cells in the absence of additional HTLV gene products. We show that transduction of Tax1 into human CD34+ cells results in marked suppression of multilineage hematopoiesis in vitro, in contrast to transduction of Tax2. Expression of Tax1 was not sufficient for initiation of apoptosis in T-cell lines, suggesting that Tax1 inhibits the maturation and differentiation of CD34+ cells rather than inducing programmed cell death.
MATERIALS AND METHODS
Cell lines.
293T and HeLa cells were cultured in Dulbeccos modified Eagle medium (DMEM) (Gibco-BRL, Grand Island, N.Y.) with 10% heat-inactivated fetal bovine serum (FBS; Gemini, Calabasas, Calif.), 2 mM l-glutamine (Gibco-BRL), and 100 U of penicillin/ml and 100 μg of streptomycin/ml (Pen/Strep; Gemini). Jurkat and CEM cell lines were cultured in Iscove modified Dulbecco medium (IMDM) (Gibco-BRL), supplemented with 10% FBS, 2 mM l-glutamine, and Pen/Strep at 37°C in a humidified incubator with 5% CO2.
Tax transactivation assay.
Lentiviral vector constructs were cotransfected with pHTLV-1-LTR-CAT or pHTLV-2-LTR-CAT into 293T cells, constructs encoding the bacterial chloramphenicol acetyltransferase (CAT) gene expressed from the HTLV-1 LTR or HTLV-2 LTR, respectively. Then, 2.5 μg of each lentiviral vector construct was cotransfected with 2.5 μg of pHTLV-1-LTR-CAT or pHTLV-2-LTR-CAT into 293T cells (5.0 × 105) by using the calcium phosphate DNA precipitation method (38). Cell pellets were lysed by using the freeze-thaw method, and 5 μg of the cell extract was analyzed for CAT activity. Cell extracts were tested by using a Bradford assay, and CAT reactions were normalized for the amounts of protein, as previously described (8). Acetylated products were measured by using a Molecular Dynamics (Sunnyvale, Calif.) PhosphorImager 445SI and then analyzed for CAT activity by using the Molecular Dynamics ImageQuant 5.1 program.
Lentiviral vector construction.
The pHR′CMV-Tax1/GFP and pHR′CMV-Tax1(−)/GFP were constructed as previously described (97). The HTLV-2 tax cDNA was isolated by PCR amplification from BC20.2 (a gift from Irvin S. Chen, University of California, Los Angeles) (42), by using forward primer 25-mer (5′-TGCGCTCGAGACCACCAACACCATG-3′) and reverse primer 25-mer (5′-TGGGATCCCTAGTCGCCATTGTCAT-3′). The amplified cDNA fragment was subcloned into the PCR cloning vector pCR2.1 (Invitrogen, Carlsbad, Calif.), creating pCR2.1Tax2. The Tax2 fragment was isolated by BamHI and XhoI restriction digestion of pCR2.1Tax2 and ligated into pHR′CMV-Tax1/GFP, which was first digested with XhoI and BamHI to liberate the tax1 gene and then purified by gel electrophoresis. Sequencing of pHR′CMV-Tax2/GFP confirmed the insertion of the tax2 gene. pHR′CMV-Vpr/GFP was a gift from Vicente Planelles (University of Utah, Salt Lake City), and pCMV-Tax1 was a gift from William Wachsman (University of California at San Diego).
Generation of VSV-G-pseudotyped lentivirus vectors.
Vesicular stomatitis virus protein G (VSV-G)-pseudotyped lentiviral vector virus stocks were generated as previously described (14, 97). Briefly, a three-plasmid system was used; transfer plasmid (pHR′CMV-GFP, pHR′CMV-Tax1/GFP, pHR′CMV-Tax1(−)/GFP, pHR′CMV-Tax2/GFP, or pHR′CMV-Vpr/GFP), the pCMVΔR8.2ΔVPR (9) packaging vector, and pHCMV-G (15) were cotransfected into 2 × 107 293T cells by calcium phosphate precipitation. Cells were incubated in DMEM supplemented with 100 μM chloroquine for 24 h; the medium was then removed and replaced with 25 ml of DMEM plus 10% FBS. Supernatants were harvested at 3 and 5 days posttransfection, filtered (0.45-μm-pore-size filter), pooled, and subjected to ultracentrifugation (50,000 × g for 2 h) by using an SW27 rotor (Beckman, Palo Alto, Calif.). The pellet was resuspended in 1/100 initial volume in serum-free DMEM overnight at 4°C and then pooled and frozen at −80°C. Titers of virus stocks were determined by infecting HeLa cells (3 × 105) with virus stocks that were serially diluted (1:10, 1:100, and 1:500) in serum-free DMEM. Cells were analyzed for GFP at 72 h postinfection by flow cytometry. pA18G-BHK-21 cells were infected concurrently with serially diluted viral stocks and assayed for β-galactosidase activity at 72 h postinfection, as previously described (5, 97). Virus titers generally ranged from 106 to 107 transducing units per ml.
Apoptosis analysis.
Jurkat and CEM cells (106) were infected (multiplicity of infection [MOI] = 3) in a final volume of 3 ml of DMEM containing 8 μg of Polybrene/ml (Sigma, St. Louis, Mo.). Cells were exposed to virus for 4 h at 2,500 rpm (555 × g) in a Beckman-Coulter GPR centrifuge at room temperature. Cells were resuspended in 20 ml of IMDM plus 10% FBS. At 48 and 72 h postinfection, 5 × 105 cells were stained with phycoerythrin (PE)-conjugated Annexin V (Biovision, Mountain View, Calif.) and 7-amino actinomycin D (7-AAD; Calbiochem, La Jolla, Calif.), as previously described (87). Cells were washed twice with 5 ml of phosphate-buffered saline (PBS) and suspended in 500 μl of binding buffer and 5 μl of Annexin V-PE. Cells were incubated at room temperature for 15 min in darkness and then washed again with 5 ml of PBS. Cells were then resuspended in 1 ml of PBS with 1 μl of 7-AAD (1.0 mg/ml). Cells were washed once with 5 ml of PBS and analyzed on a FACSSTAR flow cytometer (Becton Dickinson, Mountain View, Calif.). The data was analyzed by using WinMidi software.
Isolation of hematopoietic progenitor (CD34+) cells.
Human CD34+ cells were prepared as previously described (31). Briefly, fragments of fetal liver tissues were washed in PBS, and a single-cell suspension was obtained by digestion in collagenase (507 U/ml), hyaluronidase (2,400 U/ml), and DNase (300 Kunitz units/ml) in serum-free AIM-V medium (Gibco-BRL) for 4 h at 37°C. Erythrocytes were removed by centrifugation at 1,500 rpm (200 × g) in a Beckman GPR centrifuge for 15 min at room temperature over a Ficoll-Hypaque (Sigma) gradient. Mononuclear cells were incubated with anti-CD34 magnetic beads (Miltenyi Biotec, Calabasas, Calif.) for 30 min at room temperature. CD34+ cells were purified by passage of the cell suspension through a MidiMACS magnetic column (Miltenyi Biotec). The purity of isolated CD34+ cells was analyzed with a PE-conjugated monoclonal antibody (MAb) against CD34+ (Becton Dickinson) and found to be at least 92% CD34 positive.
Transduction of CD34+ cells with lentiviral vectors.
Enriched CD34+ cell populations were infected with VSV-G-pseudotyped lentivirus vectors as previously described (4). Briefly, purified CD34+ cells (3 × 106) were infected (MOI = 3) in a final volume of 3.0 ml containing 8 μg of Polybrene/ml. Cells were infected by centrifugation, as described above, and then resuspended in 3.0 ml of IMDM supplemented with 30 μl of StemSpan cytokine cocktail CC100 (StemCell Technologies, Vancouver, British Columbia, Canada) containing Flt-3 ligand (100 ng/ml), stem cell factor (100 ng/ml), IL-3 (20 ng/ml), and IL-6 (20 ng/ml). After 3 days, the cell culture was incubated with a PE-conjugated anti-CD34 MAb and CD34+/GFP+ cells were purified by fluorescence-activated cell sorting (FACS) with a FACSVantage flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The CellQuest program (Becton Dickinson Immunocytometry Systems) was used for data analysis.
Clonogenic colony-forming assays.
Sorted CD34+/GFP+ cells (103) were cultured in 2 ml of Methocult medium H4433 (StemCell Technologies) at 37°C in a humidified atmosphere with 5% CO2. Clonogenic granulocyte-macrophage (CFU-GM), erythroid burst (BFU-E), and highly proliferative pluripotent (HPP-CFC) CFU were identified by morphology at 12 to 14 days postplating and counted under an inverted fluorescent microscope (Leica DMIL) (90). Colonies were randomly isolated by aspiration, and DNA and RNA were subjected to PCR analysis. Fluorescent photomicrographs were taken with a digital camera attached to an inverted fluorescent microscope (Eclipse TE300; Nikon). Each assay was done in triplicate. Statistics were performed by single-factor analysis of variance (ANOVA) analysis (P < 0.005).
PCR analysis of clonogenic colonies.
DNA and RNA were extracted from clonogenic colonies by the urea lysis method as previously described (31, 101, 102). Quantitative DNA PCR for HTLV tax-rex sequences (nucleotides 7336 to 7495; primers 670 and 671) and human β-globin sequences (LA1/LA2) were determined as previously described (31-33). Uninfected human peripheral blood mononuclear cell DNA and linearized pH6neo (a plasmid containing an HTLV-2 infectious proviral clone) were serially diluted and analyzed in parallel as controls. RNA was extracted from randomly chosen clonogenic colonies by using Trizol (Gibco-BRL) and analyzed by reverse transcription-PCR (RT-PCR) analysis according to the manufacturer's protocol. RNA was resuspended in 100 μl of RNase-free water containing 1 μl (1 U/μl) of DNase (Promega, Madison, Wis.). Detection of tax-rex mRNA transcripts was performed with primers 670 and 671 described above and the OneStep RT-PCR kit (Qiagen, Valencia, Calif.). Briefly, primers were labeled with 32P by the T4 kinase reaction. A total of 10 μl of mRNA was combined with 10 μl of 5× RT-PCR buffer, 2 μl of deoxynucleoside triphosphate mix (a 10 mM concentration of each deoxynucleoside triphosphate), 1 μl of each of the 3′ and 5′ primers (primers 670 and 671), 2 μl of RT-PCR enzyme mix, and 24 μl of RNase-free water according to the manufacturer's instructions. Each reaction was programmed for 30 min at 50°C for the reverse transcriptase reaction, followed by 15 min at 95°C to inactivate the reverse transcriptase and 40 cycles of 1 min at 94°C, 30 s at 60°C, and 1 min at 72°C. Products from the PCRs were analyzed on a 6% acrylamide gel.
Real-time quantitative PCR of clonogenic colonies.
Quantification of tax DNA sequences and human β-globin sequences in clonogenic colonies was assayed by real-time quantitative PCR (Q-PCR) by using the SYBR-GREEN quantitative real-time PCR kit (Qiagen). PCR was performed in a final volume of 25 μl consisting of a master mix containing 12.5 μl of SYBR-GREEN mix, 2.5 μl of 10× buffer, forward and reverse primers at 4 ng/μl, 11 μl of water, and 5 μl of template DNA according to the manufacturer's instructions. The tax gene was amplified with the primers described above. All real-time PCRs were performed in 25 μl of SmartCycler tubes on a SmartCycler system (Cepheid, Sunnyvale, Calif.). The amount of sampled DNA was from ca. 5 to 20 human cells per PCR. The thermal cycling conditions consisted of 40 cycles at 95°C for 30 s, 60°C for 1 min, 72°C for 1 min, and 78°C for 45 s. The fluorescence signal increase of SYBR-GREEN was automatically detected during the 78°C phase of the PCR. For each sample, the SmartCycler system provided an amplification curve constructed by relating the fluorescence signal intensity (ΔRn) to the cycle number. Cycle threshold (Ct) was defined as the cycle number at which the fluorescence signal was more than 30 fluorescent units above the mean background noise collected from the 5th to the 10th cycle. The standard curves for the calculation of tax and β-globin sequences were accomplished by using the identical standards used in the Q-PCR analysis. Reaction conditions were programmed on a Dell computer (Dell, Roundrock, Tex.) linked directly to the SmartCycler system. Analysis of cycle data was performed on the software developed by Cepheid.
RESULTS
Construction and characterization of bicistronic lentiviral vectors expressing HTLV Tax.
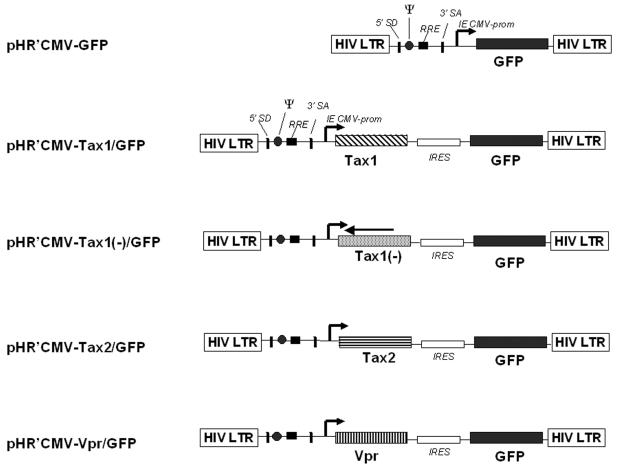
We designed lentivirus vectors by incorporating a strategy of insertion of a translational cis-acting element, the internal ribosome entry site sequence, from encephalomyocarditis virus (Fig. 1). We previously described the feasibility and advantages of employing a bicistronic lentivirus vector (LV) containing two open reading frames (97, 103). The particular advantages of using LVs for the present study are (i) the efficient infection of actively dividing, as well as nondividing cells (i.e., hematopoietic stem and progenitor cells); (ii) lack of expression of human immunodeficiency virus type 1 (HIV-1) gene products that would interfere with analysis of apoptosis; and (iii) stable gene transduction and expression. Viral genes are coexpressed with the GFP marker gene from a bicistronic mRNA initiated at an internal cytomegalovirus (CMV) immediate-early promoter (Fig. 1) (97, 103). Transient-transfection (CAT) assays with 293T cells demonstrated that HTLV-1 Tax and HTLV-2 Tax were each capable of transactivating transcription from the HTLV-1 LTR (LTR-1-CAT), as well as the HTLV-2 LTR (LTR-2-CAT) (Table 1). These results are in agreement with previous reports demonstrating that Tax1 and Tax2 can each transactivate transcription from the reciprocal viral LTR (84, 85, 89). A vector containing the tax1 gene in the antisense orientation (pHR′CMV-Tax1(−)/GFP) was constructed as a control and did not code for functional Tax protein. LV stocks pseudotyped with VSV-G envelope protein were generated by cotransfection of 293T cells with a HIV-1 packaging construct that lacks the HIV-1 Vpr gene (pCMVΔR8.2ΔVpr, a gift from Irvin Chen, University of California at Los Angeles) and pHCMV-G, as previously described (4, 97). Titers of virus stocks were determined by infection of HeLa cells and by flow cytometric analysis of GFP expression. pA18G-BHK-21 cells harbor a stable integration of an HTLV-1 LTR/β-galactosidase construct (5). Infection of pA18G-BHK-21 cells with HR′CMV-Tax1/GFP and HR′CMV-Tax2/GFP was used to confirm viral titers and to demonstrate functional Tax1 and Tax2 transcriptional transactivation activity (data not shown) (97).
FIG. 1.
Schematic representation of multigene lentiviral vectors. Multigene vectors based on the use of an internal ribosomal entry site sequence from the encephalomyocarditis virus (80). The target gene and reporter gene are driven by an immediate-early CMV promoter (IE CMV prom). pHR′CMV-Tax1(−)/GFP contains the HTLV-1 tax gene inserted in the antisense orientation and does not encode for functional Tax1. pHR′CMV-Vpr/GFP encodes the HIV-1 vpr gene (Vicente Planelles). SA, splice acceptor; SD, splice donor; RRE, HIV-1 Rev response element; Ψ, psi packaging signal for the transfer vector.
TABLE 1.
CAT activities of lentiviral expression vectors
| Vector construct | % Acetylationa
|
|||||
|---|---|---|---|---|---|---|
| pHTLV-1-LTR-CATb
|
pHTLV-2-LTR-CATc
|
|||||
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | Expt 5 | Expt 6 | |
| pHR′CMV-Tax1/GFP | 100 | 100 | 100 | 100 | 100 | 100 |
| pHR′CMV-Tax2/GFP | NT | NT | 48 | 57 | 125 | 117 |
| pHR′CMV-Tax1(−)/ GFP | NT | 3.3 | NT | 8 | NT | 18 |
| pHR′CMV/GFP | 3 | NT | 11 | 8 | 25 | 5 |
| pCMV-Tax1d | 300 | 100 | NT | NT | NT | NT |
| BC20.2e | NT | NT | 202 | 199 | 1150 | 180 |
pHTLV-1-LTR-CAT and pHTLV-2-LTR-CAT expression plasmids (2.5 μg) were cotransfected by calcium phosphate precipitation with lentiviral vector constructs (2.5 μg) into 5 × 105 293T cells. GFP expression was monitored for each transfection, and >80% of the cells were transfected in each experiment. Cells were harvested and assayed for CAT activity 48 h posttransfection as described in Materials and Methods. The numbers represent the average percent acetylation values and are normalized to the value for pHR′CMV-Tax1/GFP for each experiment (set at 100%). NT, not tested.
pHTLV-1-LTR-CAT contains the HTLV-1 LTR initiating transcription of the bacterial CAT gene.
pHTLV-2-LTR-CAT contains the HTLV-2 LTR initiating transcription of the bacterial CAT gene.
pCMV-Tax1 encodes the HTLV-1 tax gene expressed from the immediate early CMV promoter (a gift from William Wachsman, University of California at San Diego).
BC20.2 is the tax/rex cDNA clone of HTLV-2 (42).
Ectopic expression of HTLV Tax1 fails to induce apoptosis in T lymphoid cells.
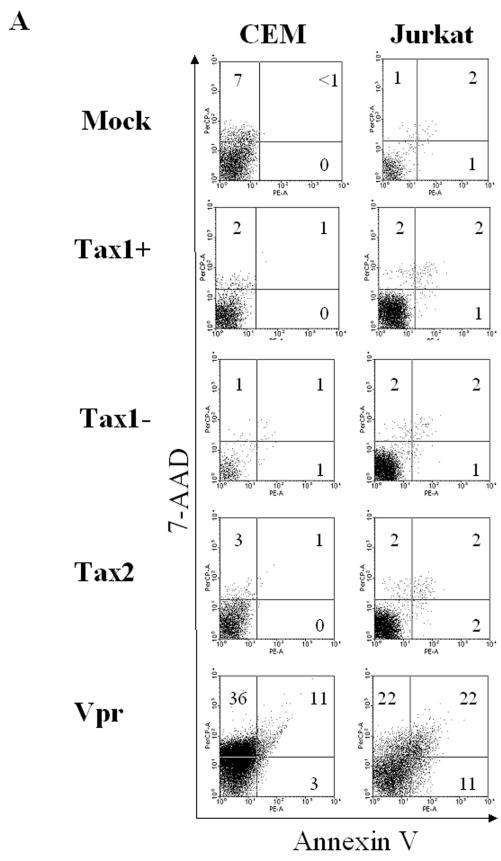
Previous reports have indicated that expression of Tax1 is sufficient to induce apoptosis in some immortalized cell lines (21-23, 44, 51, 54, 64, 75, 81, 98). We used LV-mediated transduction to establish whether ectopic expression of Tax1 or Tax2 in T lymphoid cells could induce programmed cell death. As a positive control for the induction of apoptosis, a LV construct encoding the HIV-1 Vpr gene was used. It has been previously documented that HIV-1 Vpr induces G2 arrest and extensive apoptosis in cells (7, 49, 77, 92, 93). CEM and Jurkat T lymphoid cell lines were infected with HR′CMV-Tax1/GFP, HR′CMV-Tax2/GFP, HR′CMV-Tax1(−)/GFP, HR′CMV-Vpr/GFP, or HR′CMV-GFP (MOI = 3), and cells which expressed GFP were analyzed for staining with Annexin V and the dead cell exclusion dye 7-AAD (Fig. 2).
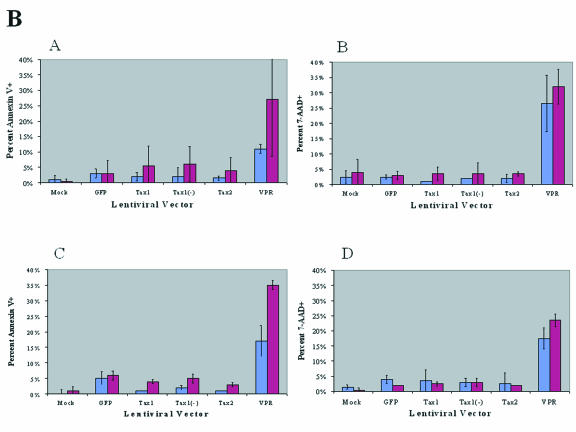
FIG. 2.
Flow cytometric analysis of CEM and Jurkat T cells transduced with HTLV Tax. Jurkat or CEM cells (106) were infected with lentiviral vectors (MOI = 3) by centrifugation (4 h, 2,500 rpm) in the presence of 8 μg of Polybrene/ml. CEM and Jurkat cells (106) were mock infected as a negative control for apoptosis. Cells were resuspended in IMDM with 10% FBS, stained with Annexin V-PE (AV-PE) and 7-AAD, and analyzed by flow cytometry at 48 and 72 h postinfection. (A) Representative flow cytometric analysis of LV-transduced CEM and Jurkat cells at 72 h postinfection. GFP+ cells were gated and analyzed for AV (horizontal axis) and 7-AAD staining (vertical axis). Representative panels of transduced CEM (left column) and Jurkat (right column) cells. The percentage of individual subpopulations in each quadrant is indicated. Cells that are AV-PE single positive (lower right quadrant) and AV-PE+ 7-AAD+ positive (upper right quadrant) are early- and late-stage apoptotic cells, respectively. (B) Annexin V and 7-AAD staining of Jurkat and CEM cells transduced with LVs. Jurkat and CEM cells were transduced with LVs and GFP+ cells were analyzed for AV and 7-AAD staining by flow cytometry. CEM cells (subpanels A and B) and Jurkat cells (subpanels C and D) were analyzed at 48 h (blue bars) or 72 h (purple bars) postinfection. The percentage of GFP+ cells that were positive for AV staining (subpanels A and C) or which stained for 7-AAD (subpanels B and D), after infection with HR′CMV-Tax1/GFP transduction (Tax1), HR′CMV-Tax1(−)/GFP [Tax1(−)], HR′CMV-Tax2/GFP (Tax2), or HR′CMV-GFP (GFP) are indicated. Values of AV and 7-AAD expression with HR′CMV-Vpr/GFP (VPR) were significantly higher than those obtained with the other vectors, which in turn were higher than those obtained with medium alone (Mock). The data were compiled from three independent experiments. Statistical analysis was performed by ANOVA (P < 0.005).
No significant levels of apoptosis were detected in CEM cells expressing Tax1 or Tax2. The percentage of Tax1- or Tax2-transduced (GFP+) cells that stained for Annexin V ranged from 1 and 2% at 72 h postinfection, similar to levels displayed by mock-transduced CEM cells (Fig. 2A). In contrast, CEM cells transduced with HIV-1 Vpr showed significantly elevated levels of Annexin V (14%), confirming that expression of Vpr is sufficient to induce apoptosis. Between 3 and 4% of Jurkat cells transduced with Tax1, Tax2, or the antisense Tax1 vector [Tax1(−)] displayed Annexin V staining in contrast to 33% of Jurkat cells transduced with Vpr at 72 h postinfection. The lack of apoptosis after Tax1 or Tax2 transduction was also reflected in the percentage of cells staining with 7-AAD. Between 3 and 4% of CEM cells infected with LVs encoding Tax1, Tax2, or Tax1(−) showed staining with 7-AAD at 72 h postinfection in comparison to 48% of cells transduced with Vpr. In Jurkat cells, 4% of the cells transduced with either Tax1 or Tax2 stained for 7-AAD versus 44% of Vpr-transduced cells. These data demonstrate that ectopic expression of Tax1 or Tax2 from LVs is not sufficient for induction of apoptosis in human lymphoid cells. Our results are in agreement with observations by other investigators who have also demonstrated that expression of Tax1 is not sufficient for induction of apoptosis (44, 50, 51).
Tax1 transduction of human CD34+ cells suppresses hematopoiesis in vitro.
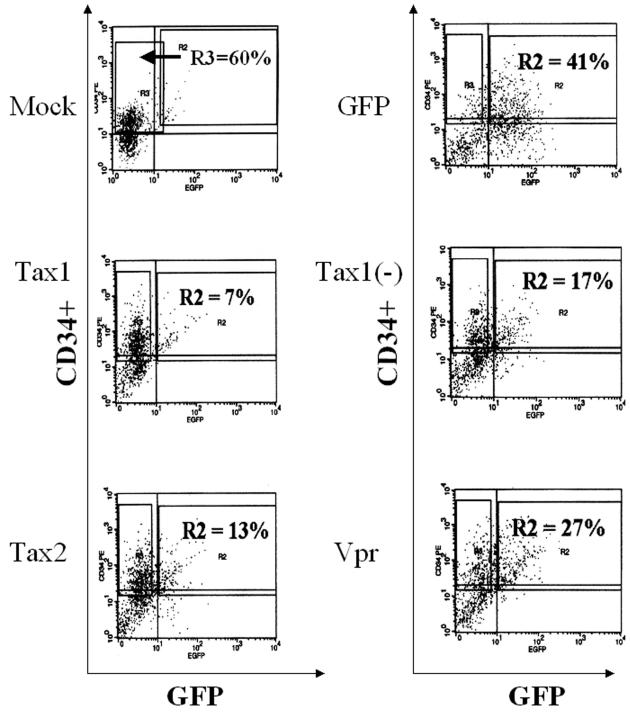
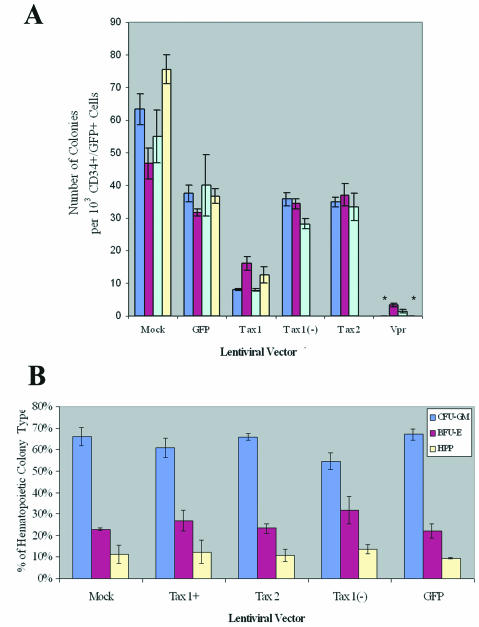
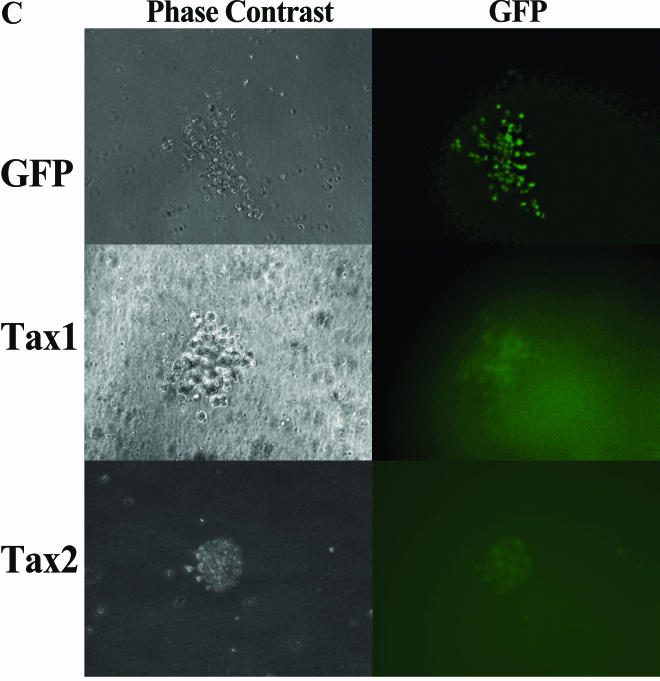
We previously demonstrated that HTLV-1 was able to infect human hematopoietic progenitor (CD34+) cells and that lymphopoiesis was perturbed in SCID-hu mice reconstituted with HTLV-1-infected CD34+ cells (31). Since CD34+ cells have the capability of differentiating into multiple hematopoietic lineages, we determined whether expression of HTLV-1 or-2 Tax could alter maturation patterns or differentiation of human CD34+ cells in culture. Purified CD34+ cells were infected with LVs (MOI = 3) and purified by FACS for coexpression of CD34+ and GFP (Fig. 3). GFP expression levels revealed transduction frequencies of between 7 and 41% in CD34+ cells, similar to transduction levels previously reported employing LVs (3, 4). Purified CD34+ GFP+ cells were plated in a semisolid medium permissive for the propagation of erythroid (BFU-E), myeloid (CFU-GM, CFU-M, and CFU-G), and primitive progenitor (HPP-CFC and CFU-GEMM) colony-forming cells. Colonies were identified by morphology and enumerated at 12 to 14 days postplating. CD34+ cells transduced with Tax1 demonstrated a two- to fivefold reduction in clonogenic colony-forming activity in vitro, in comparison with CD34+ cells transduced with LVs encoding Tax2, Tax1(−), or only GFP (Fig. 4A). Although Tax1 mediated a suppression of hematopoiesis in colony-forming activity, no significant alteration of the ratio of myeloid to erythroid colony types was detected (Fig. 4B). A number of CFU-GM arising from CD34+ cells transduced with Tax1 demonstrated detectable GFP expression when analyzed by fluorescence microscopy, suggesting that Tax1 expression could be sustained during the differentiation of CD34+ cells in vitro (Fig. 5C). As expected, transduction of CD34+ cells with HIV-1 Vpr resulted in the elimination of colony-forming activity in vitro. The limited number of colonies which developed from Vpr-transduced CD34+ cells in experiments 2 and 3 were not analyzed for proviral integrations but were presumably the result of a small number of untransduced CD34+ cells that contaminated the FACS-purified cell population. These data demonstrate that Tax1 expression suppresses the maturation and differentiation of human CD34+ cells in vitro. Notably, the ratios of hematopoietic colony types that develop from Tax1-transduced CD34+ cells was not altered compared to the ratio of colony types that developed from CD34+ cells transduced with Tax2, Tax1(−) or GFP, suggesting that the suppressive effect exerted by Tax1 on hematopoiesis occurs relatively early in differentiation and prior to lineage commitment of CD34+ cells. Alternatively, Tax1 may inhibit differentiation of hematopoietic progenitor cells at latter stages of hematopoiesis independently of the specific hematopoietic lineage.
FIG. 3.
FACS isolation of CD34+ cells infected with lentiviral vectors. Purified CD34+ cells (3 × 106) were infected with lentiviral vectors (MOI = 3) by centrifugation (4 h at 2,500 rpm) in a Beckman GPR centrifuge. Cells were incubated for 72 h in IMDM supplemented with 10% FBS and 30 μl of cytokine cocktail StemSpan CC100. Cells were incubated with a PE-conjugated human-specific MAb against the CD34 marker, and cell sorting was performed with a FACSVantage flow cytometer. An additional cell sample was incubated with a mouse immunoglobulin G γ isotype control antibody to set compensation and gates for FACS. The R2 gate in each panel represents the gate set to isolate CD34+ GFP+ cells. Note that cells transduced with LVs encoding a bicistronic mRNA display approximately 5- to 10-fold-lower levels of GFP in comparison with cells transduced with HR′CMV-GFP, as has previously been reported (103). The percentage of CD34+ GFP+ cells is displayed in the upper right panel. Mock-transduced CD34+ cells were isolated only on the basis of CD34+ expression and are represented by the R3 gate. LVs used in each transduction are as follows: GFP, HR′CMV-GFP; Tax1, HR′CMV-Tax1/GFP; Tax1(−), HR′CMV-Tax1(−)/GFP; Tax2, HR′CMV-Tax2/GFP; and Vpr, HR′CMV-Vpr/GFP. The purity of the sorted cell populations was not determined after isolation due to the limited numbers of cells recovered by FACS.
FIG. 4.
Clonogenic colony-forming activity of lentiviral vector- transduced CD34+ cells. GFP+ CD34+ cells were purified by FACS, and isolated cells (103) were plated in 10-mm dishes containing 2 ml of MethoCult H4433. Mature colony types were identified by morphology as granulocyte-macrophage CFU (CFU-GM), burst forming unit-erythroid (BFU-E), or highly proliferative pluripotent type (HPP-CFC), as previously described (31). (A) Total number of myeloid, erythroid and pluripotential colonies per CD34+ GFP+ cells (103) plated was determined at 14 days postplating. Purified CD34+ GFP+ cell samples were plated in triplicate. Each column represents a separate sorting experiment. Colony-forming activities were assayed four times for each transduction, except for Tax1(−) and Tax2-transduced CD34+ cells, which were assayed three times (sorting experiments 2, 3, and 4). Transduction of CD34+ cells with HR′CMV-Vpr/GFP resulted in no colony formation in FACS experiments 1 and 4 and is indicated by an asterisk. (B) Relative distribution of clonogenic colonies. Colonies were analyzed by morphology and characterized as CFU-GM, BFU-E, or HPP-CFC. The average numbers of CFU-GM colonies that arose per 103 purified CD34+ GFP+ cells plated were 38.8 (Mock), 24.4 (GFP), 7.3 (Tax1), 21.2 [Tax1(−)], and 21.4 (Tax2). The average numbers of BFU-E colonies arising per 103 CD34+ GFP+ cells plated were 14.9 (Mock), 9.0 (GFP), 2.8 (Tax1), 8.0 [Tax1(−)], and 8.3 (Tax2). The average numbers of CFU-HPP colonies arising per 103 CD34+ GFP+ cells plated were 6.0 (Mock), 3.6 (GFP), 1.2 (Tax1), 3.2 [Tax1(−)], and 3.3 (Tax2). Statistical analysis was performed by ANOVA (P < 0.005).
FIG. 5.
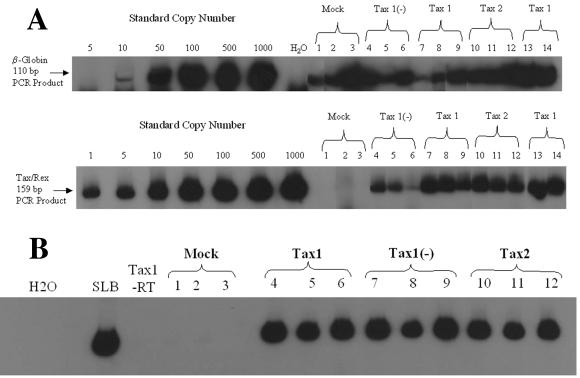
PCR analysis of clonogenic colonies. Clonogenic colonies were cultivated from LV-transduced CD34+ cells isolated by FACS on the basis of CD34 and GFP coexpression, as described in Fig. 3. Colonies were randomly isolated by aspiration from the methycellulose medium under an inverted microscope (Leica DMIL). (A) Q-PCR analysis of high-molecular-weight DNA from clonogenic colonies arising from lentiviral vector- transduced CD34+ cells. Clonogenic colonies were identified by morphology after 12 to 14 days in culture, and DNA was processed for PCR analysis as previously described (31). DNA from cells was assayed for the presence of HTLV-1 tax/rex (nucleotides 7336 to 7495) and human β-globin sequences. The numbers of cells in each colony varied and were reflected by the β-globin signal obtained. The amplified HTLV-1 and β-globin products were 159 and 110 bp, respectively. The hematopoietic colonies were identified as follows: lanes 1 to 3, 6, 8, and 12, CFU-GM; lanes 4, 9, 10, and 13, BFU-E; and lanes 7 and 14, HPP-CFC. Colonies 1, 6, 8, and 12 were from FACS experiment 1; colonies 2, 4, 11, and 13 were from FACS experiment 2; colonies 3, 5, and 9 were from FACS experiment 3; and colonies 7, 10, and 14 were from FACS experiment 4. “Mock” represents clonogenic colonies derived from sorted CD34+ cells that were mock infected and sorted only on the basis of CD34 expression. The colony analyzed in lane 6 was scored as negative for tax/rex sequences, suggesting that this colony arose from maturation of a nontransduced CD34+ cell, which presumably contaminated the FACS-purified cell population. (B) RT-PCR analysis of RNA from clonogenic colonies. RNA was isolated from clonogenic colonies by using Trizol, and a 10-μl aliquot of RNA representing 25% of the total sample was analyzed by RT-PCR with primers 670 and 671 and a OneStep RT-PCR kit (Qiagen). “Tax1-RT” represents the sample from lane 4 assayed with heat-inactivated reverse transcriptase and was used as a negative control for this experiment. RNA extracted from 104 SLB-1 cells, an HTLV-1 transformed T-cell line, was used as a positive control (SLB). Colonies 1, 2, and 4 to 8 were CFU-GM; colonies 3, 9, and 10 were BFU-E; and colonies 11 and 12 were from HPP-CFC. Colonies 1, 4, 7, and 10 were from FACS experiment 2; colonies 2, 5, 8, and 11 were from FACS experiment 3; and colonies 3, 6, 9, and 12 were from FACS experiment 4. (C) Low-field (×20) light and fluorescent micrographs were taken of a CFU-GM arising from CD34+ cells infected with HR′CMV-GFP (GFP, top panel), HR′CMV-Tax1/GFP (Tax1, middle panel), or HR′CMV-Tax2/GFP (Tax2, bottom panel) at 14 days postplating. Note that GFP expression is generally 5- to 10-fold lower in cells infected with the bicistronic LVs than in cells infected with HR′CMV-GFP, as has previously been reported (102).
Molecular characterization of clonogenic colonies.
To verify the presence of proviral sequences in clonogenic colonies arising from LV-transduced CD34+ cells, high-molecular-weight DNA was isolated and analyzed for tax sequences by PCR. Colonies (generally representing from 150 to 300 cells) were randomly isolated from Methocult by aspiration, and DNA was extracted and analyzed by Q-PCR with oligonucleotide primers specific for the HTLV tax gene (primers 670 and 671), as previously described (31-33). The number of cells per colony was determined by Q-PCR with primers specific for the human β-globin gene (LA1/LA2) (Fig. 5A). It should be noted that, because of the sequence homology between the tax1 and tax2 genes, primers 670 and 671 are capable of detecting both viral genes, as well as the antisense Tax1 vector sequences [Tax1(−)]. Almost all clonogenic colonies which arose from CD34+ cells transduced with LVs encoding Tax1, Tax2, or Tax1(−) (27 of 30 colonies analyzed) displayed the presence of integrated HTLV tax sequences when assayed by Q-PCR (Fig. 5B). Clonogenic colonies generally displayed between one to four copies of the tax gene per cell, as determined by real-time PCR analysis, although a few colonies demonstrated as many as eight proviral integrations (data not shown). Tax RNA expression was detected in all colonies (n = 20) arising from CD34+ cells transduced with Tax1 or Tax2 and in 80% of Tax1(−) transduced colonies (n = 10) when analyzed by RT-PCR analysis (Table 2). GFP expression was detected by fluorescence microscopy in some of the clonogenic colonies examine, confirming the expression of LV sequences in differentiated cells (Fig. 5C). These results demonstrate that clonogenic colonies which arise from LV-transduced CD34+ cells are capable of maintaining expression of Tax1 after maturation and differentiation.
TABLE 2.
RT-PCR analysis of clonogenic colonies arising from lentiviral vector-transduced CD34+ cells
| Vector construct | No. of colonies positive for HTLV tax RNA/total no. of colonies testeda
|
||||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | Total | |
| pHR′CMV-Tax1/GFP | 2/3 | 2/2 | 2/2 | 3/3 | 9/10 |
| pHR′CMV-Tax1(−)/GFP | NT | 3/3 | 2/4 | 3/3 | 8/10 |
| pHR′CMV-Tax2/GFP | NT | 3/3 | 4/4 | 3/3 | 10/10 |
Clonogenic colonies arising from LV-transduced CD34+ cells were randomly isolated by aspiration from the MethoCult medium. RNA was purified from each colony by using Trizol and analyzed for tax RNA expression by using the One- Step RT-PCR kit and primers 670 and 671 end labeled with 32P (34). PCR products were analyzed on a 6% polyacrylamide gel. Tax expression was scored as positive if the signal was at least 10-fold higher than the signal from a mock-infected colony. NT, not tested.
DISCUSSION
Hematopoietic progenitor cells have been proposed to serve as reservoirs for HTLV-1 infection in vivo, and maintenance of viral infection in these cell types may be important in the manifestation of the disease (40, 59, 70). It can be envisioned that HTLV-1 infection of hematopoietic stem cells may occur during neonatal or perinatal viral transmission and that progenitor cells may play a role in maintaining a persistent viral infection in the infected individuals. Hematopoietic progenitor cells are relatively quiescent, and the establishment of viral latency in these cells may also allow HTLV to evade immune surveillance mechanisms. Since HTLV Tax has a pivotal role in deregulation of lymphocyte growth and immortalization, the present study was undertaken to assess the sensitivity of human CD34+ hematopoietic progenitor cells to Tax expression. We previously demonstrated that human CD34+ cells were capable of supporting HTLV-1 infection (31). Reconstitution of T lymphopoiesis with HTLV-1-infected CD34+ cells in SCID-hu mice resulted in the perturbation of thymopoiesis and an aberrant display of thymocyte subpopulations. Our current data demonstrate that HTLV-1 Tax-transduced CD34+ cells have a reduced capacity to undergo development and maturation in vitro. Human hematopoietic cells bearing the CD34 marker are a heterogeneous cell population encompassing both hematopoietic progenitor cells and hematopoietic stem cells. Hematopoietic stem cells are predominantly in the quiescent phase of the cell cycle (34, 39, 78). The cyclin-dependent kinase inhibitor p21cip1/waf1 (p21) is a key molecular regulator governing entry of hematopoietic stem cells into the cell cycle, and it was recently shown that the abolition of p21 expression in CD34+ cells results in a notable expansion of stem cell numbers (94). Since Tax1 upregulates expression of p21, we predict that expression of Tax1 in CD34+ cells may inhibit the proliferation, but not the differentiation profile, of hematopoietic stem cells (1, 19, 28, 94). Indeed, the lack of alteration of the ratio of the development of myeloid versus erythroid colonies seen in Tax1-transduced CD34+ cells suggests that Tax1 affects proliferation, rather than the impairment of differentiation, of human hematopoietic progenitor cells. Preliminary data from our laboratory suggest that Tax1 is more effective at upregulating transcription from a p21 promoter construct than is Tax2 in a transient-transfection assay (A. Tripp and G. Feuer, unpublished observations). The differences in the activation of p21 gene expression by Tax1 and Tax2 in CD34+ cells may account for the specificity of suppression of hematopoiesis displayed by HTLV-1 Tax.
Although we do not detect the induction of apoptosis in T lymphoid cell lines after Tax1 expression, we cannot completely exclude the possibility that Tax1 may suppress hematopoiesis by sensitization of a subset of CD34-bearing cells to apoptosis during cultivation in vitro. Tax1 has been shown to inhibit cellular DNA repair activity by suppression of nucleotide excision repair (50-54). Furthermore, downregulation of DNA repair activity has been reported to induce apoptosis in human CD34+ cells (16). Tax1 expression may indirectly sensitize a subset of CD34+ cells to programmed cell death by inhibition of DNA repair activity. Previous reports have also demonstrated that transduction of cell lines with Tax1 result in the arrest of cells in G2/M (44, 61, 63). Further investigations will be required to decipher the precise mechanism of hematopoietic suppression by Tax1. It is noteworthy that HTLV-2 infection of a human CD34+ cell line was previously shown to promote survival and an increase in telomerase activity in human CD34+ cells (10, 18, 79). In contrast, infection of human CD34+ cells with HIV-1 and measles virus has been demonstrated to disrupt hematopoiesis (20, 27, 36, 55, 56, 68). It remains to be established whether HTLV-1 infection of CD34+ cells can inhibit hematopoiesis.
The different abilities of HTLV-1 and HTLV-2 Tax to suppress hematopoiesis of human CD34+ cells provides a model system to characterize and contrast the functions of these two viral proteins in primary human cells. The employment of LVs encoding HTLV Tax allows for the transduction of quiescent cells and for the ability to dissect and characterize the effect of Tax expression in the absence of other HTLV gene products. Although Tax1 and Tax2 have ca. 77 to 85% homology at the amino acid level and the transcriptional activation profiles of both proteins are remarkably similar, distinct biological differences that differentiate these proteins have previously been described (60, 89, 91). Tax1 is longer than Tax2 (353 and 331 amino acids, respectively). Tax1 has been shown to inhibit cellular p53 function more efficiently and displays a higher transformation capacity in rat fibroblast cells than does Tax2 (29, 65). Tax1 is also capable of inducing micronuclei in simian cells, in contrast to Tax2, which lacks this function (89). The ability to suppress the maturation and development of human CD34+ cells in vitro identifies an important property that distinguishes Tax1 from Tax2 activity in primary human hematopoietic cells. The divergence between Tax1 and Tax2 in their respective ability to suppress hematopoiesis in vitro may ultimately provide insight into the different pathogenic potential of HTLV-1 and -2 infection in humans. It is interesting to speculate that the induction of cell cycle arrest or the prevention of egress of infected hematopoietic stem cells from G0 may promote the survival of HTLV-1-infected CD34+ cells by maintaining viral latency.
Although several reports have linked HTLV-1 Tax expression with the initiation of programmed cell death (21-23, 43, 54, 74, 81, 98), we failed to detect apoptosis in T lymphoid cell lines transduced with LVs expressing Tax1. It is unlikely that the failure to detect apoptosis can be attributed to variations in cell lines or vectors, since expression of the HIV-1 Vpr protein reproducibly induced apoptosis in all cells analyzed. Furthermore, the ability to monitor GFP expression implies that Tax is also expressed from the bicistronic mRNA in transduced cells. It should be noted that our results are in agreement with reports from other laboratories demonstrating that Tax1 expression is not sufficient to induce apoptosis in cell lines (12, 24, 50, 86). Previous evidence does, however, suggest that Tax1 sensitizes cells to programmed cell death when cells are subjected to DNA damage (12, 24, 44, 50, 51, 53, 86). It is noteworthy that the functions of Tax1 and Tax2 may be significantly different when assayed in transformed cell lines in comparison to primary hematopoietic cells, particularly with respect to the ability to initiate programmed cell death. The elucidation of the mechanisms involved in regulating cell growth and apoptotic pathways in HTLV-infected hematopoietic progenitor cells will provide important insights into the initiation of HTLV-1-associated diseases.
Acknowledgments
This work was supported by the National Institutes of Health (1R29 CA77567 and RO1 RR14324) and the Leukemia Research Foundation.
We thank Edward Barker and Vicente Planelles for critical reading of the manuscript.
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Akagi, T., H. Ono, N. Tsuchida, and K. Shimotohno. 1997. Aberrant expression and function of p53 in T cells immortalized by HTLV-I Tax1. FEBS Lett. 406:263-266. [DOI] [PubMed] [Google Scholar]
- 3.An, D. S., S. K. Kung, A. Bonifacino, R. P. Wersto, M. E. Metzger, B. A. Agricola, S. H. Mao, I. S. Chen, and R. E. Donahue. 2001. Lentivirus vector-mediated hematopoietic stem cell gene transfer of common gamma-chain cytokine receptor in rhesus macaques. J. Virol. 75:3547-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An, D. S., R. P. Wersto, B. A. Agricola, M. E. Metzger, S. Lu, R. G. Amado, I. S. Chen, and R. E. Donahue. 2000. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J. Virol. 74:1286-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astier-Gin, T., J. P. Portail, F. Lafond, and B. Guillemain. 1995. Identification of HTLV-I- or HTLV-II-producing cells by cocultivation with BHK-21 cells stably transfected with a LTR-lacZ gene construct. J. Virol. Methods 51:19-29. [DOI] [PubMed] [Google Scholar]
- 6.Ballard, D. W., E. Bohnlein, J. W. Lowenthal, Y. Wano, B. R. Franza, and W. C. Greene. 1988. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science 241:1652-1655. [DOI] [PubMed] [Google Scholar]
- 7.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumgarten, H. 1985. A simple microplate assay for the determination of cellular protein. J. Immunol. Methods 82:25-37. [DOI] [PubMed] [Google Scholar]
- 9.Blomer, U., L. Naldini, T. Kafri, D. Trono, I. M. Verma, and F. H. Gage. 1997. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71:6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bovolenta, C., E. Pilotti, M. Mauri, M. Turci, P. Ciancianaini, P. Fisicaro, U. Bertazzoni, G. Poli, and C. Casoli. 2002. Human T-cell leukemia virus type 2 induces survival and proliferation of CD34+ TF-1 cells through activation of STAT1 and STAT5 by secretion of interferon-gamma and granulocyte macrophage-colony-stimulating factor. Blood 99:224-231. [DOI] [PubMed] [Google Scholar]
- 11.Brady, J., K. T. Jeang, J. Duvall, and G. Khoury. 1987. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J. Virol. 61:2175-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauweiler, A., J. E. Garrus, J. C. Reed, and J. K. Nyborg. 1997. Repression of bax gene expression by the HTLV-1 Tax protein: implications for suppression of apoptosis in virally infected cells. Virology 231:135-140. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. A., F. B. Nelson, E. L. Reinherz, and D. J. Diamond. 1991. The human interferon-gamma gene contains an inducible promoter that can be transactivated by tax I and II. Eur. J. Immunol. 21:1879-1885. [DOI] [PubMed] [Google Scholar]
- 14.Buchschacher, G. L., Jr., and F. Wong-Staal. 2000. Development of lentiviral vectors for gene therapy for human diseases. Blood 95:2499-2504. [PubMed] [Google Scholar]
- 15.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buschfort-Papewalis, C., T. Moritz, B. Liedert, and J. Thomale. 2002. Down-regulation of DNA repair in human CD34+ progenitor cells corresponds to increased drug sensitivity and apoptotic response. Blood 100:845-853. [DOI] [PubMed] [Google Scholar]
- 17.Cann, A. J., J. D. Rosenblatt, W. Wachsman, N. P. Shah, and I. S. Chen. 1985. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation. Nature 318:571-574. [DOI] [PubMed] [Google Scholar]
- 18.Casoli, C., M. C. Re, P. Monari, G. Furlini, G. Tosi, C. Gradozzi, P. P. Dall'Aglio, P. P. Bertazzoni, and R. S. Accolla. 1998. Human T-cell leukemia virus type II directly acts on CD34+ hematopoietic precursors by increasing their survival potential: envelope-associated HLA class II molecules reverse this effect. Blood 91:2296-2304. [PubMed] [Google Scholar]
- 19.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann, G. Franchini, and M. E. Klotman. 1996. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood 88:1551-1560. [PubMed] [Google Scholar]
- 20.Chelucci, C., H. J. Hassan, C. Locardi, D. Bulgarini, E. Pelosi, G. Mariani, U. Testa, M. Federico, M. Valtieri, and C. Peschle. 1995. In vitro human immunodeficiency virus-1 infection of purified hematopoietic progenitors in single-cell culture. Blood 85:1181-1187. [PubMed] [Google Scholar]
- 21.Chen, X., V. Zachar, M. Zdravkovic, M. Guo, P. Ebbesen, and X. Liu. 1997. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T-cell lymphotropic virus type I Tax transactivator. J. Gen. Virol. 78:3277-3285. [DOI] [PubMed] [Google Scholar]
- 22.Chlichlia, K., M. Busslinger, M. E. Peter, H. Walczak, P. H. Krammer, V. Schirrmacher, and K. Khazaie. 1997. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene 14:2265-2272. [DOI] [PubMed] [Google Scholar]
- 23.Chlichlia, K., G. Moldenhauer, P. T. Daniel, M. Busslinger, L. Gazzolo, V. Schirrmacher, and K. Khazaie. 1995. Immediate effects of reversible HTLV-1 tax function: T-cell activation and apoptosis. Oncogene 10:269-277. [PubMed] [Google Scholar]
- 24.Copeland, K. F., A. G. Haaksma, J. Goudsmit, P. H. Krammer, and J. L. Heeney. 1994. Inhibition of apoptosis in T cells expressing human T-cell leukemia virus type I Tax. AIDS Res. Hum. Retrovir. 10:1259-1268. [DOI] [PubMed] [Google Scholar]
- 25.Cowan, E. P., R. K. Alexander, S. Daniel, F. Kashanchi, and J. N. Brady. 1997. Induction of tumor necrosis factor alpha in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax. J. Virol. 71:6982-6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crenon, I., C. Beraud, P. Simard, J. Montagne, P. Veschambre, and P. Jalinot. 1993. The transcriptionally active factors mediating the effect of the HTLV-I Tax transactivator on the IL-2R alpha kappa B enhancer include the product of the c-rel proto-oncogene. Oncogene 8:867-875. [PubMed] [Google Scholar]
- 27.Davis, B. R., and G. Zauli. 1995. Effect of human immunodeficiency virus infection on haematopoiesis. Baillieres Clin. Haematol. 8:113-130. [DOI] [PubMed] [Google Scholar]
- 28.de La Fuente, C., F. Santiago, S. Y. Chong, L. Deng, T. Mayhood, P. Fu, D. Stein, T. Denny, F. Coffman, N. Azimi, R. Mahieux, and F. Kashanchi. 2000. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-infected cells and its association with cyclin A/cdk2. J. Virol. 74:7270-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endo, K., A. Hirata, K. Iwai, M. Sakurai, M. Fukushi, M. Oie, M. Higuchi, W. W. Hall, F. Gejyo, and M. Fujii. 2002. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J. Virol. 76:2648-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 31.Feuer, G., J. K. Fraser, J. A. Zack, F. Lee, R. Feuer, and I. S. Chen. 1996. Human T-cell leukemia virus infection of human hematopoietic progenitor cells: maintenance of virus infection during differentiation in vitro and in vivo. J. Virol. 70:4038-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feuer, G., S. A. Stewart, S. M. Baird, F. Lee, R. Feuer, and I. S. Chen. 1995. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J. Virol. 69:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feuer, G., J. A. Zack, W. J. Harrington, Jr., R. Valderama, J. D. Rosenblatt, W. Wachsman, S. M. Baird, and I. S. Chen. 1993. Establishment of human T-cell leukemia virus type I T-cell lymphomas in severe combined immunodeficient mice. Blood 82:722-731. [PubMed] [Google Scholar]
- 34.Fleming, W. H., E. J. Alpern, N. Uchida, K. Ikuta, G. J. Spangrude, and I. L. Weissman. 1993. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J. Cell Biol. 122:897-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fouchard, N., B. Flageul, M. Bagot, M. F. Avril, O. Hermine, F. Sigaux, H. Merle-Beral, X. Troussard, J. F. Delfraissy, G. de The, et al. 1995. Lack of evidence of HTLV-I/II infection in T CD8 malignant or reactive lymphoproliferative disorders in France: a serological and/or molecular study of 169 cases. Leukemia 9:2087-2092. [PubMed] [Google Scholar]
- 36.Geissler, R. G., O. G. Ottmann, K. Kleiner, U. Mentzel, A. Bickelhaupt, D. Hoelzer, and A. Ganser. 1993. Decreased haematopoietic colony growth in long-term bone marrow cultures of HIV-positive patients. Res. Virol. 144:69-73. [DOI] [PubMed] [Google Scholar]
- 37.Good, L., S. B. Maggirwar, and S. C. Sun. 1996. Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 15:3744-3750. [PMC free article] [PubMed] [Google Scholar]
- 38.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gothot, A., R. Pyatt, J. McMahel, S. Rice, and E. F. Srour. 1997. Functional heterogeneity of human CD34+ cells isolated in subcompartments of the G0/G1 phase of the cell cycle. Blood 90:4384-4393. [PubMed] [Google Scholar]
- 40.Grant, C., K. Barmak, T. Alefantis, J. Yao, S. Jacobson, and B. Wigdahl. 2002. Human T-cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell Physiol. 190:133-159. [DOI] [PubMed] [Google Scholar]
- 41.Grassmann, R., C. Dengler, I. Muller-Fleckenstein, B. Fleckenstein, K. McGuire, M. C. Dokhelar, J. G. Sodroski, and W. A. Haseltine. 1989. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc. Natl. Acad. Sci. USA 86:3351-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green, P. L., Y. M. Xie, and I. S. Chen. 1991. The Rex proteins of human T-cell leukemia virus type II differ by serine phosphorylation. J. Virol. 65:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall, A. P., J. Irvine, K. Blyth, E. R. Cameron, D. E. Onions, and M. E. Campbell. 1998. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J. Pathol. 186:209-214. [DOI] [PubMed] [Google Scholar]
- 44.Haoudi, A., and O. J. Semmes. 2003. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology 305:229-239. [DOI] [PubMed] [Google Scholar]
- 45.Himes, S. R., L. S. Coles, R. Katsikeros, R. K. Lang, and M. F. Shannon. 1993. HTLV-1 tax activation of the GM-CSF and G-CSF promoters requires the interaction of NF-κB with other transcription factor families. Oncogene 8:3189-3197. [PubMed] [Google Scholar]
- 46.Hjelle, B., and R. Chaney. 1992. Sequence variation of functional HTLV-II tax alleles among isolates from an endemic population: lack of evidence for oncogenic determinant in tax. J. Med. Virol. 36:136-141. [DOI] [PubMed] [Google Scholar]
- 47.Hjelle, B., R. Mills, S. Swenson, G. Mertz, C. Key, and S. Allen. 1991. Incidence of hairy cell leukemia, mycosis fungoides, and chronic lymphocytic leukemia in first known HTLV-II-endemic population. J. Infect. Dis. 163:435-440. [DOI] [PubMed] [Google Scholar]
- 48.Jeang, K. T., S. G. Widen, O. J. Semmes IV, and S. H. Wilson. 1990. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 49.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kao, S. Y., F. J. Lemoine, and S. J. Mariott. 2000. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene 19:2240-2248. [DOI] [PubMed] [Google Scholar]
- 51.Kao, S. Y., F. J. Lemoine, and S. J. Marriott. 2001. p53-independent induction of apoptosis by the HTLV-I tax protein following UV irradiation. Virology 291:292-298. [DOI] [PubMed] [Google Scholar]
- 52.Kao, S. Y., F. J. Lemoine, and S. J. Marriott. 2000. Suppression of DNA repair by human T-cell leukemia virus type 1 Tax is rescued by a functional p53 signaling pathway. J. Biol. Chem. 275:35926-35931. [DOI] [PubMed] [Google Scholar]
- 53.Kao, S. Y., and S. J. Marriott. 1999. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 73:4299-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitajima, I., T. Nakajima, T. Imamura, I. Takasaki, K. Kawahara, T. Okano, T. Tokioka, Y. Soejima, K. Abeyama, and I. Maruyama. 1996. Induction of apoptosis in murine clonal osteoblasts expressed by human T-cell leukemia virus type I tax by NF-κB and TNF-α. J. Bone Miner. Res. 11:200-210. [DOI] [PubMed] [Google Scholar]
- 55.Koka, P. S., J. K. Fraser, Y. Bryson, G. C. Bristol, G. M. Aldrovandi, E. S. Daar, and J. A. Zack. 1998. Human immunodeficiency virus inhibits multilineage hematopoiesis in vivo. J. Virol. 72:5121-5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koka, P. S., B. D. Jamieson, D. G. Brooks, and J. A. Zack. 1999. Human immunodeficiency virus type 1-induced hematopoietic inhibition is independent of productive infection of progenitor cells in vivo. J. Virol. 73:9089-9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korber, B., A. Okayama, R. Donnelly, N. Tachibana, and M. Essex. 1991. Polymerase chain reaction analysis of defective human T-cell leukemia virus type I proviral genomes in leukemic cells of patients with adult T-cell leukemia. J. Virol. 65:5471-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemoine, F. J., S. Y. Kao, and S. J. Marriott. 2000. Suppression of DNA repair by HTLV type 1 Tax correlates with Tax trans-activation of proliferating cell nuclear antigen gene expression. AIDS Res. Hum. Retrovir. 16:1623-1627. [DOI] [PubMed] [Google Scholar]
- 59.Levin, M. C., M. Krichavsky, R. J. Fox, T. Lehky, S. Jacobson, C. Fox, F. Kleghorn, J. White, N. Young, R. J. Edwards, N. E. Jack, and C. Bartholomew. 1997. Extensive latent retroviral infection in bone marrow of patients with HTLV-I-associated neurologic disease. Blood 89:346-348. [PubMed] [Google Scholar]
- 60.Lewis, M. J., N. Sheehy, M. Salemi, A. M. VanDamme, and W. W. Hall. 2002. Comparison of CREB- and NF-κB-mediated transactivation by human T lymphotropic virus type II (HTLV-II) and type I (HTLV-I) tax proteins. Virology 295:182-189. [DOI] [PubMed] [Google Scholar]
- 61.Liang, M. H., T. Geisbert, Y. Yao, S. H. Hinrichs, and C. Z. Giam. 2002. Human T-lymphotropic virus type 1 oncoprotein Tax promotes S-phase entry but blocks mitosis. J. Virol. 76:4022-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lindholm, P. F., R. L. Reid, and J. N. Brady. 1992. Extracellular Tax1 protein stimulates tumor necrosis factor-β and immunoglobulin kappa light chain expression in lymphoid cells. J. Virol. 66:1294-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu, B., M.-H. Liang, Y.-l. Kuo, W. Liao, I. Boros, T. Kleinberger, J. Blancato, and C.-Z. Giam. 2003. Human T-lymphotropic virus type 1 oncoprotein tax promotes unscheduled degradation of Pds1p/securin and Clb2p/cyclin B1 and causes chromosomal instability. Mol. Cell. Biol. 23:5269-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Los, M., K. Khazaie, K. Schulze-Osthoff, P. A. Baeuerle, V. Schirrmacher, and K. Chlichlia. 1998. Human T-cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J. Immunol. 161:3050-3055. [PubMed] [Google Scholar]
- 65.Mahieux, R., C. A. Pise-Masison, P. F. Lambert, C. Nicot, L. De Marchis, A. Gessain, P. Green, W. Hall, and J. N. Brady. 2000. Differences in the ability of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 Tax to inhibit p53 function. J. Virol. 74:6866-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahieux, R., C. A. Pise-Masison, C. Nicot, P. Green, W. W. Hall, and J. N. Brady. 2000. Inactivation of p53 by HTLV type 1 and HTLV type 2 Tax trans-activators. AIDS Res. Hum. Retrovir. 16:1677-1681. [DOI] [PubMed] [Google Scholar]
- 67.Majone, F., O. J. Semmes, and K. T. Jeang. 1993. Induction of micronuclei by HTLV-I Tax: a cellular assay for function. Virology 193:456-459. [DOI] [PubMed] [Google Scholar]
- 68.Manchester, M., K. A. Smith, D. S. Eto, H. B. Perkin, and B. E. Torbett. 2002. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J. Virol. 76:6636-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martin, M. P., R. J. Biggar, G. Hamlin-Green, S. Staal, and D. Mann. 1993. Large granular lymphocytosis in a patient infected with HTLV-II. AIDS Res. Hum. Retrovir. 9:715-719. [DOI] [PubMed] [Google Scholar]
- 70.Mikovits, J., F. Ruscetti, W. Zhu, R. Bagni, D. Dorjsuren, and R. Shoemaker. 2001. Potential cellular signatures of viral infections in human hematopoietic cells. Dis. Markers 17:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miyake, H., T. Suzuki, H. Hirai, and M. Yoshida. 1999. Trans-activator Tax of human T-cell leukemia virus type 1 enhances mutation frequency of the cellular genome. Virology 253:155-161. [DOI] [PubMed] [Google Scholar]
- 72.Mori, N., N. Mukaida, D. W. Ballard, K. Matsushima, and N. Yamamoto. 1998. Human T-cell leukemia virus type I Tax transactivates human interleukin 8 gene through acting concurrently on AP-1 and nuclear factor-κB-like sites. Cancer Res. 58:3993-4000. [PubMed] [Google Scholar]
- 73.Muraoka, O., T. Kaisho, M. Tanabe, and T. Hirano. 1993. Transcriptional activation of the interleukin-6 gene by HTLV-1 p40tax through an NF-κB-like binding site. Immunol. Lett. 37:159-165. [DOI] [PubMed] [Google Scholar]
- 74.Nicot, C., and R. Harrod. 2000. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol. Cell. Biol. 20:8580-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicot, C., R. Mahieux, S. Takemoto, and G. Franchini. 2000. Bcl-XL is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood 96:275-281. [PubMed] [Google Scholar]
- 76.Paul, N. L., M. J. Lenardo, K. D. Novak, T. Sarr, W. L. Tang, and N. H. Ruddle. 1990. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-κB. J. Virol. 64:5412-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Planelles, V., J. B. Jowett, Q. X. Li, Y. Xie, B. Hahn, and I. S. Chen. 1996. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J. Virol. 70:2516-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Randall, T. D., and I. L. Weissman. 1997. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood 89:3596-3606. [PubMed] [Google Scholar]
- 79.Re, M. C., P. Monari, D. Gibellini, P. Ciancianaini, P. P. Dall'Aglio, M. Vignoli, G. Furlini, E. Ramazzotti, U. Bertazzoni, and C. Casoli. 2000. Human T-cell leukemia virus type II increases telomerase activity in uninfected CD34+ hematopoietic progenitor cells. J. Hematother. Stem Cell Res. 9:481-487. [DOI] [PubMed] [Google Scholar]
- 80.Rees, S., J. Coote, J. Stables, S. Goodson, S. Harris, and M. G. Lee. 1996. Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. BioTechniques 20:102-110. [DOI] [PubMed] [Google Scholar]
- 81.Rivera-Walsh, I., M. Waterfield, G. Xiao, A. Fong, and S. C. Sun. 2001. NF-κB signaling pathway governs TRAIL gene expression and human T-cell leukemia virus-I Tax-induced T-cell death. J. Biol. Chem. 276:40385-40388. [DOI] [PubMed] [Google Scholar]
- 82.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosen, C. A., R. Park, J. G. Sodroski, and W. A. Haseltine. 1987. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc. Natl. Acad. Sci. USA 84:4919-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross, T. M., M. Narayan, Z.-Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 Tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 74:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 70:5194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saggioro, D., S. Barp, and L. Chieco-Bianchi. 2001. Block of a mitochondrial-mediated apoptotic pathway in Tax-expressing murine fibroblasts. Exp. Cell Res. 269:245-255. [DOI] [PubMed] [Google Scholar]
- 87.Schmid, I., C. H. Uittenbogaart, B. Keld, and J. V. Giorgi. 1994. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J. Immunol. Methods 170:145-157. [DOI] [PubMed] [Google Scholar]
- 88.Semmes, O. J., and K.-T. Jeang. 1996. Localization of human T-cell leukemia virus type 1 Tax to subnuclear compartments that overlap with interchromatin speckles. J. Virol. 70:6347-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semmes, O. J., F. Majone, C. Cantemir, L. Turchetto, B. Hjelle, and K. T. Jeang. 1996. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology 217:373-379. [DOI] [PubMed] [Google Scholar]
- 90.Serke, S., S. Sauberlich, Y. Abe, and D. Huhn. 1991. Analysis of CD34-positive hemopoietic progenitor cells from normal human adult peripheral blood: flow-cytometrical studies and in-vitro colony (CFU-GM, BFU-E) assays. Ann. Hematol. 62:45-53. [DOI] [PubMed] [Google Scholar]
- 91.Shimotohno, K., W. Wachsman, Y. Takahashi, D. W. Golde, M. Miwa, T. Sugimura, and I. S. Chen. 1984. Nucleotide sequence of the 3′ region of an infectious human T-cell leukemia virus type II genome. Proc. Natl. Acad. Sci. USA 81:6657-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shostak, L. D., J. Ludlow, J. Fisk, S. Pursell, B. J. Rimel, D. Nguyen, J. D. Rosenblatt, and V. Planelles. 1999. Roles of p53 and caspases in the induction of cell cycle arrest and apoptosis by HIV-1 vpr. Exp. Cell Res. 251:156-165. [DOI] [PubMed] [Google Scholar]
- 93.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stier, S., T. Cheng, R. Forkert, C. Lutz, D. M. Dombkowski, J. L. Zhang, and D. T. Scadden. 2003. Ex vivo targeting of p21Cip1/Waf1 permits relative expansion of human hematopoietic stem cells. Blood 102:1260-1266. [DOI] [PubMed]
- 95.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity toward CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 96.Uittenbogaard, M. N., H. A. Giebler, D. Reisman, and J. K. Nyborg. 1995. Transcriptional repression of p53 by human T-cell leukemia virus type I Tax protein. J. Biol. Chem. 270:28503-28506. [DOI] [PubMed] [Google Scholar]
- 97.Wrzesinski, S., R. Seguin, Y. Liu, S. Domville, V. Planelles, P. Massa, E. Barker, J. Antel, and G. Feuer. 2000. HTLV type 1 Tax transduction in microglial cells and astrocytes by lentiviral vectors. AIDS Res. Hum. Retrovir. 16:1771-1776. [DOI] [PubMed] [Google Scholar]
- 98.Yamada, T., S. Yamaoka, T. Goto, M. Nakai, Y. Tsujimoto, and M. Hatanaka. 1994. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J. Virol. 68:3374-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao, J., and B. Wigdahl. 2000. Human T-cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front. Biosci. 5:D138-D168. [DOI] [PubMed] [Google Scholar]
- 100.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 102.Zack, J. A., A. M. Haislip, P. Krogstad, and I. S. Chen. 1992. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J. Virol. 66:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu, Y., G. Feuer, S. L. Day, S. Wrzesinski, and V. Planelles. 2001. Multigene lentiviral vectors based on differential splicing and translational control. Mol. Ther. 4:375-382. [DOI] [PubMed] [Google Scholar]