Abstract
The current inactivated influenza virus vaccines induce antibodies that protect against closely related virus strains. They do not, however, protect against antibody-escape variants of seasonal influenza A viruses or new pandemic influenza A viruses emerging from non-human reservoirs. Might boosting influenza A virus–specific CD8+ T cell memory diminish the danger posed by these variant viruses? Pre-existing CD8+ T cell–mediated immunity directed at peptides from conserved internal proteins of the influenza A virus does not prevent infection, but it can promote early virus clearance and decrease morbidity in mice. In this issue of the JCI, Lee et al. show that people who have not been exposed to avian influenza A (H5N1) viruses have cross-reactive CD8+ T cell memory to a wide range of H5N1 peptides (see the related article beginning on page 3478). These peptides could be used to add a CD8+ T cell component to current antibody-focused vaccine strategies with a view to reducing the impact of infection with novel influenza A viruses.
The recent spread of the extremely virulent avian influenza A subtype H5N1 viruses, herein referred to as H5N1, through Asia and to North Africa and Europe has raised serious concerns about the possibility of a novel human influenza pandemic (1, 2). Though the severe disease that can develop in humans exposed to H5N1-infected birds is rare and sustained human-to-human transmission of the virus has not yet been observed, the three influenza pandemics of the 20th century were all caused by influenza A viruses that originated from birds (3). Variant influenza A (H1N1), A (H3N2), and B viruses also cause regular seasonal epidemics that are associated with substantial morbidity and economic loss. It is bad enough that some 250,000–500,000 (particularly elderly) people die annually from influenza, but what if we should face an event like the 1918–1919 influenza pandemic? That pandemic killed in excess of 40 million people worldwide — before the era of rapid air travel and at a time when the global population was less than a third of that today.
Limitations of current influenza vaccines
Inactivated influenza vaccines elicit neutralizing antibody responses that provide reasonable protection against the homologous H1N1, H3N2, and B viruses (4). However, antibody-mediated selection drives changes (known as antigenic drift) in the viral HA (H) and neuraminidase (NA; N) surface glycoproteins, which in turn dictate the frequent production of a new vaccine, sometimes as often as annually, as has been the case in each of the last five years. The WHO recommends candidate vaccine virus strains that have been identified among the collections of emerging field isolates supplied by a global network of 124 WHO National Influenza Centers and other diagnostic laboratories and characterized by the four WHO Collaborating Centers for Influenza (in London, Atlanta, Melbourne, and Tokyo). The WHO’s recommendations also inform the composition of the live vaccines produced from “cold-adapted” viruses that are selected to grow at the lower temperature of the human nose and upper respiratory tract. These vaccines have been used for many years in Russia and the former Soviet Union and are now available internationally, but are not approved for Western use in the very young or the elderly.
Apart from the lack of protection against variant viruses, the use of traditional inactivated vaccines in a future influenza pandemic will be constrained by the need for specialized egg-based production facilities and long production times required for the creation of a new vaccine. Most influenza vaccines are made from viruses grown in the allantoic cavity of embryonated hen’s eggs. The viruses are then inactivated, purified, and in most cases “split” to produce vaccines that consist largely of HA and NA subunits. Recent advances include the use of reverse genetics to express the HA and NA of extremely virulent viruses, such as H5N1, with the internal proteins of standard vaccine strains (5) in order to enable the virus to replicate without killing the chick embryo, as well as the development of cell culture systems for high-titer virus growth. Even with these advances, however, it is likely to be many months before a new vaccine based on a pandemic virus can be deployed globally.
Cross-protection by CTLs
While nobody is suggesting that we abandon the current antibody-based strategy, there is increasing interest in the possibility that it might be useful to add a CD8+ T cell–activating component to the trivalent seasonal influenza vaccines (6). The early finding (7, 8) that influenza A virus–specific CTLs are broadly cross-reactive for cells of the same MHC class I type infected with serologically distinct H1N1 and H3N2 viruses was initially greeted with scepticism, then by indifference. The argument is that if seasonal influenza infection does promote cross-reactive T cell responses, then why do so many people get sick every one or two years? The counter-argument is, of course, that the majority of individuals may be protected from more serious disease by their T cell response. Healthy adults do not usually die from influenza, and it is the very young, who have not previously been exposed to the virus, and the frail elderly who are particularly at risk. Adoptive T cell transfer or cross-priming experiments with H1N1 and H3N2 viruses in mice have demonstrated conclusively that established CD8+ T cell memory is protective (9). Primed mice may still show substantial weight loss and morbidity, but they clear serologically different influenza A viruses from the lung more rapidly than mice that are not primed and can even survive respiratory challenge with highly virulent H7N7 and H5N1 viruses (9).
The identity of the antigen recognized by influenza A virus–specific CTLs remained obscure for the best part of a decade until Alain Townsend and John Skehel in the United Kingdom and Jon Yewdell, Jack Bennink, Geoff Smith, and Bernie Moss at the NIH showed (10, 11) that the cross-reactive CTLs generated in a number of mouse strains of different MHC class I types recognize the conserved internal influenza A virus nucleoprotein (NP). Subsequently, Townsend and colleagues broke open the CTL/MHC class I story by demonstrating that NP-specific CD8+ T cells recognize short viral peptides bound by the MHC class I molecules (12). This was a paradigm-shifting experiment that led to the creation of the field of research on protein/peptide processing in the cytoplasmic compartment. Subsequently, a number of other internal proteins in the virus, particularly the acid and basic polymerases (PA and PB1, respectively) and the structural matrix protein 1 (M1), have been found to provide additional peptides that make up the peptide–MHC class I complexes recognized by influenza virus–specific CTLs in both mice and humans (13).
The case for CTL-mediated cross-protective vaccines
The article by Lee et al. in this issue of the JCI presents a detailed analysis, in individuals in the United Kingdom and Viet Nam, of the specificities of pre-existing CD8+ and CD4+ T cells for peptides encompassing all the proteins of an H5N1 and an H3N2 virus (14). Although citizens of the United Kingdom are not likely to have encountered an H5N1 virus, and the Vietnamese subjects were laboratory workers who were seronegative for H5, most subjects in both groups did possess memory T cells that were able to recognize cells displaying H5N1 peptides. A broad spectrum of peptides was recognized, predominantly from the viral NP and M1 proteins and, as subjects were not HLA typed, presumably reflecting a range of MHC class I–restriction specificities. Some, but by no means all, of these peptides were conserved between the H3N2 and H5N1 strains used.
Would it be worthwhile to boost this T cell memory in order to strengthen cross-protective immunity (Figure 1)? Though CD8+ CTL immunotherapy can control Epstein-Barr virus–induced lymphomagenesis in humans (15), experience with vaccines that function exclusively by promoting CD8+ T cell–mediated immunity in higher primates and humans has largely been restricted to the HIV and SIV lentiviruses. The results have generally been disappointing. Experiments with SIV vaccines have shown, for example, that the virus is controlled for a time following vaccination but then mutates to avoid CD8+ T cell surveillance (16). However, the situation with influenza A viruses is different, as these viruses neither integrate cDNA into the host genome nor persist in the host in any form. Enhanced control of influenza viruses in the short term is thus likely to be sufficient to limit disease and reduce transmission. Although expansion of the population of pre-existing memory T cells may only serve to change the disease profile from mortality to morbidity, the impact could be substantial in the event of a pandemic or a severe seasonal epidemic.
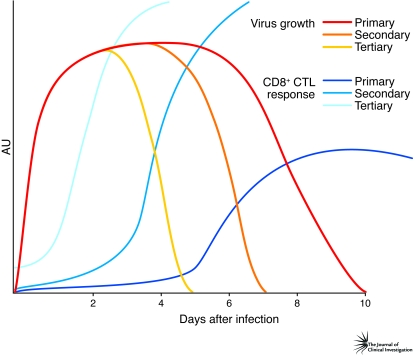
Figure 1. The CTL response to influenza A virus infection.
Influenza A viruses rapidly grow to very high titers in the lungs of infected mice (primary virus growth). Virus clearance is only enhanced (secondary virus growth) by approximately 2–3 days (21) in those animals that have memory CD8+ CTL numbers at what might be considered normal, physiological prevalence (<0.5% in spleen). Boosting those CTL counts (to >5%) a few weeks before viral challenge by some form of secondary stimulation can cause the period before the virus is successfully eliminated to be shortened by 48 hours or more (tertiary virus growth) (22). As shown in this issue of the JCI in the study by Lee et al. (14), most people already possess memory CTLs specific for the influenza A viruses. In the face of a rapidly emerging seasonal influenza epidemic, or a pandemic caused by a novel influenza A virus, a possible future strategy to mitigate the impact would be to stockpile a vaccine for emergency use that increases CD8+ CTL numbers.
What type of vaccine might be appropriate to induce cross-protective CTLs? Inactivated vaccines induce negligible CD8+ T cell responses, and live, cold-adapted vaccines can induce CD8+ T cell memory cells, but the numbers are low. For repeated administration, the most practicable possibilities would be the use of either DNA encoding, particularly, the whole M1 and NP proteins (17), or of a spectrum of immunogenic peptides from these and other viral components (18). Another approach would be to separate NP and M1 proteins made in the current vaccine production protocol and then to deliver them with an adjuvant or linker molecule to promote their entry into, and degradation via, the cytoplasmic processing pathway for presentation with MHC class I molecules. However, repeated use of viral proteins or viral vectors (e.g., vaccinia viruses or alphaviruses) has the problem that bound antibodies would tend to target the NP or M1 protein for lysosomal degradation so that they do not enter the cytoplasmic processing pathway. Whatever strategy is used, a major advantage of a broad-spectrum CD8+ T cell vaccine is its potential for large-scale stockpiling prior to need.
Are there risks in giving regular doses of a vaccine that promotes CD8+ T cell responses? Some have expressed concern that primed CD4+ and CD8+ T cells might contribute to the excessive immune response known as a cytokine storm (19), which can cause early mortality in those individuals infected with the H5N1 and (most likely) the H1N1 virus responsible for the 1918 influenza pandemic. However, as the analysis of this systemic shock phenomenon progresses, it appears that the chemokines and cytokines involved are produced by elements of the innate response, especially monocytes and neutrophils (20). The first goal must therefore be to remove the virus from the equation as soon as possible after infection, an effect that can be mediated by a rapidly emerging recalled CD8+ T cell response. All in all, as suggested by Lee et al. in their current study (14), the possibility of developing a vaccination strategy for the regular boosting of influenza A virus–specific T cell–mediated immunity would seem eminently worthy of further consideration and experiment.
Acknowledgments
The Melbourne WHO Collaborating Centre for Reference and Research on Influenza is supported by the Australian Government Department of Health and Ageing. P.C. Doherty is funded by the NIH, the Australian National Health and Medical Research Council, and The American Lebanese Syrian Associated Charities (ALSAC) at St. Jude Children’s Research Hospital.
Footnotes
Nonstandard abbreviations used: M1, matrix protein 1; NA, neuraminidase; NP, nucleoprotein.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3273–3275 (2008). doi:10.1172/JCI37232.
See the related article beginning on page 3478.
References
- 1.Li K.S., et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 2.Russell C.J., Webster R.G. The genesis of a pandemic influenza virus. Cell. 2005;123:368–371. doi: 10.1016/j.cell.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R.B. The origins of pandemic influenza — lessons from the 1918 virus. N. Engl. J. Med. 2005;353:2209–2211. doi: 10.1056/NEJMp058281. [DOI] [PubMed] [Google Scholar]
- 4.Palese P. Making better influenza virus vaccines? Emerg. Infect. Dis. 2006;12:61–65. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann E., Krauss S., Perez D., Webby R., Webster R.G. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/S0264-410X(02)00268-2. [DOI] [PubMed] [Google Scholar]
- 6.Epstein S.L., et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Effros R.B., Doherty P.C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J. Exp. Med. 1977;145:557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zweerink H.J., Askonas B.A., Millican D., Courtneidge S.A., Skehel J.J. Cytotoxic T cells to type A influenza virus; viral hemagglutinin induces A-strain specificity while infected cells confer cross-reactive cytotoxicity. Eur. J. Immunol. 1977;7:630–635. doi: 10.1002/eji.1830070910. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P.G., Keating R., Hulse-Post D.J., Doherty P.C. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend A.R., Skehel J.J. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J. Exp. Med. 1984;160:552–563. doi: 10.1084/jem.160.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yewdell J.W., Bennink J.R., Smith G.L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend A.R., et al. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-X. [DOI] [PubMed] [Google Scholar]
- 13.Gotch F., McMichael A., Smith G., Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 1987;165:408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee L.Y.-H., et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 2008;118:3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rooney C.M., et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–1555. [PubMed] [Google Scholar]
- 16.Barouch D.H., et al. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 17.Epstein S.L., et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg. Infect. Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day E.B., et al. The context of epitope presentation can influence functional quality of recalled influenza A virus-specific memory CD8+ T cells. . J. Immunol. 2007;179:2187–2194. doi: 10.4049/jimmunol.179.4.2187. [DOI] [PubMed] [Google Scholar]
- 19.Cheung C.Y., et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/S0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 20.Szretter K.J., et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flynn K.J., Riberdy J.M., Christensen J.P., Altman J.D., Doherty P.C. In vivo proliferation of naive and memory influenza-specific CD8(+) T cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen J.P., Doherty P.C., Branum K.C., Riberdy J.M. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J. Virol. 2000;74:11690–11696. doi: 10.1128/JVI.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]