Abstract
Fetal loss induced by antiphospholipid antibodies (aPLs) in mice is a complement-driven inflammatory condition. Engagement of the complement receptor C5aR on neutrophils induces expression of the principal initiator of the blood clotting mechanism, tissue factor (TF), and blocking this downstream event of complement activation prevents antibody-induced fetal loss. In this issue of the JCI, the study by Redecha et al. clarifies that in mice, the contribution of TF to this pathogenic mechanism is independent of its role in coagulation and thrombosis, but involves inflammatory signaling through the receptor PAR2 (see the related article beginning on page 3453). The study not only sheds light on a critical effector mechanism of aPL-induced fetal loss, but also suggests that treatment with statins, which decrease TF and PAR2 expression, may hold promise as a therapeutic approach to antiphospholipid syndrome–associated pregnancy complications.
Antiphospholipid syndrome (APS) is characterized by the presence of autoreactive antiphospholipid antibodies (aPLs), which recognize specific plasma proteins that possess an affinity for anionic phospholipids, combined with clinical evidence of thrombosis. In pregnant females, aPLs trigger severe pregnancy complications, such as miscarriage, intrauterine growth restriction, and fetal death, and also increase the likelihood of preeclampsia (1–3). Infusion of aPLs isolated from human patients into pregnant mice is sufficient to reproduce fetal loss and fetal growth restriction. A remarkable series of genetic and pharmacologic intervention studies in this mouse model has led to a detailed understanding of the pathogenesis underlying aPL-induced fetal loss (1, 4, 5).
Clinically, the most relevant antigen recognized by aPLs is β2-glycoprotein I (β2GPI), a plasma protein with poorly characterized functions. Binding of aPL induces the formation of β2GPI dimers with increased affinity for anionic phospholipids. Binding to negatively charged membrane surfaces is also essential for the function of key coagulation proteins, including prothrombin. Competition between aPL/β2GPI complexes and coagulation factors for binding to phosphatidylserine explains the anticoagulant effect exerted by aPLs in in vitro coagulation assays. The affinity for negatively charged membranes directs the selective allocation of aPL/β2GPI complexes to the phosphatidylserine-rich surface of fetal trophoblast cells in the placenta.
A critical milestone in understanding aPL-induced fetal loss was the observation of massive accumulation of complement component C3 in the placenta and the finding that that genetic deficiency of maternal C3 or administration of the C3 inhibitor Cryy-IgG prevents fetal loss (6). It has since been shown that downstream of C3 in this signaling pathway, interactions between complement component C5a and the C5a receptor (C5aR), rather than the classic cell-destroying formation of the C5b-C9 membrane attack complex, are responsible for fetal loss (7).
Thus far, 2 specific consequences of C5a-C5aR engagement are known to contribute to fetal loss: augmented release of TNF (8) and induction of tissue factor (TF) expression by maternal neutrophils (9). TNF-α may exert direct cytotoxic effects on trophoblast cells that line maternal blood spaces in the placenta, or sustain the activation of immune cells in the placenta. Accordingly, genetic ablation or functional inhibition of TNF-α and its interactions with its receptors prevents aPL-induced fetal loss (8). More recent work shows that the aPL-induced expression of TF on neutrophils is a critical second link between C5aR engagement and fetal loss (9). C5aR-deficient neutrophils fail to increase TF expression in response to aPL, and pharmacologic or genetic suppression of TF in maternal neutrophils is sufficient to prevent the formation of reactive oxygen species and fetal loss.
TF mediates fetal loss independent of thrombosis
In the study by Redecha et al. in this issue of the JCI (10), the authors distinguish the relative role of the 2 known functions of TF. TF is a transmembrane receptor for the circulating coagulation Factor VII (FVII) (11). Upon formation of the TF/FVII complex, TF gains affinity for plasma coagulation Factor X (FX) and converts it — via partial proteolysis — from an inactive zymogen to the active protease form (FXa). After release from the ternary TF/FVIIa/FXa complex, FXa assembles with FVa on negatively charged membranes to form the principal enzymatic complex (prothrombinase) driving the generation of thrombin. Formation of the ternary complex of TF, FVIIa, and FX is regulated at the level of TF expression as well as by the redox status of preformed TF (12). With few exceptions, constitutive TF expression is limited to cell types that are sequestered from access to blood and blood components by the endothelial cell barrier of blood vessels. Injury to the endothelial cell barrier facilitates physical contact of TF and FVII and thereby triggers the blood coagulation reaction. Inflammatory mediators compromise endothelial barrier function and induce expression of TF in endothelial cells and various immune cell populations. This activates blood coagulation at sites of inflammation. However, the TF/FVIIa/FXa complex not only initiates coagulation, but can also elicit cellular signaling processes via proteolytic activation of G protein–coupled receptors protease-activated receptor 1 (PAR1) or PAR2, coexpressed on the same cell surface with TF. Signaling via this TF/PAR2 axis couples the initiation of blood coagulation to the stimulation of inflammatory immune cell function.
The ability of activated complement to induce TF expression has been recognized for some time and seemed to explain how thrombosis might contribute to aPL-induced fetal loss: “Simply put . . . [blood] clots form, placental infarction ensues, and embryos die” (13). The study by Redecha and coworkers makes elegant use of genetically altered mice to test and dismiss this hypothesis (10). In order to distinguish the relative role of TF-initiated cell signaling from TF function in blood coagulation, the authors use 3 kinds of genetically altered mice. They show that mice expressing a variant of murine TF that possesses normal procoagulant function, but is defective with respect to signaling as a result of deletion of the cytoplasmic domain, are protected against aPL-induced fetal loss. Likewise, aPL infusion fails to trigger pregnancy failure in mice lacking the candidate downstream receptor target of TF, PAR2. A third approach relies on humanized transgenic mice expressing near-normal levels of human TF in lieu of the murine protein. This clever idea enabled the use of anti–human TF antibodies that preferentially either block the procoagulant function of TF or inhibit its cell signaling function. Only the inhibition of TF’s cell signaling function rescued pregnancies, recreating the results seen in mice expressing the signaling-defective TF variant or those lacking PAR2. Notably, pharmacologic suppression of complement-induced expression of TF and PAR2 by simvastatin and pravastatin was nearly as effective as genetic TF inhibition in preventing fetal loss. This observation provides a strong rationale for further exploring the therapeutic potential of statin drugs in aPL-induced pregnancy complications (Figure 1).
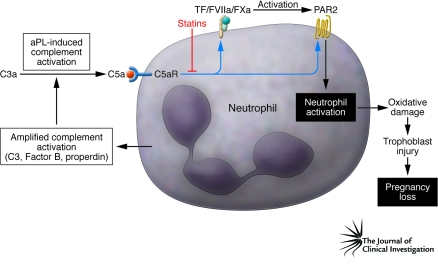
Figure 1. Role of TF/PAR2 signaling in aPL-induced fetal loss.
Maternal aPLs activate the complement system in the placenta. Engagement of C5aR triggers expression by neutrophils of the blood coagulation initiator TF, resulting in generation of reactive oxygen species, injury to fetal trophoblast cells of the placenta, and ultimately loss of the fetus. TF binds and activates coagulation factors FVII and FX circulating in maternal blood to form the ternary TF/FVIIa/FXa complex. The study by Redecha et al. in this issue of the JCI (10) shows that TF-mediated neutrophil activation proceeds through engagement of PAR2 via this initiation complex of blood coagulation. The statin drugs simvastatin and pravastatin prevent C5a-induced upregulation of TF and PAR2 expression, thereby inhibiting the release of reactive oxygen species and suppressing amplification of complement activation by factors released from neutrophils. Statins may hold promise as a therapeutic approach to APS-associated pregnancy complications.
The findings reported here by Redecha et al. (10) are entirely consistent with earlier indications that thrombosis, defined as the pathological formation of blood clots, plays a minor role — if any — in the mouse model of aPL-induced fetal loss. Thrombosis or placental infarctions are conspicuously absent from the placentas of aPL-infused mice, and stringent anticoagulation therapy with heparinoids (fondaparinux) or the selective thrombin inhibitor hirudin cannot prevent fetal loss in mice. Moreover, previous work also shows that the ability of unfractionated or low–molecular weight heparin to prevent aPL-induced fetal loss is not based on its anticoagulant mechanism of action, but on its inhibition of complement activation (14).
While placental thrombosis is not a prominent feature of aPL-induced fetal loss in mice, it is a reasonable assumption that increased TF expression — whether on immune cells in the placenta or on other cells on which complement activation occurs — not only engages PAR2, but also increases the likelihood of thrombosis. Acknowledging that there are limits in transferring findings from the mouse model to humans, the data to date suggest that thrombosis and placental infarction seen in a subset of pregnancies in women with APS may reflect more of a complicating epiphenomenon rather than the causative pathogenic mechanism of aPL-mediated disorders of pregnancy.
aPLs and thrombosis
This stimulating work by Redecha et al. (10) has naturally raised more questions regarding the mechanism of thrombosis caused by aPLs in the absence of pregnancy. As summarized in a recent review (15), inhibition of complement at the level of C3, C5, or C5a ameliorates aPL-induced thrombosis in animal models, clearly implicating complement activation as a contributing factor to the pathogenesis of aPL-mediated thrombophilia (16). However, the picture is probably more complex and may involve TF-independent mechanisms. β2GPI/aPL complexes interact with specific receptors on the surface of platelets, such as the LDL receptor family member ApoE receptor 2/LDLR-related protein 8 and the platelet adhesion receptor GP1bα. Binding to these receptors causes platelet activation (17, 18). These receptors have recently emerged as targets of the key component of the protein C anticoagulant pathway, activated protein C/protein C (19), and the presence of aPL is (at least in a subset of patients) associated with resistance to the anticoagulant activity of activated protein C (non-Leiden activated protein C resistance; ref. 20). Tracing this emerging link between the coagulation mechanism and antibody-mediated inflammation will likely shed more light on the thrombotic pathogenesis of APS.
Footnotes
Nonstandard abbreviations used: -a, activated; aPL, antiphospholipid antibody; APS, antiphospholipid syndrome; β2GPI, β2-glycoprotein I; C5aR, C5a receptor; FVII, Factor VII; PAR, protease-activated receptor; TF, tissue factor.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3276–3278 (2008). doi:10.1172/JCI37243.
See the related article beginning on page 3453.
References
- 1.Salmon J.E., de Groot P.G. Pathogenic role of antiphospholipid antibodies. Lupus. 2008;17:405–411. doi: 10.1177/0961203308090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Laat B., Mertens K., de Groot P.G. Mechanisms of disease: antiphospholipid antibodies-from clinical association to pathologic mechanism. Nat. Clin. Pract. Rheumatol. 2008;4:192–199. doi: 10.1038/ncprheum0740. [DOI] [PubMed] [Google Scholar]
- 3.Levine J.S., Branch D.W., Rauch J. The antiphospholipid syndrome. N. Engl. J. Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- 4.Salmon J.E., Girardi G. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J. Reprod. Immunol. 2008;77:51–56. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmon J.E., Girardi G., Lockshin M.D. The antiphospholipid syndrome as a disorder initiated by inflammation: implications for the therapy of pregnant patients. Nat. Clin. Pract. Rheumatol. 2007;3:140–147. doi: 10.1038/ncprheum0432. [DOI] [PubMed] [Google Scholar]
- 6.Holers V.M., et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girardi G., et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman J., Girardi G., Salmon J.E. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J. Immunol. 2005;174:485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 9.Redecha P., et al. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redecha P., Franzke C.-W., Ruf W., Mackman N., Girardi G. Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J. Clin. Invest. 2008;118:3453–3461. doi: 10.1172/JCI36089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler. Thromb. Vasc. Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 12.Versteeg H.H., Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J. Biol. Chem. 2007;282:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson J.P. Complement system on the attack in autoimmunity. J. Clin. Invest. 2003;112:1639–1641. doi: 10.1172/JCI20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girardi G., Redecha P., Salmon J.E. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat. Med. 2004;10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 15.Salmon J.E., Girardi G. Theodore E. Woodward Award: antiphospholipid syndrome revisited: a disorder initiated by inflammation. Trans. Am. Clin. Climatol. Assoc. 2007;118:99–114. [PMC free article] [PubMed] [Google Scholar]
- 16.Pierangeli S.S., et al. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 17.Pennings M.T., et al. Platelet adhesion to dimeric beta-glycoprotein I under conditions of flow is mediated by at least two receptors: glycoprotein Ibalpha and apolipoprotein E receptor 2’. J. Thromb. Haemost. 2007;5:369–377. doi: 10.1111/j.1538-7836.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 18.Shi T., et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 2006;54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 19.White T.C., et al. Protein C supports platelet binding and activation under flow: role of glycoprotein Ib and apolipoprotein E receptor 2. . J. Thromb. Haemost. 2008;6:995–1002. doi: 10.1111/j.1538-7836.2008.02979.x. [DOI] [PubMed] [Google Scholar]
- 20.Kassis J., et al. Antiphospholipid antibodies and thrombosis: association with acquired activated protein C resistance in venous thrombosis and with hyperhomocysteinemia in arterial thrombosis. Thromb. Haemost. 2004;92:1312–1319. doi: 10.1160/TH04-03-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]