Abstract
A peripheral B cell tolerance checkpoint appears to be operative during the germinal center (GC) reaction. We previously showed that a transgenic BCR clonotype that is ‘dual reactive’ for the hapten arsonate (Ars) and nuclear auto-antigens is stimulated to enter the GC response via Ars immunization. However, the participation of this clonotype in this response wanes with time and it gives rise to few memory B cells capable of mounting a secondary anti-Ars IgG response. Enforced expression of Bcl-2 partially rescues the GC and memory B cell responses of this clonotype, suggesting that apoptotic pathways are involved in the action of the GC tolerance checkpoint. Since GC B cells substantially up-regulate levels of expression of the Fas apoptotic death receptor, we determined whether an intrinsic Fas deficient could rescue the participation of this clonotype in the GC response. It could not, strongly indicating that Fas expression by autoreactive GC B cells is not necessary for their elimination. In addition, experiments in which Fas-sufficient dual reactive clonotype B cells were transferred to Fas-deficient hosts revealed an absence of participation of these B cells in the GC and IgG anti-Ars responses. We present data consistent with the idea that T cells in Fas-deficient hosts are primed to express elevated levels of FasL and eliminate antigen-activated B cells that up-regulate Fas.
Keywords: B cell, Fas death receptor, Fas ligand, germinal center, tolerance
Introduction
Studies on the origin of auto-antibodies that arise in autoimmune disease have provided insights into how central and peripheral B cell tolerance operate, by revealing the outcome of defects in these pathways (1–6). These studies paint a picture of subversion of a normal T cell-dependent germinal center (GC) and memory B cell response. The general character of the auto-antibodies produced by DNA, snRNPs, antibody Fc regions and nucleosomes in systemic autoimmune states is indistinguishable from antibodies produced by foreign antigen-driven B cells in the GC–memory pathway. The critical exception is that these auto-antibodies have high affinity for a characteristic group of auto-antigens, while the memory compartment elicited by foreign antigens does not.
Levels of apoptosis are high in GCs and it has been speculated that this results from a combination of the lack of positive selection of B cells with reduced affinity for antigen due to somatic mutation and the negative selection of autoreactive GC B cells (7–13). Consistent with this idea, GC B cells express very low levels of anti-apoptotic factors of the Bcl-2 family such as Bcl-2 and Bcl-xL and high levels of the apoptotic death receptor Fas (CD95) (7). Fas clearly plays a central function in the regulation of autoimmunity, and most data indicate a primary function for Fas in the action of peripheral tolerance checkpoints (14–16). However, while some past studies have indicated that Fas is involved in the affinity maturation process (17, 18), experiments on a possible role for this death receptor in the negative selection of autoreactive B cells during the GC reaction have been limited. In a previous study in which injection of large boluses of soluble antigen were used to induce apoptosis of antigen-specific GC B cells, no effect of a Fas deficiency on this process was detected (9). Moreover, while GC B cells express high levels of a preformed Fas death receptor complex, the activity of this complex appears to be suppressed in many GC B cells by c-FLIP (19–21).
We have previously described two lines of VH ‘knock-in’ mice that differ only in the presence or absence of a single mutation to arginine (R) at position 55 in the CDR2 subregion of the VH gene used to replace the endogenous JH locus (22, 23). We term these lines of mice HKIR and HKI65, respectively. Both versions of this VH gene, in combination with a single, endogenous kappa light-chain gene (Vκ10A), encode antibodies that we term ‘canonical’. Canonical antibodies bind the hapten arsonate (Ars) and can be specifically detected using the monoclonal anti-clonotypic antibody E4 (24, 25). Antibodies with the R55 form of the V domain also display reactivity for nuclear auto-antigens such as chromatin and can cause kidney dysfunction via glomerular deposition in vivo (17). B cells expressing BCRs containing either type of V domain develop to mature follicular (FO) phenotype, reside in follicles and are not short lived (23, 26). However, canonical HKIR B cells are not ignorant of auto-antigen, as they express very low levels of surface BCR (sBCR). Moreover, these levels can be dramatically up-regulated by blocking the interaction of auto-antigen with this BCR using a monovalent form of Ars during primary development of this clonotype (27).
Canonical HKI65 and HKIR B cells mount early anti-Ars primary responses that are indistinguishable (28). This includes homing to follicles, migration to the T–B interface and induction of co-stimulatory molecules, proliferation, differentiation to primary antibody-forming cells (AFCs), heavy (H)-chain class switching and entry into GCs and somatic hypermutation. However, canonical HKIR B cells display substantially reduced participation in the latter stages of the GC response and in the anamnestic AFC response (28). These data suggest that while the nuclear antigen reactivity of this type of B cell does not result in anergy, these B cells are subjected to the action of a GC tolerance checkpoint. In support of this idea, enforced Bcl-2 expression partially rescues the participation of HKIR B cells in the ongoing GC response (28).
To determine if Fas plays a role in the action of the putative GC tolerance checkpoint operative on canonical HKIR B cells, we generated new lines of HKI65 and HKIR mice containing the lpr Fas deficiency locus. Analysis of chimeric mice created by transferring lpr or Fas-sufficient HKI65 or HKIR B cells into Fas-sufficient hosts that were subsequently immunized with Ars revealed no apparent influence of a Fas deficiency on the GC response of these clonotypes. Surprisingly, however, when lpr hosts were used for analogous experiments, dramatically reduced participation of both canonical HKI65 and HKIR clonotypes in the GC and IgG responses was observed. We discuss the implications of these data for an understanding of the mechanism of action of GC peripheral tolerance checkpoints and the role of the Fas–FasL axis in lymphocyte homeostasis.
Methods
Mice
C57BL/6 mice, C57BL/6.SJL (B6.CD45.1) and mice homozygous for the lymphoproliferative spontaneous mutation Faslpr (C57BL/6J-lpr/lpr) were originally purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and then bred in the Thomas Jefferson University mouse facilities. Ig VH chain knock-in mice (termed HKI65 and HKIR) have been previously described (22, 23). The double Ig transgenic HKI65/Vκ10A mouse line was created via breeding of HKI65 mice to a line of conventional Vκ10A light-chain transgenic mice (a kind gift of Larry Wysocki). HKI65.lpr and HKIR.lpr mice were created by breeding of C57BL/6J-lpr/lpr mice to HKI65 or HKIR mice, respectively. All mice were housed under pathogen-free conditions and were given autoclaved food and water. The use of mice in these studies was approved by the Institutional Animal Care and Use Committee.
Adoptive transfers and immunizations
Total splenocytes (5 × 106) or 2 × 106 MACS-enriched B cells (CD3e−, Thy1.2−, F4/80−, GR1− and CD11b−) from mice of 7–10 weeks of age were injected into the retro-orbital sinus of syngeneic C57BL/6J or C57BL/6J-lpr/lpr (hereafter referred to as B6 and B6.lpr, respectively) recipients, also 7–10 weeks of age. Recipient mice were either left naive or immunized 12 h later with 100 μg of Ars–keyhole limpet hemocyanin (KLH) in alum intra-peritoneally (i.p.). Naive recipient mice were sacrificed 1 or 7 days after cell transfer; immunized mice were sacrificed 6 days after immunization (i.e. 7 days after cell transfer).
Antibodies and other staining reagents
Antibodies and other reagents used for immunohistochemistry and flow cytometry included the following: anti-GL7-FITC; anti-B220 (clone RA3-6B2) labeled with biotin, FITC or PE; anti-IgD-PE (clone 11-26); anti-CD21/35-FITC (7G6); anti-CD23-PE (B3B4); PE and biotin-anti-mouse CD45.2 (clone 104), biotin-anti-mouse CD45.1 (clone A20), anti-IgMa-PE (DS-1), anti-IgMb-FITC (AF6-78) and anti-Fas (CD95)-PE (all BD Biosciences); metallophilic macrophage-1-FITC (MOMA-1; Serotec); anti-mouse C1qRp-PE (AA4.1; eBiosciences); peanut lectin agglutinin (PNA)-FITC (Vector Laboratories) and donkey anti-mouse IgM-FITC (all from Jackson ImmunoResearch Laboratories) and a biotinylated form of the anti-idiotypic mAb E4 (made in-house). Staining with all biotinylated antibodies was followed by SA–PE or SA–Cychrome (BD Biosciences) staining.
Immunochemistry and immunofluorescence
Spleens were snap frozen in OCT compound and cryosections (5–6 μm) made as previously described (25). Immunofluorescence staining was performed using the antibodies and reagents listed above and analyzed using fluorescensce microscopy (Leitz Diaplan).
Flow cytometry analysis
Single-cell suspensions were prepared and stained with the antibodies listed above as described previously (23). Cells were assayed on an EPICS XL-MCL (Coulter). Data were analyzed using the FLOWJO software (Treestar).
ELISpot assay
Multiscreen 96-well filtration plates (Millipore) were coated with anti-mouse IgM, anti-mouse IgG or both (Caltag Laboratories) at concentrations of 10 μg ml−1 overnight at 4°C. Splenocytes from chimeric mice were added to the plates in serial 2-fold dilutions and incubated in RPMI 1640 medium containing 10% FBS for 6 h at 37°C. Antibodies produced by canonical B cells were detected using biotinylated anti-clonotypic E4 mAbs (made in-house) followed by SA conjugated to alkaline phosphatase (Vector Laboratories). The plates were developed using the Vector Blue Alkaline-Phosphatase Substrate kit III (Vector Laboratories). ELISpots were counted using a computerized imaging video system (Cellular Technology).
ELISA
Clonotype-positive (E4) total serum Igs were measured by ELISA on 96-well plates (Immulon-4; Thermo Electron) as previously described (29).
In vitro T cell stimulation
Spleen cells were prepared from 10-week-old C57BL/6-lpr/lpr and C57BL/6 mice. The cells were stained with Thy 1.2-biotin antibody (PharMingen) and then SA micro beads (Miltenyi Biotec) were used to positively select T cells in MACS separation. The 96-well round-bottom plates were coated with anti-CD3e antibody (eBioscience) at 10 μg ml−1 in PBS. A total of 2 × 105 T cells per well were incubated in 200 μl RPMI medium containing 10% FBS. After 4 h, cells were collected and stained for anti-CD3, anti-CD4, anti-CD8 and anti-Fas ligand–biotin (clone MFL4; BD PharMingen) and analyzed by flow cytometry.
Results
Mildly perturbed peripheral B cell development in Fas-deficient mice
HKI65 and HKIR mice homozygous for the H-chain knock-in loci [backcrossed >20 generations to the C57BL/6 (B6) background] were crossed to C57BL/6-lpr/lpr (B6.lpr) mice to create new congenic lines heterozygous for the H-chain knock-in locus and the lpr locus. These mice were backcrossed to the B6.lpr line to create mice hemizygous for the H-chain knock-in loci and homozygous for the lpr locus. These new strains are called HKI65.lpr and HKIR.lpr.
Potential influences of a Fas deficiency on the primary development of canonical HKI65 and HKIR clonotypes were investigated by flow cytometric analysis of the bone marrow (BM) and splenic B cell compartments of young mice. No significant differences were seen in developmental subset percentages or total numbers in the BM of Fas-sufficient and -deficient versions of HKI65, HKIR and B6 mice (data not shown). Figure 1(A) shows that levels of H chain allelic exclusion in HKI65 and HKIR mice and sIgM down-regulation in HKIR mice were not overtly altered by a Fas deficiency. However, somewhat reduced numbers of FO B cells were observed in the spleens of Fas-deficient HKI65 and HKIR mice (Fig. 1B), and this was accompanied by increased numbers of CD21−, CD23−, B220+ cells. Levels of marginal zone B cells (CD21high and CD23low) did not reproducibly differ in any of the lines of mice. Direct comparison of sBCR levels on E4+ B cells revealed sIgD (but not sIgM, data not shown) levels were slightly lower on canonical HKI65.lpr, HKIR.lpr and B6.lpr splenic B cells as compared with their Fas-sufficient counterparts (Fig. 1C).
Fig. 1.
Primary B cell development in Fas-deficient HKI65 and HKIR mice. (A) Splenocytes were isolated from Fas-sufficient and Fas-deficient HKI65 and HKIR hemizygous mice as well as B6 mice and were stained with anti-B220 and anti-IgMa and anti-IgMb allotypic mAbs and analyzed by flow cytometry. HKI knock-in loci encode the IgMa allotype. The data shown are from pooled cells from two mice of each genotype. (B) Splenocytes from mice of the indicated genotypes were stained with anti-B220, anti-CD21 and anti-CD23 and analyzed by flow cytometry. Follicular (CD23high CD21low) and marginal zone (CD23low CD21high) sub-populations are gated. The data shown are representative of at least three independent experiments. (C) Splenocytes from mice of the indicated genotypes were stained with anti-B220, anti-IgD and anti-clonotypic E4 mAbs and analyzed by flow cytometry. All data shown in this figure are from cells in the B220+ gate. The results are representative of at least three independent experiments.
Analysis of the frequency of transitional B cells in the spleens of HKI65.lpr and HKIR.lpr mice using the anti-C1qRp mAb AA4.1 revealed a 1.5- to 2-fold increase in this population, including the canonical B cell sub-population (Fig. 2). Taken together with the data shown in Fig. 1, these results suggest that primary peripheral B cell development is somewhat retarded in Fas-deficient HKI mice, resulting in accumulation of immature B cells in the spleen. Importantly, however, these differences are also seen in B6.lpr as compared with B6 mice (Fig. 2 right panels and data not shown), demonstrating that HKI transgene expression does not contribute to this phenomenon. Whether this slightly altered development results from B cell autonomous or environmental influences of a Fas deficiency remain to be determined. Nonetheless, both HKIR.lpr and HKIR.lpr mice have fairly normal FO B cell compartments containing a high frequency of canonical clonotypes. This allowed us to determine whether a Fas deficiency in these clonotypes influenced their participation in the GC response.
Fig. 2.
Increased numbers of splenic transitional B cells in Fas-deficient mice. Spleen cells from mice of the indicated genotypes were stained with anti-B220, anti-clonotypic E4 mAb and AA4.1 and analyzed by flow cytometry. The upper panel shows the percentage of immature AA4.1+ and mature AA4.1− B cells out of total cells in the lymphocyte gate. The lower panel shows the percentage of immature AA4.1+ cells out of gated B220+ and E4+ cells. The data shown are representative of two independent experiments.
An intrinsic Fas deficiency does not rescue canonical HKIR B cells from the action of a GC tolerance checkpoint
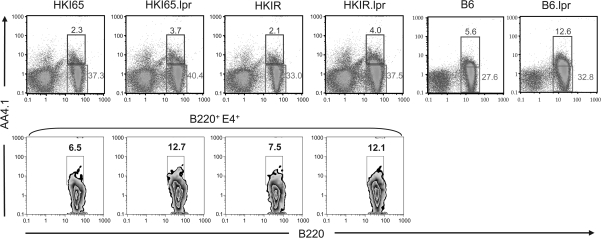
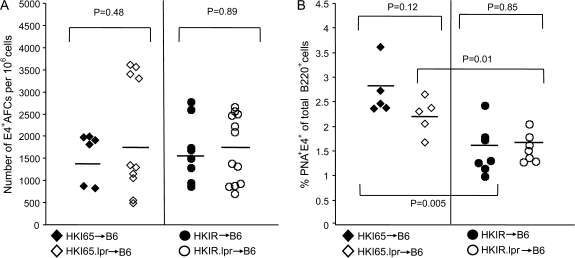
For this purpose, we employed our previously developed adoptive transfer approach (28). Splenic B cells from HKI65.lpr, HKIR.lpr mice and their Fas-sufficient counterparts were injected into (->) CD45.1 congenic B6 mice, and 1 day later the chimeric mice were immunized (i.p.) with Ars–KLH in alum. B6 mice lack the VH gene necessary to encode canonical, E4+ antibodies. At various times thereafter, chimeric mice were sacrificed and canonical clonotype participation in the AFC and GC responses were quantitated by ELISpot assay and flow cytometry, respectively.
Figure 3, left panel shows that at day 7 of the response, the number of E4+ AFCs in the spleens of all four types of chimeric mice was comparable. Thus, an intrinsic Fas deficiency in canonical B cells expressing either low (HKI65) or high (HKIR) avidity for auto-antigens does not augment the early AFC response. Figure 3, right panel, shows that, consistent with our previously reported results (28), Fas-sufficient canonical HKIR B cells mount a significantly reduced day 7 GC response as compared with Fas-sufficient canonical HKI65 B cells. A Fas deficiency did not significantly alter this outcome, demonstrating that this reduced participation of canonical HKIR B cells in the ongoing GC response is not due to Fas-induced apoptosis. A Fas deficiency also did not significantly alter the participation of canonical HKI65 B cells in the GC response (even with the one ‘outlier mouse’ in the HKI65->B6 data set, the difference in the percentage of E4+ GC B cells in HKI65->B6 and HKI65.lpr->B6 chimeras was not significantly different).
Fig. 3.
An intrinsic Fas deficiency does not influence the primary AFC and GC responses of canonical HKIR B cells. The indicated types of chimeric mice were created as described in Methods using 2 × 106 B cell-enriched splenic donor cells per recipient and immunized 12 h later with 100 μg of Ars–KLH in alum. Mice were sacrificed on day 6 after immunization and the E4+ clonotype-specific immune response was assessed using ELISpot and flow cytometry. (A) The ELISpot data shown represent the number of E4+ IgG-and IgM-producing AFCs per million splenocytes. (B) Splenocytes were stained with anti-B220, E4 and PNA and the number of PNA+, E4+ cells out of total B220+ cells evaluated by flow cytometry and the values plotted. Black filled circles and diamonds represent data obtained from Fas-sufficient HKI65 B cells in individual mice and open circles and diamonds data represent from Fas-deficient HKI65 B cells in individual mice. Horizontal bars show the mean value for each genotype. P values for differences between data from different types of chimeric mice, calculated using the Student’s t-test, are shown.
Suppression of the canonical IgG and GC responses in Fas-deficient hosts
The data above suggested that an intrinsic Fas deficiency did not overtly influence the regulation of autoreactive HKIR B cell activity during the anti-Ars response. However, many past studies have demonstrated the pivotal influence of Fas in the regulation of the development of the auto-antibody response and in certain peripheral B cell tolerance pathways (14, 15). As such, we wished to determine whether regulation of HKIR B cell participation in the AFC and GC responses was altered in a Fas-deficient environment.
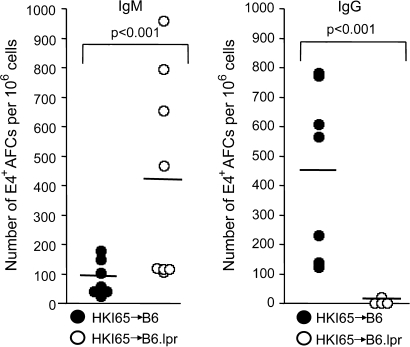
To this end, we modified our adoptive transfer approach such that Fas-sufficient HKI65 B cells were transferred into B6.lpr recipients that were subsequently immunized with Ars–KLH. Initial studies of the serum antibody and AFC responses of canonical B cells in these mice yielded the unanticipated result that many mounted a robust early canonical IgM response but a nearly undetectable canonical IgG response as compared with Fas-sufficient canonical B cells responding in Fas-sufficient hosts (Fig. 4).
Fig. 4.
Fas-sufficient canonical HKI65 B cells do not participate in the Ars-induced IgG AFC response in Fas-deficient hosts. Splenocytes from HKI65 mice were adoptively transferred into B6 or B6.lpr mice and the resulting chimeric mice immunized 12 h later with 100 μg of Ars–KLH in alum. Mice were sacrificed on day 6 after immunization and the E4 clonotype-specific immune response was assessed using ELISpot analysis of splenocytes. ELISpot membranes were coated with either anti-mouse IgG (gamma chain specific) or anti-mouse IgM (mu chain specific) and ELISpots developed with the E4 mAb. Data from individual mice are indicated by open and filled circles. P values for comparisons of data from chimeras generated using Fas-sufficient and -deficient hosts, calculated using the Student’s t-test, are shown.
Previous studies of mice in which Fas was specifically deleted in T cells produced the unexpected finding that these mice not only failed to develop high auto-antibody titers and autoimmune pathology but also became B lymphopenic (30). Much of this effect was shown to require Fas–FasL interactions and correlated with T cell activation, suggesting that lymphocyte homeostatic pathways mediated by this receptor–ligand pair were perturbed by Fas-deficient T cells. These observations provided one possible explanation for the results shown in Fig. 4, namely, that FasL expressing, activated Fas-deficient T cells were killing Fas-sufficient B cells once the latter were activated by immunization and expressed high levels of Fas.
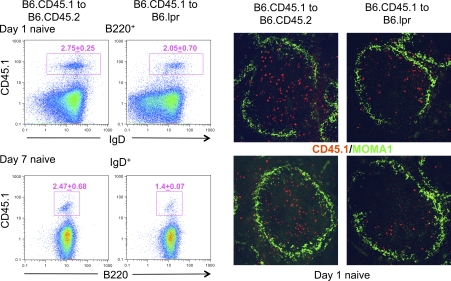
To test this idea, we first transferred B6.CD45.1 B cells into Fas-deficient or -sufficient B6.CD45.2 congenic hosts and monitored the stability of the CD45.1+ donor B cell compartment for 1 week via flow cytometry and immunohistology. Figure 5 shows that naive donor B cells were relatively stable 1 day after transfer and resided mainly in white pulp regions of the spleen. However, somewhat reduced numbers of donor B cells were observed in white pulp areas at day 7 after transfer, consistent with inefficient elimination of donor B cells expressing low levels of Fas.
Fig. 5.
Effects of a host Fas deficiency on Fas-sufficient donor B cell homing to and stability in follicles in unimmunized chimeric mice. MACS-enriched B cells from B6.CD45.1 mice were adoptively transferred into either Fas-sufficient (B6.CD45.2) or Fas-deficient (B6.lpr) recipients of the CD45.2 allotype (5 × 106 cells per mouse). Mice were sacrificed either on day 1 or day 7 after transfer. Left panels: splenocytes were isolated, stained with anti-B220, anti-IgD and anti-allotypic CD45.1 mAbs and analyzed by flow cytometry. Gated donor CD45.1+ cells are shown. The numbers shown above the gates indicate the mean percentage of CD45.1+ cells out of total B220+ or IgD+ cells and the standard deviation from three separate experiments. Right panels: immunofluorescent staining of spleen sections of mice described above on day 1 after transfer. Red staining indicates donor CD45.1 cells, while green staining with MOMA-1 delineates the border of the follicle and the marginal zone. Original magnification of images was ×140.
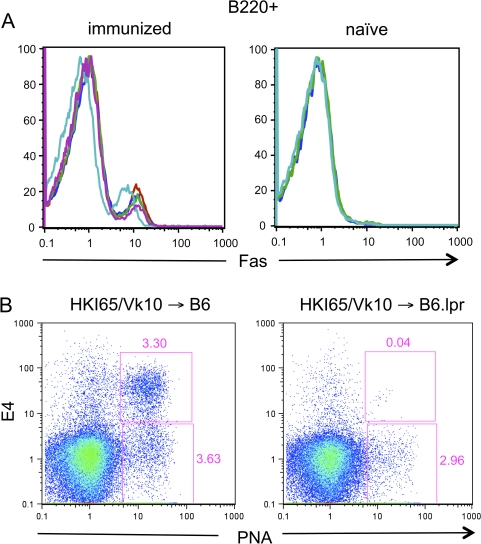
Next, Fas expression levels on B cells in chimeric mice created by injection of canonical HKI65/Vκ10A double transgenic B cells into B6.lpr mice, some of which were subsequently immunized with Ars–KLH, were evaluated by flow cytometry. Figure 6(A) shows that, as expected, a major sub-population of B cells in the immunized mice had up-regulated Fas 6 days later, but no such up-regulation was detected in unimmunized mice. These studies were followed by analysis of the contribution of canonical HKI65/Vκ10A B cells to the GC responses in such mice. Figure 6(B) illustrates that few, if any, E4+ GC B cells could be detected in the HKI65/Vκ10A->B6.lpr chimeras (upper gates). Nonetheless, E4−, host B cells continued to contribute to the GC response at this time (lower gates), consistent with the idea that expression of Fas is required for elimination of GC B cells.
Fig. 6.
Fas expression and GC responses of B cells in Fas-sufficient -> Fas-deficient chimeric mice. (A) Chimeric mice created by transfer of 2 × 106 HKI65/Vκ10A double transgenic B cells into B6.lpr hosts were either immunized the day after donor cell transfer with 100 μg of Ars–KLH in alum or left naive. On day 7 after transfer, mice were sacrificed, spleen cells isolated, stained with anti-B220 and anti-CD95 (Fas receptor) and analyzed by flow cytometry. Colored lines in the histograms represent B220-gated cells from individual mice. (B) Splenic B cells from HKI65/Vκ10A double transgenic mice were transferred into B6 or B6.lpr hosts and chimeric mice immunized as described above. Six days after immunization, chimeric mice were sacrificed, spleens cells isolated and stained with E4 and PNA and analyzed by flow cytometry. The E4+, PNA+ canonical GC B cell and E4−, PNA+ host GC B cell gates are shown.
CD8 T cells from Fas-deficient mice express elevated levels of FasL when activated via the TCR complex in vitro
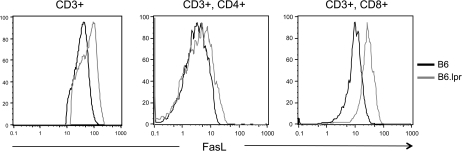
To determine if T cells in Fas-deficient mice expressed elevated levels of Fas ligand that might promote their killing of Fas-sufficient B cells, we examined such levels on the splenic T cells of naive B6 and B6.lpr mice via flow cytometry. Using this approach, we did not detect elevated levels of FasL expression on the T cells of B6.lpr mice (data not shown). As such, we stimulated enriched splenic T cells from B6 and B6.lpr mice with plate-bound anti-CD3ϵ antibody in vitro and 4 h later evaluated FasL expression on total CD3 T cells and CD8 and CD4 T cell sub-populations via flow cytometry. Figure 7 shows that total T cells and the CD8 sub-population from B6.lpr mice expressed much higher levels of surface FasL after this stimulation than such populations from B6 mice. CD4 T cells from both types of mice appeared to express similar levels of FasL after activation.
Fig. 7.
CD8 T cells in Fas-deficient mice are primed to express elevated levels of FasL upon activation. Enriched splenic T cells from B6 and B6.lpr mice were activated for 4 h by plate-bound anti-CD3e antibody as described in Methods. Cells were then stained with antibodies for the indicated markers and levels of expression of surface FasL on various sub-populations evaluated by flow cytometry. FasL levels on cells gated for expression of CD3 only, CD3 and CD4 or CD3 and CD8 are shown.
Suppression of the IgG and GC responses of Fas-deficient canonical B cells in Fas-deficient hosts
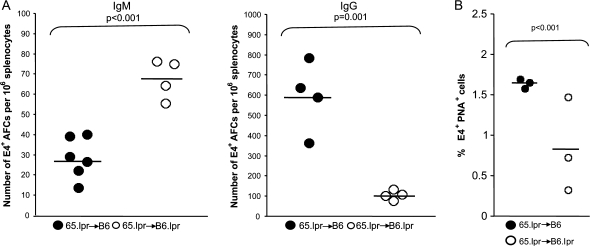
We next evaluated whether expression of Fas on donor B cells was required for suppression of the Fas-sufficient B cell GC and IgG responses in Fas-deficient hosts. We transferred HKI65.lpr B cells into B6 or B6.lpr hosts that were subsequently immunized with Ars–KLH and evaluated the E4+ IgM and IgG responses by ELISpot assay. Figure 8 shows that the IgM response of HKI65.lpr B cells was still enhanced and the IgG response reduced in Fas-deficient as compared with Fas-sufficient hosts. However, these differences were not as dramatic as those observed in chimeras containing Fas-sufficient donor B cells. These data indicate that while Fas expressed on donor B cells contributes to the suppression of the GC and IgG responses, other factors are at work as well.
Fig. 8.
A Fas deficiency in recipient mice results in an enhanced IgM response and compromised IgG AFC and GC responses from Fas-deficient donor B cells. Splenocytes from HKI65.lpr mice were adoptively transferred into B6 or B6.lpr mice (5 × 106 per mouse) and chimeric mice immunized 12 h later with 100 μg of Ars–KLH in alum. Mice were sacrificed on day 6 after immunization and the clonotype-specific E4+ immune response was assessed using ELISpot and flow cytometry as described in the legends to Figs 3 and 4. (A) The number of E4+ IgM- and IgG-producing AFCs per million splenocytes is illustrated. (B) The percentages of PNA+, E4+ B cells out of the total number of B220+ lymphocytes are shown. Filled and open circles represent transfer into Fas+-deficient or Fas-deficient recipients, respectively. Each circle represents an individual mouse. Horizontal bars show the mean values for each data set. Statistical significance was assessed by the Student’s t-test.
Discussion
Past reports on the role of Fas in the B cell-positive and -negative selective events that take place during the GC reaction have been limited and conflicting. Smith et al. (31) did not observe a measurable perturbation of the GC reaction and antibody affinity maturation in the immune response to (4-hydroxy-3-nitrophenyl)acetyl (NP) in Fas-deficient mice. In contrast, Takahashi et al., also studying the immune response to NP in such mice, did (15). Based largely on the high levels of Fas expression characteristic of GC B cells, Lindhout et al. (13) proposed a model in which autoreactive or ‘bystander’ GC B cells were killed via interaction with CD40L-expressing GC T cells. While to our knowledge this idea had not been directly tested prior to the studies reported here, Han et al. (9) did show that induction of apoptosis of GC B cells via injection of large doses of soluble, cognate antigen took place efficiently in Fas-deficient mice. This protocol was argued to recapitulate the pathway in which tolerance might be induced in the GC B cell compartment to abundant, soluble self-antigens.
In agreement with the general conclusions of Han et al., the studies we report here suggest little, if any, direct role for Fas in the negative selection of autoreactive canonical HKIR B cells during the GC response. A possible explanation for these results is that in addition to expressing high levels of Fas, many GC B cells also express elevated levels of c-FLIP, an antagonist of Fas-mediated apoptosis (19). Moreover, B cells can be rendered resistant to Fas-mediated killing by a variety of stimuli available in the GC including IL-4 (32), BCR ligation (33), CD40 ligation (34) and other factors derived from FO dendritic cells (18, 35). Many of these stimuli have been shown to induce the anti-apoptotic long isoform of c-FLIP (19, 34). Collectively, these data argue that autoreactive GC B cells may be protected from Fas-mediated apoptosis via stimuli that induce c-FLIP. To test this notion, we are currently evaluating the GC response mounted by c-FLIP-deficient canonical HKIR B cells.
In contrast, the data we obtained from the analysis of B cell chimeras generated using Fas-deficient recipient mice strongly suggest that under certain conditions, the Fas–FasL pathway can dramatically alter the outcome of a B cell immune response, including the GC reaction. These data are consistent with those of Hao et al. (30) suggesting that a Fas deficiency in T cells results in the increased potential of these cells to express FasL upon activation, a change resulting from perturbation of lymphocyte homeostasis. These T cells cannot kill themselves, but efficiently kill Fas-expressing cells of other types. We initially speculated that the absence of canonical IgG and GC responses was due to killing of Fas-sufficient canonical HKI B cells by FasL-expressing, KLH-specific CD4 T cells during Ars-KLH induced cognate interaction of these cell types. However, our data demonstrate that only CD8 T cells in B6.lpr mice are primed to express abnormally high levels of FasL upon activation, suggesting that other scenarios, such as those discussed below, may account for the suppression of canonical IgG and GC responses in B6.lpr hosts. Moreover, the increased IgM AFC responses we observed from Fas-sufficient donor B cells in Fas-deficient hosts are not easily explained by direct killing of donor B cells by host CD8 T cells. Further studies in this vein are clearly warranted.
Our experiments also demonstrated that while Fas-deficient donor B cells were capable of mounting IgG responses in Fas-deficient hosts, these responses were far from robust. Given the above rationale, this indicates that a Fas deficiency either indirectly perturbs aspects of T cell–B cell cognate interaction or additional death receptors that can productively interact with FasL are expressed on activated B cells. With regard to the first possibility, Hao et al. (30) explained the loss of Fas-deficient naive T cells in mice in which Fas was selectively deleted in T cells due to destruction of the natural lymphoid microenvironments for these cells by activated, FasL-expressing T cells. We did not observe gross changes in the size or organization of the T cell zones of the spleen in immunized HKI->B6.lpr or HKI.lpr->B6.lpr chimeras (data not shown). However, further studies will be required to determine if microenvironmental locales in which cognate T cell–B cell interaction normally takes place were altered and whether CD8 T cells expressing high levels of FasL might be responsible for such alterations. Such alteration might account for the enhanced IgM AFC response we observed from both Fas-sufficient and -deficient donor B cells in Fas-deficient hosts.
With regard to the possibility of another death receptor family ligand for FasL, Pitti et al. (36) have reported the cloning of a receptor for FasL from human lung tissue that they termed DcR3. A soluble form of this receptor inhibited Fas–FasL interactions and apoptosis, leading the authors to conclude that DcR3 was a FasL decoy receptor. However, whether DcR3 was capable of intrinsic signaling or associating with signaling proteins was not evaluated. Given these questions, it will be important to determine if murine B cells express a homologue of DcR3 and, if so, whether it is expressed on the cell surface and can mediate signal transduction.
Funding
National Institutes of Health; Commonwealth of Pennsylvania (ME-03-184) to T.M.
Acknowledgments
We acknowledge the use of the Kimmel Cancer Center Flow Cytometry and Bioimaging Facilites in this work. We thank Scot Fenn for technical help and all members of the Manser laboratory for their indirect contributions to this work.
Glossary
Abbreviations
- AFC
antibody-forming cell
- Ars
arsonate
- BM
bone marrow
- FO
follicular
- GC
germinal center
- H
heavy
- i.p.
intra-peritoneal
- KLH
keyhole limpet hemocyanin
- NP
(4-hydroxy-3-nitrophenyl)acetyl
- PNA
Peanut lectin agglutinin
- SA
streptavidin
- sBCR
surface BCR
References
- 1.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu. Rev. Immunol. 1992;10:731. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 2.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 1994;12:487. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 3.Marion TN, Tillman DM, Jou N-T, Hill RJ. Selection of immunoglobulin variable regions in autoimmunity to DNA. Immunol. Rev. 1992;128:123. doi: 10.1111/j.1600-065x.1992.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 4.Eilat D, Anderson WF. Structure-function correlates of autoantibodies to nucleic acids: lessons from immunochemical, genetic and structural studies. Mol. Immunol. 1994;27:203. doi: 10.1016/0161-5890(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 5.Stollar BD. Molecular analysis of anti-DNA antibodies. FASEB J. 1994;8:337. doi: 10.1096/fasebj.8.3.7511550. [DOI] [PubMed] [Google Scholar]
- 6.Shan H, Shlomchik MJ, Marshak-Rothstein A, Pisetsky DS, Litwin S, Weigert MG. The mechanism of autoantibody production in an autoimmune MRL/Ipr mouse. J. Immunol. 1994;153:5104. [PubMed] [Google Scholar]
- 7.MacLennan ICM. Germinal centers. Annu. Rev. Immunol. 1994;12:117. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 8.Nossal GJV. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Zheng B, Dal Porto J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl IV. Affinity dependent, antigen-driven B cell apoptosis in germinal centers as a mechanism for maintaining self-tolerance. J. Exp. Med. 1995;182:1635. doi: 10.1084/jem.182.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulendran B, Kannourakis G, Nouri S, Smith KGC, Nossal GJV. Soluble antigen can cause enhanced apoptosis of germinal-center B cells. Nature. 1995;375:331. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- 11.Shokat K, Goodnow CC. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature. 1995;375:334. doi: 10.1038/375334a0. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Zheng B, Takahashi Y, Kelsoe G. Distinctive characteristics of germinal center B cells. Semin. Immunol. 1997;9:255. doi: 10.1006/smim.1997.0081. [DOI] [PubMed] [Google Scholar]
- 13.Lindhout E, Koopman G, Pals ST, de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol. Today. 1997;18:573. doi: 10.1016/s0167-5699(97)01160-2. [DOI] [PubMed] [Google Scholar]
- 14.Elkon KB, Marshak-Rothstein A. B cells in systemic autoimmune disease: recent insights from Fas-deficient mice and men. Curr. Opin. Immunol. 1996;8:852. doi: 10.1016/s0952-7915(96)80015-x. [DOI] [PubMed] [Google Scholar]
- 15.Strasser A, Bouillet P. The control of apoptosis in lymphocyte selection. Immunol. Rev. 2003;193:82. doi: 10.1034/j.1600-065x.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubio CF, Kench J, Russell DM, Yawger R, Nemazee D. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J. Immunol. 1996;157:65. [PubMed] [Google Scholar]
- 17.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 18.Mastache EF, Lindroth K, Fernández C, González-Fernández A. Somatic hypermutation of Ig genes is affected differently by failures in apoptosis caused by disruption of Fas (lpr mutation) or by overexpression of Bcl-2. Scand. J. Immunol. 2006;63:420. doi: 10.1111/j.1365-3083.2006.001758.x. [DOI] [PubMed] [Google Scholar]
- 19.van Eijk M, Defrance T, Hennino A, de Groot C. Death-receptor contribution to the germinal-center reaction. Trends Immunol. 2001;22:677. doi: 10.1016/s1471-4906(01)02086-5. [DOI] [PubMed] [Google Scholar]
- 20.Hennino A, Bérard M, Krammer PH, Defrance T. FLICE-inhibitory protein is a key regulator of germinal center B cell apoptosis. J. Exp. Med. 2001;193:447. doi: 10.1084/jem.193.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Eijk M, Medema JP, de Groot C. Cutting edge: cellular Fas-associated death domain-like IL-1-converting enzyme-inhibitory protein protects germinal center B cells from apoptosis during germinal center reactions. J. Immunol. 2001;166:6473. doi: 10.4049/jimmunol.166.11.6473. [DOI] [PubMed] [Google Scholar]
- 22.Notidis E, Heltemes L, Manser T. Dominant, hierarchical induction of self-tolerance during foreign antigen-driven B cell development. Immunity. 2002;17:317. doi: 10.1016/s1074-7613(02)00392-8. [DOI] [PubMed] [Google Scholar]
- 23.Heltemes-Harris LM, Liu X, Manser T. Progressive surface BCR downregulation accompanies efficient development of anti-nuclear antigen B cells to mature, follicular phenotype. J. Immunol. 2004;172:823. doi: 10.4049/jimmunol.172.2.823. [DOI] [PubMed] [Google Scholar]
- 24.Manser T, Gefter ML. The molecular evolution of the immune response: idiotope specific suppression indicates that B cells express germline encoded V genes prior to antigenic stimulation. Eur. J. Immunol. 1986;16:1439. doi: 10.1002/eji.1830161120. [DOI] [PubMed] [Google Scholar]
- 25.Vora KA, Tumas-Brundage K, Manser T. Contrasting the in situ behavior of a memory B cell clone during primary and secondary immune responses. J. Immunol. 1999;163:4315. [PubMed] [Google Scholar]
- 26.Liu X, Manser T. Antinuclear antigen B cells that downregulate surface BCR during development to mature, follicular phenotype do not display features of anergy in vitro. J. Immunol. 2005;174:4505. doi: 10.4049/jimmunol.174.8.4505. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Wysocki LJ, Manser T. Autoantigen-B cell antigen receptor interactions that regulate expression of B cell antigen receptor loci. J. Immunol. 2007;178:5035. doi: 10.4049/jimmunol.178.8.5035. [DOI] [PubMed] [Google Scholar]
- 28.Alabyev B, Rahman ZSM, Manser T. Quantitatively reduced participation of anti-nuclear antigen B cells that down regulate BCR during primary development in the germinal center/memory B cell response to foreign antigen. J. Immunol. 2007;178:5623. doi: 10.4049/jimmunol.178.9.5623. [DOI] [PubMed] [Google Scholar]
- 29.Casson L, Manser T. Random mutagenesis of two CDR amino acids yields an unexpectedly high frequency of antibodies with increase affinity for both cognate antigen and autoantigen. J. Exp. Med. 1995;182:743. doi: 10.1084/jem.182.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Z, Hamplel B, Yagita H, Rajewsky K. T cell-specific ablation of Fas leads to Fas ligand-mediated lymphocyte depletion and inflammatory pulmonary fibrosis. J. Exp. Med. 2004;199:1355. doi: 10.1084/jem.20032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith KG, Nossal GJ, Tarlinton DM. FAS is highly expressed in the germinal center but is not required for regulation of the B-cell response to antigen. Proc. Natl Acad. Sci. USA. 1995;92:11628. doi: 10.1073/pnas.92.25.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foote LC, Marshak-Rothestein A, Rothstein T. Inducible Fas-resistance mediated by IL-4 in activated B cells. Ann. N.Y. Acad. Sci. 1997;815:114. doi: 10.1111/j.1749-6632.1997.tb52050.x. [DOI] [PubMed] [Google Scholar]
- 33.Tumang JR, Negm RS, Solt LA, et al. BCR engagement induces Fas resistance in primary B cells in the absence of functional Bruton's tyrosine kinase. J. Immunol. 2002;168:2712. doi: 10.4049/jimmunol.168.6.2712. [DOI] [PubMed] [Google Scholar]
- 34.Troeger A, Schmitz I, Siepermann M, et al. Up-regulation of c-FLIPS+R upon CD40 stimulation is associated with inhibition of CD95-induced apoptosis in primary precursor B-ALL. Blood. 2007;110:384. doi: 10.1182/blood-2006-08-038398. [DOI] [PubMed] [Google Scholar]
- 35.Lindhout E, Lakeman A, de Groot C. Follicular dendritic cells inhibit apoptosis in human B lymphocytes by a rapid and irreversible blockade of preexisting endonuclease. J. Exp. Med. 1995;181:1985. doi: 10.1084/jem.181.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]