Abstract
The use of antibodies against the human B cell surface protein CD20 represents the most advanced therapeutic approach among the B cell–depleting armamentarium for the treatment of autoimmune disorders. However, recent evidence indicates that B cells can also be essential for suppressing unwanted autoaggressive T cell responses, and therefore, a more careful evaluation of which types of autoimmune disorders this therapy should be utilized for, and at which phases of disease this therapy should be applied, is necessary. In this issue of the JCI, Matsushita et al. report that the timing of this therapy is critical for the management of EAE, a mouse model of human MS (see the related article beginning on page 3420). The results suggest the existence of two opposite actions executed by B cells during the course of autoimmune pathology; CD1dhiCD5+ regulatory B cells suppress EAE induction, whereas B cells are required for the expansion of autoantigen-specific T cells during disease progression. Given the existence of such regulatory B cells in humans, these findings not only resolve previously unexplained contradictions with respect to the outcome of B cell–depleting therapy but also provide insight into the best regimen for this treatment approach.
The role of B lymphocytes in autoimmune diseases has been thought to be associated largely with the capacity of plasmablasts and plasma cells to produce self-damaging antibodies. Indeed, in some human autoimmune diseases, recapitulation of disease pathology through the transfer of pathogenic antibodies has been well documented (1). These autoantibodies bind self-antigens and can interfere with normal cellular functions as well as harness immune effector mechanisms to generate autoimmune pathology (2).
However, it has been revealed that most complex autoimmunity involves additional B cell functions. These functions include the ability of B cells and their secreted products (in addition to antibodies and immune complexes) to modulate T cells and DCs through antigen presentation and costimulation. The first experimental evidence of such B cell function was obtained by the generation of autoimmune disease–prone MRL/lpr mice with compromised B cell functions (3). MRL/lpr mice completely deficient in mature B cells (resulting from an IgM heavy chain knockout mutation in MRL/lpr mice) exhibited no signs of autoantibodies or glomerulonephritis and exhibited improvement in mortality compared with MRL/lpr mice. Surprisingly, however, membrane IgM MRL/lpr mice that express only the membrane form of IgM, thereby being incapable of antibody or autoantibody secretion, developed significant glomerulonephritis or mortality, although this was less severe compared with that in MRL/lpr mice (4). These data indicate that antibody-independent mechanisms, in addition to the autoantibodies themselves, also play important pathogenic roles in autoimmune disorders and may involve important inflammatory roles of B cells in cytokine production as well as in directing T cell and DC functions. In contrast to the inflammatory role of B cells demonstrated in MRL/lpr mice, in the mouse model of human MS, EAE, Janeway and colleagues provided the first suggestive evidence that B cells may also play an antiinflammatory role, in that B10.PL mice lacking B cells suffered an unusually severe and chronic form of EAE (5). This observation raised the possibility that B cell–depleting therapy might exacerbate, rather than ameliorate, disease pathology. Thus, the fact that rituximab (a genetically engineered anti-CD20 monoclonal antibody), the most widely used chimeric monoclonal antibody for human B cell depletion, has already been used in a phase II trial to treat MS patients (6) appears to be inconsistent with the above observations at first glance, because MS has close similarities to EAE. In this issue of the JCI, Matsushita and colleagues have tackled this paradox by carefully evaluating the efficacy of anti-CD20 antibody treatment in the mouse EAE model and describe the existence of two opposing functions of B cells and that the nature of these functions is dependent on the course of disease pathology. Specifically, B cell function during the phase of disease initiation was found to differ from that during the phase of disease progression (7).
Initiation phase of EAE
To understand how to treat EAE, it is helpful to consider how EAE pathology is initiated and maintained. EAE is not a spontaneous autoimmune disease and thus requires either direct immunization with antigenic myelin components such as myelin oligodendrocyte glycoprotein (MOG) or adoptive transfer of anti-myelin–specific CD4+ T cells. It has been thought that T cells, which are able to recognize myelin components, are activated in the periphery, migrate to the CNS, and cause autoinflammation that leads to paralysis. In resting states, the CNS is regarded as a system that is protected against the immune response because of the tight endothelial junctions of the blood-brain barrier, the absence of DCs in CNS parenchyma, and the presence of an immunosuppressive microenvironment characterized by the production of CNS antiinflammatory cytokines. Thus, autoantigen-activated T cells are thought to lead to a breakdown of the blood-brain barrier and enable the recruitment of other inflammatory cells such as monocytes as well as components of both the B cell response (B cells, antibodies, and complement factors) or other T cell responses (e.g., CD8+ T cells) (8).
One of the key findings from the current study reported by Matsushita et al. (7) in this issue of the JCI is that B cell depletion by anti-CD20 antibody before EAE induction exacerbates the disease symptoms of EAE, consistent with the previous report using IgM-knockout mice (5). More importantly, they show that the adoptive transfer of the IL-10–producing CD1dhiCD5+ B cell subset from the spleen, but not of other B cell subsets, can prevent exacerbation of EAE pathogenesis, clearly demonstrating that CD1dhiCD5+ B cells from the spleen are responsible for the antiinflammatory effect of B cells. CD5 or CD1d have been suggested to represent good markers for tracing such regulatory B cell subsets, in that CD5+ peritoneal B-1 cells are able to produce particularly large amounts of IL-10 following LPS stimulation (9) and the spleen B cells regulating ulcerative colitis express CD1d (10). Based on such suggestive observations, Yanaba and colleagues previously demonstrated that CD1dhiCD5+ B cells in the spleen can produce a high amount of IL-10 and that this subset is able to inhibit the pathology of augmented contact hypersensitivity (11), which is an inflammatory immune reaction that is primarily mediated by T cells (12).
Does this B cell subset require antigen-specificity as well as IL-10 production? The answer seems to be yes, because a previous study by Fillatreau et al. showed that B cells specific for an irrelevant antigen (hen-egg lysozyme) were unable to resolve EAE and that IL-10 production by B cells was required for EAE suppression (13). Moreover, B cells from mice that had recovered from EAE can produce IL-10 upon in vitro stimulation with specific antigen (e.g., MOG) and anti-CD40 antibody together (13). A suppressive effect of IL-10 produced by B cells has also been demonstrated in a model of inflammatory bowel disease (14) and in collagen-induced arthritis (15), suggesting a more general role for IL-10–producing B cells in homeostasis. More recently, IL-10–producing B cells have been identified in humans (16), and preliminary evidence shows that B cells from patients with MS produce decreased amounts of IL-10 (17).
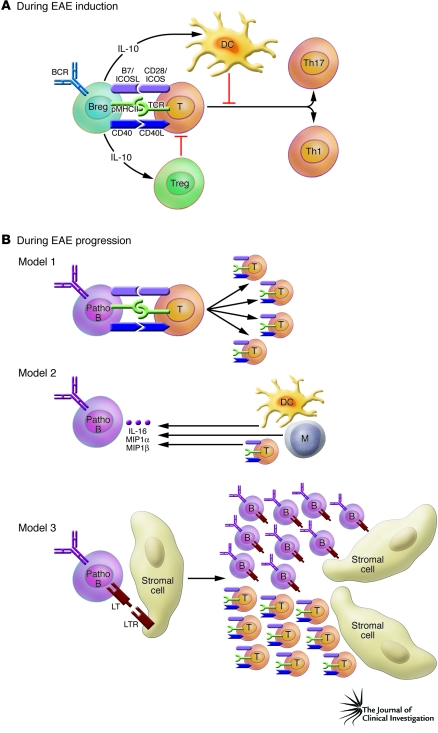
How and where do IL-10–producing B cells suppress autoimmune disease? Given diverse etiologies, multiple mechanisms of action might be in operation. Now, two potential target cells are postulated to be involved — DC cells and Tregs (Figure 1A). IL-10 has potent activity in limiting DC function, and therefore, IL-10–producing B cells may exert their effects on T cell responses indirectly. Indeed, DCs from B cell–compromised mice produce higher amounts of IL-12 after immunization, and this drives a stronger Th1-cell response (18). Furthermore, IL-10 from B cells can repress the production of IL-6 and IL-12 by DCs, thereby inhibiting the differentiation of Th17 and Th1 cells, respectively (19). In the case of Tregs, only circumstantial evidence is available; in B cell–deficient mice and mice in which B cells cannot produce IL-10, a transient reduction in Foxp3 mRNA levels in the CNS during the early course of EAE disease has been reported (20).
Figure 1. Potential mechanisms of pro- and antiinflammatory effects of B cells.
(A) In this issue of the JCI, Matsushita et al. (7) show the importance of the antiinflammatory role of CD1dhiCD5+ regulatory B cells during EAE initiation. In the initiation phase of EAE, antigen-specific CD1dhiCD5+ B cells (Breg) produce IL-10, which limits DC function and/or augments Treg function. Consequently, these DCs and Tregs could inhibit the differentiation of Th17 and Th1 T cells. (B) During the progression phase of EAE, three potential models for the mechanism of action of B cells can be envisaged. In the first model, pathogenic B cells (Patho B) can function as antigen-presenting cells and enhance the proliferation of pathogenic T cells and their differentiation. In the second model, antigen-activated pathogenic B cells produce IL-16, macrophage inflammatory protein 1α (MIP1α), and MIP1β, which can modulate the migration and function of DCs, monocytes (M), and pathogenic T cells. In the third model, interactions between B cells and stromal cells via TNF/TNF receptor family networks (such as interaction of lymphotoxin [LT] with the LT receptor [LTR]) induce lymphoid-like follicle formation in the CNS, which might provide the optimal site for expansion of pathogenic T cells. B, B cell; BCR, B cell receptor; CD40L, CD40 ligand; ICOSL, ICOS ligand; pMHCII, peptide–MHC class II; T, T cell.
In regard to the location where B cells exert their suppressive functions, B cell–deficient mice and mice with IL-10–deficient B cells showed heightened lymph node Th1-cell responses to immunization. Therefore, B cell–dependent inhibitory regulation of EAE appears to start in the draining lymph nodes within days of immunization (18).
Progression phase of EAE
In addition, Matsushita et al. (7) demonstrated that B cell depletion during EAE disease progression dramatically ameliorates the EAE pathology. Why is that? Theoretically, pathogenic B cells and/or pathogenic autoantibodies could be responsible. Indeed, the titer of anti-MOG IgG antibody was decreased in B cell–depleted mice during EAE progression. Given the importance of activating Fcγ receptors (21) and anti-MOG sera (22) in inducing EAE, this decreased titer of anti-MOG IgG could contribute, at least to some extent, to alleviating the EAE pathology.
Rather than focus on the role of antibodies, Matsushita et al. (7) have focused on B cell functions. Their key finding that provides important mechanistic insight was obtained from the adoptive transfer of CD4+ T cells harboring transgenic TCRs specific for MOG into B cell–depleted mice during EAE progression. This type of B cell depletion significantly reduced MOG-specific CD4+ T cell expansion within the CNS and draining lymph node. Three, not necessarily mutually exclusive, possible explanations for these data can be envisaged (Figure 1B). First, B cells could function as antigen-presenting cells and promote expansion of pathogenic T cells, possibly Th1 and Th17 effector T cells. In this scenario, cognate interactions between B and T cells mediated by CD40/CD40 ligand and/or ICOS/ICOS ligand might be required. Second, B cells can secrete cytokines and chemokines, including IL-16, macrophage inflammatory protein 1α (MIP1α), and MIP1β, which can modulate the migration and function of DCs, monocytes, and pathogenic T cells. Finally, given the importance of B cells in the formation and maintenance of new lymphoid foci by using TNF/TNF receptor networks such B cell–dependent lymphoid-like follicle formation in the CNS might provide the optimal site for expansion of pathogenic T cells.
Taken together, Matsushita et al. (7) provide evidence that the balance of two opposing B cell functions shapes the normal course of EAE pathogenesis. As with all interesting observations, not only are previous unexplained contradictions accounted for, but also, perhaps more importantly, motivation for future experiments is provided. Several such experiments come to mind. First, how are the antigen-specific CD1dhiCD5+ B cells generated, maintained, and activated? Second, what is the origin of the pathogenic B cells and how do they work? Finally, what determines and changes the balance between regulatory and pathogenic B cells?
The important implication of these results with respect to the treatment of autoimmune diseases is that anti-CD20 antibody treatment could sometimes exacerbate autoimmune disease by unintentionally depleting CD1dhiCD5+ regulatory B cells. Indeed, one could speculate that this finding could underlie the results of a recent study of the treatment of patients with ulcerative colitis (23); depleting B cells by anti-CD20 treatment induced suppression of local IL-10 production and exacerbated disease symptoms, suggesting that the local IL-10 concentration could be a useful benchmark for diagnosis and prognosis of diseases. Thus, it will be very important to determine the involvement of CD1dhiCD5+ regulatory B cells in each autoimmune disease and, if there is any, to determine when this subset participates during disease initiation and progression. In this regard, this new study (7) definitely provides important insight for the development of the best regimen of anti–B cell therapy for autoimmune diseases.
Acknowledgments
I thank Masato Tanaka, Yuichi Aiba, and Takaharu Okada for instructive discussion and Mari Kurosaki for help with the references and with creating Figure 1.
Footnotes
Nonstandard abbreviations used: MOG, myelin oligodendrocyte glycoprotein.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3260–3263 (2008).doi:10.1172/JCI37099.
See the related article beginning on page 3420.
References
- 1.Ravetch, J.V. 2003. Fc receptors. In Fundamental immunology. W.E. Paul, editor. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 685–700. [Google Scholar]
- 2.Martin F., Chan A.C. B cell immunobiology in disease: evolving concepts from the clinic. Annu. Rev. Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 3.Shlomchik M.J., Madaio M.P., Ni D., Trounstein M., Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J. Exp. Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan O.T., Hannum L.G., Haberman A.M., Madaio M.P., Shlomchik M.J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf S.D., Dittel B.N., Hardardottir F., Janeway C.A., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser S.L., et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. . N. Engl. J. Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 7.Matsushita T., Yanaba K., Bouaziz J.-D., Fujimoto M., Tedder T.F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. . 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Behi M., et al. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol. Lett. 2005;96:11–26. doi: 10.1016/j.imlet.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 9.O’Garra A., et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int. Immunol. 1990;2:821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 10.Amano M., et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: beta 2-microglobulin-dependent and independent forms. J. Immunol. 1998;161:1710–1717. [PubMed] [Google Scholar]
- 11.Yanaba K., et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Wang B., et al. CD4+ Th1 and CD8+ type 1 cytotoxic T cells both play a crucial role in the full development of contact hypersensitivity. J. Immunol. 2000;165:6783–6790. doi: 10.4049/jimmunol.165.12.6783. [DOI] [PubMed] [Google Scholar]
- 13.Fillatreau S., Sweenie C.H., McGeachy M.J., Gray D., Anderton S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi A., Mizoguchi E., Takedatsu H., Blumberg R.S., Bhan A.K. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 15.Mauri C., Gray D., Mushtaq N., Londei M. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duddy M.E., Alter A., Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J. Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 17.Duddy M., et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 18.Moulin V., et al. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J. Exp. Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampropoulou V., et al. TLR-activated B cells suppress T cell-mediated autoimmunity. . J. Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 20.Mann M.K., Maresz K., Shriver L.P., Tan Y., Dittel B.N. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Majid K.B., et al. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2002;55:70–81. doi: 10.1046/j.1365-3083.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 22.Lyons J.A., Ramsbottom M.J., Cross A.H. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur. J. Immunol. 2002;32:1905–1913. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 23.Goetz M., Atreya R., Ghalibafian M., Galle P.R., Neurath M.F. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm. Bowel Dis. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]